Abstract
We present new high-resolution absorption data for the important sesquioxide laser material Yb:Lu2O3 for the spectral range of 880–1020 nm, at various temperatures between 8 and 300 K, and for the zero-phonon region from 960 to 990 nm, at temperatures from 8 to 300 K. We have experimentally observed the C3i (0,1)–(1,3) transition for the first time, located at 880.7 nm at 8 K. Based on high confidence fitting functions to the experimental data, we provide the first complete compilation of all observed electronic and electronic–vibrational transitions. Detailed fitting and plots of the C2 and C3i zero-line data show an evolution of the linewidth from being predominantly electronic below about 100 K to being dominated by thermal processes above 100 K. We have also found evidence for a “soft” phase transition between 80 and 100 K that changes the local coordination environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction and background
Lutetia is one of three well-known isomorphic sesquioxide host crystals, Lu2O3, Y2O3, and Sc2O3, whose development has been recently driven by the Petermann and Huber groups at The University of Hamburg in Germany [1,2,3]. These crystals were developed as possible alternatives, with more favorable physical properties, to the well-developed legacy oxide host YAG (Y3Al5O12). A number of experimental laser demonstrations have appeared in the literature, mostly exploring the usage of Yb:Lu2O3 as single crystals or transparent ceramics for CW and mode-locked lasers [4,5,6,7,8,9,10,11,12]. The high thermal stability, cubic structure, easy doping, and most importantly, the high thermal conductivity of the doped crystals make it an ideal material for extremely high-power solid-state cryolasers. A particular advantage of Lu2O3 over the other sesquioxide hosts Y2O3 and Sc2O3 is its retention of high thermal conductivity upon doping with Yb3+, given the similarity in mass between Lu3+ and Yb3+ [5, 13]. To accelerate the design and development lutetia-based devices leveraging these advantages, a detailed understanding of the spectroscopy of the Yb3+ ions in this relatively novel lattice is essential. The results of previous collaborations by members of our group have appeared in a number of publications that focused primarily on Yb:Lu2O3 [14,15,16]. In [15] in particular, we presented Yb:Lu2O3 absorption measurements obtained at 80, 150, 200, 250, and 298 K. While we were able to successfully show the presence of the C3i site in the short wavelength shoulder of the main C2 zero-phonon line at 976.21 nm, that became more pronounced as temperature was lowered, our data were noisy and we were unable to fully resolve the zero-phonon linewidth, or to lower the temperature any further. To further explore the variation of the overall spectral absorption, and particularly the evolution of the C3i and C2 absorption features near the zero-phonon line, in this paper, we report the results of our collaboration with researchers at the US Army Research Laboratory whom were successful in obtaining very detailed high-resolution absorption data at a large number of temperatures from 8 to 300 K.
2 Lu2O3 crystal properties
For convenience, the crystalline properties of Lu2O3 are shown in Table 1 [1, 4, 17]. Lu2O3 is an optically isotropic host that provides a strong, robust, easily doped host for trivalent rare-earth ions, which substitute into body-centered Lu sites. Unlike YAG, however, which has one substitutable rare-earth site, Lu2O3 has two six-coordinated lutetium sites, with local point group symmetries of C2 and C3i. The former site has no inversion center, while the latter has a center of inversion. According to the LaPorte selection rule, electric dipole transitions are allowed in the C2 site, while for the C3i site, such transitions are forbidden. Indeed, this conclusion is found to be largely obeyed experimentally, where C2 radiative sites predominate, while the C3i transitions are much weaker. In this paper, however, we show for the first time a systematic substantial variation in the strength of both the C2 and C3i site absorption as temperature is varied between 8 and 300 K, indicating that the local transition probability or oscillator strength of both sites change with temperature, likely due to changes in the local crystal field induced by slowly varying changes in the lattice parameter.
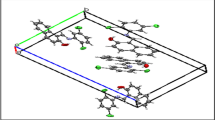
The Lu2O3 unit cell has been found to contain 32 cations, with 24 in the C2 sites and 8 in the C3i sites. In Table 1, we also provide nearest Lu–Lu inter-ion distances and Lu–O distances for both the C2 and C3i sites, as these are significant in the detailed spectral interpretation below. Figure 1 shows a structural rendering of the adjoining C3i (left) and C2 (right) Lu sites, with the site on the left having inversion symmetry, while the site on the right does not. In Fig. 2, the simple generic energy level structure of the Yb3+ ion in a crystalline host is shown; the 2F7/2 ground-state consists of four discrete Kramers doublet energy levels, while the 2F5/2 excited-state consists of three Kramers doublet discrete levels. Also shown is the labeling of each level used in this paper.
3 Crystal growth and polishing
Crystal growth from hydrothermal solution took place in closed, pure silver reaction tubes containing the powdered feedstock along with aqueous mineralizer to dissolve and transport the material during the growth cycle. In the case of Yb:Lu2O3 discussed here, rare-earth oxide powders (HEFA Rare Earth, 99.997%) of Lu2O3 and Yb2O3 were measured in the desired doping ratio (3%) such that the total powdered material was approximately 1.5 g and added directly to ¼” OD × 7” long silver tubes that had been carefully cleaned, weld-sealed, and inspected for integrity. The powdered feedstock, along with 3 mL of a 20 M aqueous KOH solution, was added before the tubes were welded closed. These loaded tubes were themselves inserted into Inconel autoclaves equipped with two external, individually controlled ceramic band heaters with temperature probes and pressure gauge assemblies to monitor temperatures and pressure throughout the entire growth process. Water added inside the autoclaves provides the counter pressure necessary to prevent bursting of reaction tubes when heated. The fully loaded and prepared autoclaves were then lowered into insulating pits and covered up to the pressure gauge in vermiculite. A temperature gradient was established with the band heaters of 670 °C for the top, growth zone, and 700 °C in the lower, dissolution zone, for this prograde solubility system. These temperatures typically resulted in pressures of around 207 MPa (30,000 psi). The autoclaves were left undisturbed in these insulated pits during the entire growth run of between 12 and 14 days. After this time, the heat was discontinued and autoclaves were left to cool naturally over several hours or quick-quenched by an air stream. The cooled reaction tubes were then carefully cut open to reveal various-sized single crystals of up to several mm on a side. While the temperature used in the present study represents a seemingly modest increase over our previous work [16], we observe improved faceting and a greater amount of optically clear regions in the as-grown crystals, compared to those grown at 630–650 °C. We postulate that moving the growth conditions farther away from the Lu2O3/LuO(OH) phase transition around 600 °C may be the source of such positive effects.
Crystals determined to be of sufficient optical quality for spectral analysis were further prepared, if necessary, by polishing. Progressive lapidary discs were used starting with 1200 grit and ending with 100,000 mesh diamond spray. The crystal used in the present study was fabricated to dimensions of approximately 4 × 4 × 0.39 mm, as shown in Fig. 3.
4 Absorption spectrophotometer and cryostat description
We performed very high-resolution absorption measurements using a Varian Cary 6000i UV–VIS–NIR absorption spectrophotometer that incorporated a CTI Cryodyne cryogenic refrigerator that included a heater, that was used to vary the absolute temperature between 8 and 300 K. The spectral resolution desired was set by computer control of the slit widths. For the Yb:Lu2O3 absorption data reported here, the spectral resolution was set to 0.15 nm for the broad scans, and to 0.05 nm for the narrow zero-line scans. The refrigerator contained two CaF2 windows for the purpose of passing the absorbing beam through the cooled sample under high vacuum conditions to avoid water vapor condensation.
5 Yb:Lu2O3 experimental absorption data
In this paper, we present what we believe to be the first comprehensive set of high-resolution absorption data for Yb:Lu2O3 spanning the temperature range from 8 to 300 K. A thin hydrothermally grown 390 µm thick Yb:Lu2O3 single-crystal was prepared by careful polishing to avoid saturation of the zero-phonon line near 976 nm at low temperatures where the line intensities are substantially larger than at room temperature. The calculated crystal Yb3+ ion density was 3.0 at-%. In Sect. 5.1, we show obtained data in the wavelength interval from 880 to 1020 nm. The data were obtained with a spectral resolution of 0.15 nm, and a sampling interval of 0.05 nm. In Sect. 5.2, we show detailed zero-line data around 976 nm, obtained with a spectral resolution of 0.05 nm and a sampling interval of 0.02 nm. All data reported in this paper are for wavelengths in air, and have not been converted to vacuum wavelengths.
5.1 Overview and discussion of absorption data from 880 to 1020 nm
Figures 4 and 5 plot the absorption coefficient as a function of wavelength for the absolute temperatures of 8, 30, 50, 75, 100, 150, 200, 250, and 300 K. Using the absorbance from the spectrophotometer, and correcting for the two Fresnel reflections of the Yb:Lu2O3 crystal using the Sellmeier equation of [18], and the four Fresnel reflections of the CaF2 windows using the Sellmeier equation of [19], and the sample thickness, we obtained absorption coefficients for each temperature.
Transitions observed in the region from about 890–980 nm are attributable to three well-known transitions from the ground-state 2F7/2 manifold to the sole excited-state 2F5/2 manifold near 905 nm, 945 nm, and 975 nm [the (0,0) electronic zero-line]. Those transitions occur in Yb:Lu2O3 near 905 nm, 949 nm, and 976 nm as may be observed in Figs. 3, 4, 5, 6, 7, 8, 9, 10 and 11. A multitude of smaller peaks can be seen on the Stokes side of the 949 nm transition, and are likely electronic–vibrational in nature, with more than one vibrational mode contributing to the observed spectra. The sharp peak at 949.2 nm is the first (0, 1) vibrational zero-phonon line. Assuming that a number of vibrational modes contribute to the observed progressions, the peak at 949.2 nm is then viewed as the first zero (0–1) vibrational line with a phonon wing, followed by a number of additional (0–2), and perhaps (0–3) vibrational zero-lines and their phonon wings since the pure electronic zero-phonon line is common to all modes [20].
Variation in center wavelength with temperature for the nine peaks defined in Fig. 6
Variation of absorption coefficient with temperature for the nine peaks defined in Fig. 6
It is noteworthy that vibrational–electronic transitions are also observed on the anti-Stokes side of the 905 nm transition, albeit with much smaller amplitudes than those associated with the 949 nm transition. It is possible that those transitions are in fact an extension of the Stokes electronic-vibrational progressions associated with the 949 nm line. In addition, two weak broad (diffuse) transitions are observed at about 957.0 nm and 964.3 nm that have an unknown origin. Their proximity to the (0, 0) zero-phonon-line near 976 nm, however, suggests that a weak coupling between the zero-phonon electronic transition and an unknown vibrational mode may be occurring.
A number of interesting features can be observed as temperature is varied. The 905 nm transition is most well resolved at lower temperatures, and gradually (particularly above about 150 K) transitions to a more smoothed profile as temperature increases to 300 K. The center wavelength is nearly constant as the temperature increases, but above about 150 K the amplitude is diminished and the FWHM bandwidth increases substantially. For the 945 nm transition, the lines are best resolved at low temperatures, with all peaks having a near constant value up to about 100 K. At higher temperatures, the main peak as well as the secondary peaks diminish as temperature continues to rise. In addition, as with the 905 nm transition, all peaks display smoothing, an increase in FWHM bandwidth, and a substantial reduction in amplitude as temperature rises to 300 K.
The zero-line at about 976 nm displays the most interesting behavior. There are two distinct lines in the region that can be observed from 8 K all the way up to 300 K. The smaller line has been previously identified as the C3i zero-phonon line [1]. That substantial intensity is observed is due to LaPorte’s rule not being completely satisfied for the C3i site (i.e. it does not identically have complete inversion symmetry). The obtained data show conclusively that the C3i and the C2 lines remain adjacent throughout the entire temperature range, and that at about 75 K appear to begin merging, a process that continues to 300 K. The merging, however, appears to be due to the increased linewidth of the C2 transition, as well as to the increased amplitude. The C2 transition displays an amplitude that is nearly constant up to about 75 K; thereafter, the amplitude rapidly increases until 150 K, and then continuously decreases up to 300 K. We observed this behavior previously [15], but the higher resolution data presented here allow a much more detailed look at the evolution of both the C3i and C2 sites as temperature is varied over a wide range. Up to liquid nitrogen temperature near 77 K, the C3i and C2 sites can be assumed to be mostly independent of one another, and cross-sections can be assigned to each since the C3i contains ¼ of all Yb ions, and the C2 site ¾. At room temperature, however, the merging of the absorption transitions of both sites means that both contribute to the total absorption and a means of assigning the contribution of each can produce good fitting functions to the observed profiles.
5.2 Variation of center wavelength and amplitudes with temperature
In Fig. 6, we identify nine lines in the 880–980 nm region that we chose to examine to determine the variation in the center wavelength and amplitude with temperature. The nine lines include the three 2F7/2–2F5/2 electronic absorption transitions (1, 7, 9), the major electronic-vibronic transitions (2, 3, 4, 5, 6), both associated with the C2 site, and the C3i zero-phonon transition (8). Table 2 shows the measured values for the center wavelength for nine temperatures for each of the lines. Also shown are the average value, the standard deviation, and the percent variation over the temperature interval. Note that for lines 4, 5, 6, and 8, wavelength values could not be determined beyond that temperature at which the line became difficult to measure because the amplitude had merged with the growing background. Figure 7 is a plot of the variation of the center wavelength with temperature, and used the data of Table 2. The standard deviations and percent change over the temperature intervals are seen to be very small for all nine lines. The C3i zero-phonon line (8) has no variation with temperature at all, and the C2 zero-phonon line has a very small change. Line 1 displays a relatively large change in wavelength with temperature, and the vibrational line 3 the largest change.
Table 3 shows the variation of the absorption coefficient with temperature for all nine lines; the data are all plotted in Fig. 8. The most pronounced variation was found for the C2 zero-phonon line (9), that began to increase at about 50 K, peaks at about 150 K, and then continuously decreases to 300 K. The C3i zero-phonon line (8) also increased with temperature starting at about 75 K, and then increased up until 200 K. A large variation is also seen in line 7, the zero-phonon vibrational line, which increases up to about 75 K, and then continuously decays as the temperature increases. The remaining lines (1–6) display minor changes with temperature, with all decreasing above about 150 K. In general, lines 2–6 are associated with vibrational bands with amplitudes that decrease with temperature above about 50–75 K. The C2 and C3i zero-phonon lines, however, undergo changes that seem to be primarily thermally driven above about 150–200 K. From the lowest temperatures to 150–200 K, however, the amplitudes grow significantly, indicating that important changes (distortions) in the local crystalline field are taking place. In Sect. 5.6 of this paper, we present more detained data for the zero-phonon lines at 15 temperatures, and further examine this point.
5.3 Determination of Yb:Lu2O3 energy levels and comparison to previously obtained results
In Table 4, we show the energies of the four (0, 1), (0, 2), (0, 3), and (0, 4) 2F7/2 levels in the ground-state manifold and the three (1, 1), (1, 2), and (1, 3) levels in the 2F5/2 excited-state manifold, obtained from a number of prior publications at the temperatures shown. All values have units of (cm−1). Rows 1 and 2 show the results reported in the Dissertation of Peters [2] for both the C2 and C3i sites, but at unknown temperatures (U). The most recent tabulation of values, found in the paper by Guyot et al.[21], are shown in rows 3–4 for the C2 and C3i sites, respectively, at 10 K. We also show in rows 5 and 6 values for the (1, 1) level in both the C2 and C3i sites, as reported by Petermann at 10 K [3]. Finally, we show values for the (1, 1), (1, 2), and (1, 3) C2 and C3i levels in rows 7 and 8 as determined by the authors of the present paper at 8 K. The energy levels quoted by Guyot et al. [21] align very closely to our values, and in rows 9 and 10, we show the differences between our values and those of [21] for C2 (ΔC2) and C3i (ΔC3i), respectively. The energies agree particularly well for the C2 site, although the C3i energy levels are close as well. The maximum disagreement for the (1, 3) levels amounts to only 0.15%.
The values we determined for the zero-phonon line C2 and C3i sites of 10,250.10 and 10,262.73 cm−1 were obtained from the data of Table 2 at 8 K. By comparison of our absorption data with that of [21], we expected to find a low-intensity line at 882.07 nm. Close examination of the baseline data of Fig. 3 yielded the peak shown in Fig. 9 at 8 K, which we determined to be located at 880.70 nm. To measure the (1, 2) energy for the C2 site at 8 K, we use the value shown in Table 2 of 949.2 nm, (10,535.19 cm−1). To identify the C3i energy for the (1, 2) level, we used a complete fit of all the electronic and phonon transitions in the range of 925–955 nm discussed in Sect. 5.4 of this paper. Again, using the previously published value of [21], we expected to see a transition at about 942.06 nm; examination of the fits shown later in Fig. 12 revealed a low-intensity transition at 941.4 nm, or 10,622.48 cm−1. It should be noted that the location of the (1, 3) line is not exactly at 903.80 nm; the real location is 903.907 nm, since it should likely have a Gaussian linewidth as do the other C2 (1, 1) and (1, 2) transitions.
5.4 Detailed fitting of All Yb:Lu2O3 transitions outside zero-phonon line region at 8 K
5.4.1 Fitting functions and peak identification for Yb:Lu2O3 825–926 nm region
We performed extensive interactive fits of all the peaks identified in the 825–956 nm spectral region that contains the C2 (0, 1)—(1, 3) electronic transition as well as a multitude of electronic-vibrational transitions. Most of our fits were generated using Fityk, which enables users to specify any number of standard fitting functions including Gaussian, Lorentzian, Voight, Pearson, and many others. In the work described here, for simplicity we restricted ourselves to standard Gaussian and Lorentzian functions only, and found that more than adequate results could be obtained with excellent chi-squared fitting values.
Figure 10 shows the obtained results: plots of the experimental data, each transition used to fit the total curve, and the resulting total fit which is nearly indistinguishable from the starting absorption coefficient data. The χ2 value obtained for this set of functions was 99.986%. The best overall fit was found to contain one Gaussian and 19 Lorentzian functions. It should be pointed out, however, that while the fit shown here is very good, there is no guarantee of uniqueness and many other fits may exist that give similar results. Our purpose here is thus to provide one set of functions that fit the experimental data very well, that other researchers may use as a guide in developing a more rigorous approach. In Table 5, we show the transition number, the fitting function type and number, the center wavelength, peak absorption coefficient, FWHM bandwidth, and the center wavenumber for each fitting transition used, and have added the C3i transition at 880.677 nm (transition number 1) as well, that was found to be best fitted as a Gaussian with the parameters shown. Transition number 4 corresponds, we believe, to the C2 (0, 1)–(1, 3) transition.
If we remove the total absorption coefficient, the fit to the absorption coefficient, and the C2 transition, the remaining transitions are shown in Fig. 11, and are believed to be primarily electronic-vibrational in origin.
5.4.2 Fitting functions and peak identification for Yb:Lu2O3 925–954 nm region
We performed the same type of fitting analysis of the 925–954 nm region (Fig. 12). Table 6 shows the various Lorentzian and Gaussian functions used and their parameters The χ2 value obtained for this set of functions was 99.950%. A total of 30 Lorentzian functions and a single Gaussian function were used. After removing the electronic functions, the results shown in Fig. 13 are obtained: all transitions are believed to be electronic–vibrational in nature.
5.5 Yb:Lu2O3 electronic–vibrational transitions
It has been noted in the literature [22] that Yb-doped laser materials display strong electron–phonon coupling, as do a number of other ions at the beginning and end of the lanthanide series. A decrease in the shielding of the 4f electrons by 5 s and 5p is considered to be the main factor for the large electron–phonon coupling for Yb3+. It has also been pointed out that Stark splittings of rare-earth ions in crystals are on the order of typical lattice optical phonon, so that in addition to normal electron–phonon coupling, near-resonance processes may appear and couple the vibronic state associated with one electronic level to those of another.
Here, we see that for Yb:Lu2O3, the vibrational structure is rich and dense. Through detailed modeling with fitting software, we identified the many (46) vibrational lines in the Yb:Lu2O3 absorption spectral region from 880 to 954 nm at 8 K by subtracting out the known C2 and C3i electronic transitions. A detailed analysis of the observed vibrational lines using the formulation of [22] could likely yield insights into the observed vibrational structure, but such an analysis is beyond the scope of this paper. There are, however, a number of interesting features that we will point out here. First, the vibrational lines are all (with the exception of the two small diffuse bands observed) observed to occur between the (0, 1)–(1, 2) and (1, 3) electronic transitions at 949.19 and 902.83 nm, with the largest number of transitions found closer to the 949.19 nm line. The vibrational lines are Stokes shifted from the 949.19 nm line, and anti-Stokes with respect to the 902.83 nm line. Because anti-Stokes lines are less probable than Stokes lines, it is possible that most of the observed vibrational lines originate from the coupling of multiple vibrational modes to the (0, 1)–(1, 2) electronic transition. In addition, multiple vibrational modes can produce additional modes with energies at multiples of the fundamental vibrational frequencies. Compilations of the Raman-determined modes, including those at most fundamental modes have been tabulated in [23, 24] for Lu2O3, and span the wavenumber range from 65 to 612 cm−1. The line at 949.189 nm is very likely the first vibrational (0,1) zero-phonon line, and the peaks identified as lines 4, 5, and 6 in Fig. 6 are likely part of the phonon tail; lines 2 and 3 may be additional vibrational zero-phonon lines (0, 2) and (0, 3), also with accompanying phonon tails. The wavenumber difference between the 949.19 nm and 902.83 nm transitions is about 541 cm−1, or close to an Fg vibration at 499 cm−1, and twice a known vibration at 264 cm−1.
To help identify the origin of the vibrational modes, Table 7 lists all the identified vibrational modes in the spectral region between 880 and 949.189 nm, the associated wavelengths and wavenumbers, the vibrational offset between the vibrational mode and the zero-vibrational mode at 949.190 nm, and the known Raman-determined vibrational modes for Lu2O3. Several of the offsets are very close to known vibrational modes. Without detailed modeling of the coupled electronic and vibrational states, however, we cannot be certain whether the near agreement between the offsets and known vibrational modes is simply fortuitous or has a deeper foundation.
Finally, it is notable there are no significant vibrational modes associated with the zero-phonon line at 975.584 nm, although as we shall discuss in Sect. 5.6 of this paper, the zero-line at low temperatures can be fit to show the existence of three separate lines, one Gaussian believed to be the electronic transition, and two additional Lorentzian lines whose origin we discuss.
5.6 Complete compilation of all identified Yb:Lu2O3 transitions at 8 K
For convenience, we combined the data from Tables 6 and 7 into Table 8, and also added the diffuse transitions near 957.50 and 964.35 nm, and the fitting parameters for the C3i and C2 zero-phonon lines, each of which could be decomposed to one Gaussian and two Lorentzian functions. The detailed zero-phonon spectra will be discussed in Sect. 5.6 of this paper. We can see, therefore, that the complete spectrum of Yb:Lu2O3 at 8 K consists of 59 discrete electronic and vibrational lines.
5.7 Zero phonon line region data and fits
Figures 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 and 26 show the measured absorption coefficients at a range of temperatures from 8 to 300 K for the zero-phonon region of 974–977 nm. The region includes both the C2 and C3i lines. We performed extensive fitting for each temperature, and found that excellent agreement between the experimental data and the fits can be obtained using Gaussian and Lorentzian fitting functions. For all cases examined the goodness of fit, as measured by the calculated χ2 value, exceeded 99.9%. We did not examine whether equivalent or better fits might be obtained with a Voigt or other common functions. We note that the total fit and absorption coefficient data have a high degree of overlap, and in some of Figures 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 and 26 are indistinguishable from one another. Table 9 lists, for each temperature, the site type, fitting function type, and the center wavelength, FWHM bandwidth, and absorption coefficient for each individual function.
The fitting function parameters of Table 9 can be used to generate a number of interesting plots, and to provide for the first time a detailed look at the variation in parameters with temperature for both the C2 and C3i transitions. Figure 27 shows the absorption coefficient and FWHM bandwidth as a function of temperature for the Gaussian component of the C2 line. The Gaussian component corresponds, we believe, to the pure electronic part of the linewidth, and is maximum at 8 K. It gradually decreases to about 100 K, and then dramatically reduces in intensity between 100 and 150 K, remaining constant thereafter. The FWHM bandwidth is seen to remain constant up to about 100 K, and then to continually increase up to 300 K. In Fig. 28, we show the C2 Lorentzian absorption coefficient and FWHM bandwidth; the Lorentzian component, that we believe to be primarily a measure of the thermal part of the bandwidth, increases beginning at the lowest temperatures monotonically until 90 K, where a sudden drop occurs. Thereafter, the intensity increases to 150 K, where the intensity decreases dramatically up to 300 K. The FWHM bandwidth also increases up to 90 K where a sudden drop occurs, and thereafter substantially increases up to 300 K. In Fig. 29, we plot the absorption coefficient and FWHM intensity for the smaller Lorentzian C2 component located at 975.371 nm at 8 K. This transition gradually reduces in intensity and increases in bandwidth until 80 K, whereupon it disappears completely up to 300 K. This transition is likely the result of a C2–C2 cation nearest-neighbor interaction, resulting in a Stokes shifted component, as has been found recently by Guzik et al. [25]. Because the nearest-neighbor interaction is not strong for Yb:Lu2O3, the Stokes shift occurs inside the zero-line profile, unlike for Nd:Lu2O3 where the Stokes shifted line may be found in the shoulder of the zero-line profile [25]. In Fig. 30, the absorption coefficient and FWHM bandwidth are plotted for the main Gaussian electronic part of the C3i forbidden line. The absorption coefficient and bandwidth are seen to be more or less constant until between 100 and 150 K where a dramatic drop in the C3i Gaussian intensity takes place, while the FWHM bandwidth increases beyond 150 K, and becomes very large at 300 K. The behavior of the C3i Gaussian transition and the C2 Gaussian transition (Fig. 27) are in fact very similar. For the C3i main Lorentzian transition, shown in Fig. 31, we see a gradual increase in the absorption coefficient intensity up to 100 K, a large increase between 100–150 K, and then thereafter a dramatic decrease up to 300 K. The FWHM bandwidth gradually decreases until about 100 K, and then at 150 K begins to increase substantially up to 300 K. The behavior is thus very similar to the C2 Lorentzian data of Fig. 28.
In Fig. 32, we use the fitting functions for the zero-lines summarized in Table 9 to calculate the total (whether C2 or C3i) Gaussian and Lorentzian areas for each temperature, and the sum of the Gaussian and Lorentzian areas to find the total area. It is clear that at about 80–90 K, the nature of the zero-line changes from being primarily Gaussian to being predominantly Lorentzian with an intensity that exceeds the Gaussian intensity up to 300 K.
Figures 27, 28, 29, 30, 31 and 32 offer a comprehensive view of the many interesting changes that accompany the Lu2O3 structure as temperature is varied. The disappearance of the C3i small Stokes-shifted Lorentzian component at 80 K is very likely the result of the thermal expansion of the unit cell as temperature increases, which increases the nearest-neighbor distances of the cations. This view is supported by the near equality of the Gaussian and Lorentzian areas that occurs near 100 K, and the growth of Lorentzian (thermal) contributions to the linewidth over Gaussian (electronic) contributions beyond 100 K. Due to the thermal expansion of the unit cell, and the resulting changes to the local coordination environment, it also seems clear that the local crystalline field experienced by each Lu cation in the C2 site becomes weaker as temperature rises. The reduced local field can be attributed, at least partially to thermal expansion, or to changes resulting from a change in the local (distorted) environment. Between 80 and 100 K, it appears that a soft phase-transition involving the rearrangement of the local coordination environment may be taking place, resulting in a reduced local field and a lower electronic transition probability. To examine this we conducted a series of low temperature X-ray diffraction experiments described in Sect. 6.0 of this paper, and discovered that subtle but apparently important changes in the structure are indeed occurring.
6 X-ray analysis of Lu2O3 coordination environments
To help determine the cause of the unusual low temperature absorption behavior, we obtained full single-crystal data sets at a range of temperatures between 100 and 300 K by single-crystal X-ray diffraction. We wished to test whether there was any detectable distortion or symmetry reduction/alteration about the Lu sites that could be leading to the observed optical effects. We obtained good quality structure refinements consistent with the bixbyite structure type, and did not observe deviations in site symmetry or space group as a function of temperature. As expected, the lattice parameter contracts as a function of lower temperatures (Fig. 33), but we did not observe any preferential contraction/expansion of the Lu–O bond lengths that resulted in any change in site symmetry. We do, however, observe an unusual structural curiosity around 150 K. At these lower temperatures, the structure refinement was significantly improved by switching the Lu1 atom (which is the centrosymmetric C3i site) from the 8b Wyckoff site above 150 K to the 8a Wyckoff site at and below 150 K. The symmetry does not change, but this positional switch also causes the Lu2 site to move to a different 24d site (still having C2 symmetry), and the oxygen atom likewise moves to a different 48e site (Table 10). Both models (above and below 150 K) have the same local arrangements of atoms, but the structure refines significantly better when the specific arrangements are used for higher temperatures versus lower temperatures. This curious phenomenon was observed for multiple crystals tested in the same temperature range. Both arrangements are reported in the literature for oxides of the bixbyite structure type [17, 26,27,28]. We are not yet sure if this is a contributing factor to the observed optical properties that occur at or near the same temperature, but decided it at least warranted mention here.
7 Discussion and conclusions
In this paper, we have presented the results of a comprehensive study of the variation of the detailed spectroscopic absorption properties of the laser material Yb:Lu2O3 as temperature is varied between 8 and 300 K. Because of the wide range of temperatures used in our measurements, it is now possible to see in a glance the evolution of the general absorption features as a function of temperature. Our measurements, when combined with the detailed fitting functions, and a least-squares approach to fitting the experimental data, also yielded many fine details of the observed electronic-vibrational transitions, and of the zero-line data.
We experimentally observed for the first time, the C3i (0, 1)–(1, 3) transition at 880.7 nm at 8 K, and based on the detailed high confidence fits to the experimental data, present for the first time a compilation of all 59 electronic and vibrational transitions observed, including the center wavelength, bandwidth, and absorption coefficient amplitude. Our high-resolution zero-line data make it possible to observe the detailed relationship of the C2 and C3i zero-lines, and the way in which they merge at higher temperatures. Detailed fitting of the zero-lines also allowed us to identify for the first time a C2 satellite Stokes transition attributable to nearest-neighbor cation interactions, which disappears at about 80 K and is unobservable at higher temperatures. Through detailed modeling of the zero linewidths, we observed the evolution of individual lines with temperature for both the C2 and C3i sites. They also show the progression of the linewidth from having a mostly electronic (Gaussian) nature below 100 K to being dominated by thermal processes (Lorentzian) at higher temperatures. When taken as a whole, it is clear from the data that something significant happens to the Yb3+ ion in the lattice between 80–100 K, and we identified a “soft” phase-transition from our X-ray measurements of the coordination environment that support this conclusion. Furthermore, recently, a closely related crystal, Yb:Y2O3, has been found to behave in a very similar way, with the total area of the zero-line varying in much the same way as shown here for Yb:Lu2O3 (Fig. 32) [29]. In our study, a single hydrothermally grown laser crystal was used, whereas in [29], the samples were ceramic. This close similarity between the two results reduces the likelihood that the variation in the zero-line strength is due to some growth-related strain or to an inadvertent impurity.
References
K. Petermann, G. Huber, L. Fornasiero, S. Kuch, E. Mix, V. Peters, S.A. Basun, Rare-earth-doped sesquioxides. J. Lumin. 87–89, 973–975 (2000)
V. Peters, Growth and spectroscopy of ytterbium-doped sesquioxides, PhD Dissertation, University of Hamburg 2001
K. Petermann, L. Fornasiero, E. Mix, V. Peters, High melting sesquioxides: crystal growth, spectroscopy, and laser experiments. Opt. Mater. 19, 67–71 (2002)
R. Peters, C. Krankel, K. Petermann, G. Huber, Broadly tunable high-power Yb:Lu2O3 thin disk laser with 80% slope efficiency. Opt. Express 15, 7076–7082 (2007)
U. Grieber, V. Petrov, K. Petermann, V. Peters, Passively mode-locked Yb:Lu2O3 laser. Opt. Express 12, 3125–3130 (2004)
M. Tokurakawa, A. Shirakawa, K. Ueda, H. Yagi, S. Hosokawa, T. Yanagitani, A.A. Kaminskii, Diode-pumped 65 fs Kerr-lens mode-locked Yb3+:Lu2O3 and nondoped Y2O3 combined ceramic laser. Opt. Lett. 33, 1380–1382 (2008)
N. Modsching, J. Drs, J. Fischer, C. Paradis, F. Labaye, M. Gaponenko, C. Krankel, V. Wittwer, T. Sudmeyer, Sub-100-fs Kerr lens mode-locked Yb:Lu2O3 thin-disk laser oscillator operating at 21 W average power. Opt. Express 27, 16111 (2019)
K. Takaichi, H. Yagi, A. Shirakawa, K. Ueda, S. Hosokawa, T. Yanagitani, A.A. Kaminskii, Lu2O3:Yb3+ ceramics—a novel gain material for high-power solid-state lasers. Phys. Stat. Solidi (a) 202, R1–R3 (2005)
A.A. Kaminskii, S.N. Bagayev, K. Ueda, K. Takaichi, A. Shirakawa, S.N. Ivanov, E.N. Khazanov, A.V. Taranov, H. Yagi, T. Yanagitani, New results on characterization of highly transparent C-modification Lu2O3 nanocrystalline ceramics: room-temperature tunable CW laser action of Yb3+ ions under LD-pumping and the propagation kinetics of non-equilibrium acoustic phonons. Laser Phys. Lett. 3, 375–379 (2006)
A. Pirri, G. Toci, M. Vannini, First laser oscillation and broad tunability of 1 at% Yb- doped Sc2O3 and Lu2O3 ceramics. Opt. Lett. 36, 4284–4286 (2011)
J. Sanghera, W. Kim, C. Baker, G. Villalobos, J. Frantz, B. Shaw, A. Lutz, B. Sadowski, R. Miklos, M. Hunt, F. Kung, I. Aggarwal, Laser oscillation in hot pressed 10% Yb3+:Lu2O3 ceramic. Opt. Mater. 33, 670–674 (2011)
D. Yin, J. Ma, P. Liu, B. Yao, J. Wang, Z. Dong, L.B. Kong, D. Tang, Submicron-grained Yb:Lu2O3 transparent ceramics with lasing quality. J. Am. Ceram. Soc. 102, 2587–2592 (2019)
C. McMillen, D. Thompson, T. Tritt, J. Kolis, Hydrothermal single-crystal growth of Lu2O3 and lanthanide-doped Lu2O3. Cryst. Growth. Des. 11, 4386–4391 (2011)
D.C. Brown, C.D. McMillen, C. Moore, J.W. Kolis, V. Envid, Spectral properties of hydrothermally-grown Nd:LuAG, Yb:LuAG, and Yb:Lu2O3 laser materials. J. Lumin. 148, 26–32 (2014)
C.A. Moore, D.C. Brown, L.D. Sanjeewa, C.D. McMillen, J.W. Kolis, “Yb:Lu2O3 hydrothermally-grown single-crystal and ceramic absorption spectra obtained between 298 and 80 K. J. Lumin. 174, 29–35 (2016)
C.D. McMillen, L.D. Sanjeewa, C.A. Moore, D.C. Brown, J.W. Kolis, Crystal growth and phase stability of Ln:Lu2O3 (Ln=Ce, Pr, Nd, Sm, Eu, Tb, Dy, Ho, Er, Tm, Yb) in a higher temperature hydrothermal regime. J. Cryst. Growth 452, 146–150 (2016)
M. Guzik, J. Pejchal, A. Yoshikawa, A. Ito, T. Goto, M. Siczek, T. Lis, G. Boulon, Structural investigations of Lu2O3 as single crystal and transparent ceramic. Cryst. Growth Des. 14, 3327–3334 (2014)
D.E. Zelmon, J.M. Northridge, N.D. Haynes, D. Perlov, K. Petermann, Temperature-dependent Sellmeier equations for rare-earth sesquioxides. Appl. Opt. 52, 3824–3827 (2013)
H.H. Li, J. Phys. Chem. Ref. Data 9, 161–289 (1980)
M. Orrit, Single molecular spectroscopy, in Quantum optics and nanophysics, ed. by C. Fabry, V. Sandoghdan, N. Treps, L.F. Cugliandolo (Oxford University Press, Oxford, 2017)
Y. Guyot, M. Gusik, G. Alombert-Goget, J. Pejchl, A. Yoshikawa, A. Ito, T. Goto, G. Boulon, “Assignment of Yb3+ energy levels in the C2 and C3i centers of Lu2O3 sesquioxide either as ceramics or as crystal. J. Lumin. 170, 513–519 (2016)
A. Lupei, A. Lupei, C. Presura, V.N. Enaki, A. Petraru, Electron-phonon coupling effects on Yb3+ spectra in several laser crystals. J. Phys. Condens. Matter 11, 3769–3778 (1999)
L. Laversenne, Y. Guyot, C. Goutaudier, MTh Cohen-Adad, G. Boulon, Optimization of spectroscopic properties of Yb3+-doped refractory sesquioxides: cubic Y2O3, Lu2O3, and monoclinic Gd2O3. Opt. Mater. 16, 475–483 (2001)
M.V. Abrashev, N.D. Todorov, J. Geshev, Raman spectra of R2O3 (R-rare earth) sesquioxides with C-type bixbyite crystal structure: a comparative study. J. Appl. Phys. 116, 103508-1–103508-6 (2014)
M. Guzik, G. Alombert-Goget, Y. Guyot, J. Pejchal, A. Yoshikawa, A. Ito, T. Goto, G. Boulon, “Spectroscopy of C3i and C2 sites of Nd3+-doped Lu2O3 sesquioxide either as ceramics or crystal. J. Lumin. 169, 606–611 (2016)
S. Geller, Structures of α-Mn2O3, (Mn0.983Fe0.017)2O3 and (Mn0.37Fe0.63)2O3 and relation to magnetic ordering. Acta Cryst. B27, 821–828 (1971)
L. Pauling, M. Shappell, The crystal structure of bixbyite and the C-modification of the sesquioxides. Z. Kristallogr. 75, 128–142 (1930)
H. Kohlmann, The crystal structure of cubic C-type samarium sesquioxide, Sm2O3. Z. Naturforsch. B 74, 433–435 (2019)
L.D. Merkle, G.A. Newburgh, N. Ter-Gabrielyan, A. Michael, M. Dubinskii, Temperature-dependent lasing and spectroscopy of Yb:Y2O3 and Yb:Sc2O3. Opt. Commun. 281, 5855–5861 (2008)
Acknowledgements
Work at Clemson was supported by the National Science Foundation through grant NSF DMR-1808371
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Brown, D.C., Fleischman, Z., Merkle, L.D. et al. Yb:Lu2O3 hydrothermally grown single-crystal high-resolution absorption spectra obtained between 8 and 300 K . Appl. Phys. B 126, 62 (2020). https://doi.org/10.1007/s00340-020-7412-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00340-020-7412-8