Abstract
Recently, we reported on the development of a series of Dy-doped organic molecular crystals (with analogous material properties to energetic organic molecular crystals) for use as two-color thermometry phosphors in thermometric imaging of heterogeneous materials under shock compression. In this study, we demonstrate thermometric imaging of one of these phosphors—Dy:Y(acac)\(_3\)(DPEPO)—under pulsed laser heating and compare its performance to the well-known two-color thermometry phosphor Dy:YAG. Based on these comparisons, we indirectly confirm that Dy:Y(acac)\(_3\)(DPEPO) is a suitable two-color thermometric phosphor for shock compression experiments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Shock compression of heterogenous materials is known to produce hot spots through mechanisms such as pore collapse [1], binder/crystal interface friction, crystal/crystal friction, microfractures, and plastic deformation [2,3,4]. These hot spots are believed to be the initiating mechanism of detonation in plastic-bonded explosives [1,2,3,4,5,6,7,8,9,10,11,12,13,14]. While hot-spot formation has been studied extensively, it is currently unknown how important each mechanism is to thermally initiated decomposition given specific loading conditions and material compositions [3].
To better understand hot-spot formation in heterogenous materials, it is necessary to perform simultaneous imaging thermometry and microstructural imaging on the ns–\(\upmu\)s time scale. Currently, the ability to perform microstructural imaging under dynamic shock (using synchrotron X-ray phase contrast imaging [15,16,17,18,19]) exists at the Dynamic Compression Sector of the Advanced Photon Source [20, 21]. Therefore, the next technique to develop is imaging thermometry with the required spatiotemporal resolution. To address this need, we have proposed using two-color thermometric imaging (TCTI), which has previously been applied to slower heating events (e.g., furnaces and turbine engines) [22,23,24,25,26,27,28].
TCTI determines temperature by measuring the temperature-dependent photoluminescence (PL) from a phosphor that has two closely spaced excited state energy levels. When this phosphor is excited using an appropriate wavelength, the two energy levels become populated, with their ratio being given by a Boltzmann distribution:
where \(n_i\) is the population of the \(i{\text {th}}\) level, T is the temperature, k is Boltzmann’s constant, and \(\Delta E\) is the energy spacing between the excited states. When these two states relax they emit PL with the intensity ratio of their emissions being proportional to their population ratio:
where A is a proportionality factor that depends on the material and spectroscopy system. Therefore we can determine the temperature of the phosphor by measuring this spectral ratio.
While there are many different phosphors that are used for two-color thermometry (TCT) [23, 24, 29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49], one of the most common classes is Dy\(^{3+}\)-doped materials [22, 24,25,26,27,28,29, 33, 34, 37, 49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71]. These materials are attractive TCT phosphors as the Dy\(^{3+}\) ion has two closely spaced excited energy levels (\({}^4F_{9/2}\) and \({}^4F_{15/2}\), \(\Delta E \approx 0.115\) eV) with a large energy difference (\(\approx 0.97\) eV) between the lowest excited state (\({}^4F_{9/2}\)) and highest ground state (\({}^6H_{15/2}\)) [72]. This large energy difference between ground and excited states ensures that the majority of energy will be emitted through radiative transitions.
Given the advantageous features of Dy\(^{3+}\)-doped materials for TCT, we recently developed a series of Dy\(^{3+}\)-doped organic crystals for use as energetic crystal analogues that are also temperature sensors [40, 41]. Of these different phosphors, we found that Dy:Y(acac)\(_3\)(DPEPO) provides the best optical properties, with the brightest PL, longest lifetime, and highest quenching temperature [41]. Note that this previous study focused on the spectral properties of the phosphors and did not evaluate their performance for TCTI.
Therefore, in this study, we investigate Dy:Y(acac)\(_3\)(DPEPO)’s potential as a TCTI phosphor by embedding it in a polymer and performing TCTI during pulsed laser heating. For comparison, we also perform the same testing using samples prepared with the well-known TCT phosphor Dy:YAG. The use of Dy:YAG allows us to characterize our TCTI system and get accurate heating profiles as its TCTI potential has been well established [22,23,24,25,26,27,28]. Note that the need to produce reproducible heating and PL from our samples to demonstrate functionality precludes using fast heating and high excitation fluences as they irreversibly damage our samples. Therefore, this demonstration is not a direct evaluation of the phosphor for the proposed application—TCTI during shock compression—but instead provides indirect validation of the technique.
2 Method
2.1 Two-color thermometric imaging setup
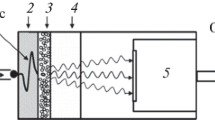
Our two-color thermometric imaging setup (shown schematically in Fig. 1) consists of a frequency tripled Nd:YAG laser (Continuum Powerlite II, 355 nm, 10 Hz, 1 mJ), a delay generator (SRS DG645), a PI-Max 4 ICCD (1024i, SB Phosphor), a Cairn Optosplit II image splitter containing two Semrock bandpass filters (Semrock BrightLine® FF01-460/14-25 and FF02-485/20-25), and various mirrors and lenses. For heating, we use a Synrad Firestar f100 CO\(_2\) laser which is directed onto the sample near the center of the 355 nm beam. Note that the CO\(_2\) laser beam is elliptical with a Gaussian profile with dimensions of 3.5 mm \(\times\) 2 mm, while the 355 nm beam is a round top-hat with a beam diameter of 10 mm.
2.2 Materials
To synthesize Dy:YAG, we use a co-precipitation technique previously reported in the literature [73]. This procedures begins by first making an aqueous ammonium hydrogen carbonate solution. This is done by adding an aqueous salt solution (0.4 M) containing dysprosium nitrate (GFS Chemicals, 99.999% purity), yttrium nitrate (Alfa Aesar, 99.99% purity), and aluminum nitrate (Alfa Aesar, >99.0% purity) to an aqueous ammonium hydrogen carbonate (Alfa Aesar, 98% purity) precipitant solution (1.5 M). The resulting Dy/Y/Al mole ratio is 0.06/2.94/5, which gives a Dy/Y ratio of 2/98. The precipitate is aged for 1 h, followed by multiple washings using deionized water and alcohol. The washed product is dried in a vacuum oven at 353 K for 12 h and then crushed with a mortar and pestle and run through a 100-mesh sieve. The resulting powder is heat treated at 1873 K for 6 h to yield the crystalline YAG phase. This phase is confirmed by XRD measurements of the material, with Fig. 2 showing the XRD spectra of the material, which is found to be consistent with the literature on crystalline YAG [56].
Synthesis of Dy:Y(acac)\(_3\)(DPEPO) begins by first synthesizing Dy:Y(acac)\(_3\). To start, we prepare a 10 mL aqueous solution of Dy(NO\(_3\))\(_3\).5H\(_2\)O/Y(NO\(_3\))\(_3\).6H\(_2\)O (0.625 M) that is mixed with a solution of acetylacetone, acac (7.5 M), in 2.5 mL methanol. To this solution, we add a 2.5 mL aqueous solution of NaOH (1.5 M) leading to precipitation of the product. The resulting suspension is aged for 2–3 h and the product is washed with deionized water and dried in vacuum oven at 80 \(^\circ\)C for \(\approx 12\) h. After preparing Dy(acac)\(_3\), we prepare Dy:Y(acac)\(_3\)(DPEPO) by mixing Dy(acac)\(_3\) and DPEPO at an equimolar ratio. This mixture is then dissolved in a methanol/DMF solution (0.06 M) and refluxed overnight. The supernatant is decanted to give the solid product, followed by drying in a vacuum oven at 80 \(^\circ\)C for \(\approx 12\) h.
To characterize the resulting molecular crystal, we measure its XRD pattern, which is shown in Fig. 2. This X-ray diffraction pattern can be fit to a single-phase monoclinic crystal structure with a space group P1, with lattice parameters of: \(a=21.20\) Å, \(b=21.20\) Å, \(c=18.78\) Å, \(\alpha =106.62^{\circ }\), \(\beta =113.39^\circ\), and \(\gamma =60.49^\circ\). The unit cell volume is 6679.69 Å\(^3\).
For our heterogeneous samples, we blend Dy:YAG or Dy:Y(acac)\(_3\)(DPEPO) crystals with hydroxyl-terminated polybutadiene/isophoronediisocyanate polyurethane (HTBP/IPDI)—containing Bis(2-ethylhexyl)adipate (DEHA) plasticizer—at a 75/25 and 50/50 crystal/polyurethane weight ratio, respectively. The crystal/polyurethane mixture is then poured onto a glass slide and cured overnight at ambient temperature.
2.3 Timing
After preparing the samples and setting up the TCTI system, the next step is to correctly determine the timing of the various components. To begin, we consider two key timing details: (1) that the ideal Q-switch delay is 295 \(\upmu\)s meaning that once the lamp fires (at a rate of 10 Hz), the sample will be excited 295 \(\upmu\)s later (as there is minimal delay between the Q-switch firing and the laser pulse exciting the sample), and (2) our temperature sensors have relatively short lifetimes compared to the heating pulse duration (10-100 ms). Given these two factors, we set up our experimental timing, such that the CO\(_2\) laser fires at \(t_0\), followed by the flashlamp at \(t_0+w-305\) \(\upmu\)s, the Q-switch at \(t_0+w-10\) \(\upmu\)s, and the ICCD gate starting at \(t_0+w\) (with an ICCD gate width of 750 \(\upmu\)s). Figure 3 shows a schematic of the timing with \(t_0\) marking the start of the CO\(_2\) laser pulse and w being the gate width of the laser pulse. Note that this timing scheme measures the PL immediately following the laser heating pulse.
3 Results
3.1 Phosphor comparison
We have previously reported on the performance of both Dy:YAG [74] and Dy:Y(acac)\(_3\)(DPEPO) [41] as TCT phosphors. Table 1 tabulates relevant TCT parameters for both materials, which include: the amplitude parameter A (Eq. 5), energy level splitting \(\Delta E\), PL lifetime, and max sensitivity defined as:
where r is the intensity ratio and the max value occurs at the minimum temperature used (i.e., 298 K). From Table 1, we find that both materials have the same calibration parameters (A and \(\Delta E\)) within uncertainty, as well as the same sensitivity. Note that these materials have similar sensitivities to other Lanthanide-doped thermometry phosphors (\(\sim\) 1–2 %/K), but are less sensitive than some organic phosphors [46], which have sensitivities reported as high as 19.6 %/K [75].
The primary difference between the two materials is the PL lifetime, with Dy:YAG having a lifetime \(\approx 40 \times\) as large. This difference in lifetime also translates into a difference in absolute intensity [72]. To demonstrate this effect, we plot the PL emission spectra of both materials using two different ICCD gate widths (500 ns and 10 ms) in Fig. 4. From Fig. 4, we find that for the 10 ms gate width, the Dy:YAG is significantly brighter, but when using the 500 ns gate width, the two materials have similar intensities. This means that while Dy:YAG is the brighter phosphor for slow heating events (e.g., furnaces and engines), the two phosphors intensities are similar for fast heating events, such as the proposed application of shock compression.
To use Dy:Y(acac)\(_3\)(DPEPO) as a TCT phosphor in shock compression, experiments will require increasing the pump laser fluence to obtain sufficient signal. However, care must be taken not to damage the sample using a single pulse. In lab, we have been able to obtain sufficient signal for imaging Dy:Y(acac)\(_3\)(DPEPO) using a 100 ns gate width, with a 15 mJ laser pulse focused into a 4 mm diameter spot. This fluence (119 mJ/cm\(^2\)) is found to take about 10 to 15 pulses before the sample shows obvious signs of damage. Additionally, we can also bin the ICCD pixels to increase the number of counts/pixel, albeit at the cost of spatial resolution.
3.2 Dy:YAG in HTPB
For the baseline test of the TCTI system, we used the well-known TCT phosphor Dy:YAG, which was dispersed into HTPB. For these trials, we limited our heating pulse widths to < 100 ms as longer durations are found to burn holes in the polymer. Figure 5 shows example two-color images for heating durations of 0 ms, 50 ms, and 100 ms. The PL emission dominates in band 1 (470–520 nm) for the ambient measurement, with the PL intensity transferring into band 2 (440–470 nm) as the sample is heated. Note that the total emission from both bands is diminished at 100 ms due to thermal quenching of the PL emission.
Having measured the two-color images for different gate widths, we next computed the spatially resolved intensity ratio for each gate width. This ratio is found to have a baseline spatial profile due to spatial inhomogeneity in the sample, excitation profile, and the optical path. Therefore, to obtain the changes in the PL band ratio due to heating, we subtract the ambient temperature ratio from each gate width’s ratio profiles. These background subtracted ratios are shown in Fig. 6.
From Fig. 6, we find several results: (1) the heating profile is elliptical, which corresponds to the CO\(_2\) laser’s known beam profile; (2) for durations < 20 ms, there is very little change in the ratio; and (3) the hottest areas (largest ratios) are not uniformly distributed. This last observation is best observed by considering the ratio profile for the 100 ms heating pulse, which displays splotchy hot spots. These hot spots could either be due to the CO\(_2\) laser beam’s quality or nonuniformity in the sample (e.g., the distribution of Dy:YAG particles in the polymer).
With the change in PL intensity ratio determined for each pulse durations, we next convert these ratio profiles into temperature profiles. To do so, we begin by writing the change in ratio \(\Delta r\) as a function of time:
where \(T_i\) is the initial temperature at time \(t_i\), A and \(\Delta E\) are material parameters, \(r_0\) is an offset parameter due to the optical setup, and \(D_i\) is a parameter related to the room temperature ratio and is given by:
For Dy:YAG, we measured the materials calibration parameters to be A = 1.71 and \(\Delta E\) = 121.2 meV, which results in \(D_i\) = 0.0141 [74].
Rearranging Eq. 5, we find the temperature as a function of ratio to be:
Taking the change in ratios from Fig. 6 and Eq. 7, we compute the temperature profiles at the different times, which are shown in Fig. 7.
From Fig. 7, we find that the heating profiles are elliptical (as expected) with the peak temperatures being near 1200 K. Comparing the different profiles at each time, we find that the heated region increases as the time increases, which is consistent with heat diffusion from the laser spot into the surrounding materials.
3.3 Dy:Y(acac)\(_3\)(DPEPO) in HTPB
Having demonstrated the TCTI technique with Dy:YAG, we next consider the performance of Dy:Y(acac)\(_3\)(DPEPO) as a TCT phosphor with the heating limited to pulse widths of 50 ms as further heating results in complete quenching of the phosphors PL. For brevity, we suppress the PL and ratio images for Dy:Y(acac)\(_3\)(DPEPO) and instead turn directly to considering the measured temperature profiles, which are shown in Fig. 8. Note that in Fig. 8, we apply an elliptical mask to hide noise outside of the region of interest.
From Fig. 8, we find that once again little-to-no heating is observed for durations \(<20\) ms. We also observe that the heated region for Dy:Y(acac)\(_3\)(DPEPO) has significantly more temperature variations than were observed for Dy:YAG. These variations are due to the Dy:Y(acac)\(_3\)(DPEPO) sample displaying more concentration inhomogeneity and a rougher surface than the Dy:YAG samples. This nonuniformity is visible to the naked eye as a rough surface and visible clumps of phosphor. Most likely, this lower uniformity is due to the Dy:Y(acac)\(_3\)(DPEPO) particles being larger than the Dy:YAG particles.
At this point, we had planned on making a direct comparison of the full heating profiles of the two materials to demonstrate Dy:Y(acac)\(_3\)(DPEPO)’s performance as compared to Dy:YAG. Unfortunately, due to uniformity issues, this is not possible. While we cannot compare the full profiles, we can make some limited comparisons if we consider the regions in Fig. 8 that correspond to the highest temperatures. The motivation for this comparison is as follows: (1) the same laser profile and power was used for both samples, which means that, for equally uniform samples, the profiles should be identical. (2) the Dy:Y(acac)\(_3\)(DPEPO) crystals are larger and doped at a lower concentration, which means that the main impact of inhomogeneity will be voids and areas of lower concentration. Both of these will result in less heating and, therefore, lower temperatures. (3) The areas with the highest concentration of Dy:Y(acac)\(_3\)(DPEPO) crystals will, therefore, be heated the most similar to the Dy:YAG sample.
With these assumptions in mind, we compare the average temperature in a 10 px circle centered at (x = 110, y = 160) for both materials, with the resulting temperatures, as shown in Fig. 9. From Fig. 9, we find good agreement between the two sensor materials, which demonstrates Dy:Y(acac)\(_3\)(DPEPO)’s suitability as a phosphor for TCTI. We also observe that both materials display an exponential heating curve, which is expected in the case of constant-power heating with heat diffusion. At early times, the energy coming into the target area is far greater than the energy being transferred out, leading to the temperature increasing linearly. However, as the temperature rises the rate of heat loss to the surrounding material increases, resulting in a net decrease in the heating rate. This continues until the rate of energy input is balanced by the heat transfer out of the region of interest, leading to a steady-state temperature. Note that we do not reach the steady state temperature as we limit the pulse width to 50 ms.
3.4 Temperature resolution
Having demonstrated Dy:Y(acac)\(_3\)(DPEPO)’s usefulness as a TCTI phosphor, we now turn to characterizing the system’s temperature resolution \(\Delta T\), which is defined as [41]:
where r(T) is the intensity ratio at temperature T (Eq. 5) and \(\sigma _r(T)\) is the systematic uncertainty in the ratio.
To characterize the systematic uncertainty in the ratio (as a function of temperature), we perform isothermal imaging measurements using Dy:YAG powder in a furnace. These measurements consist of taking ten images at each temperature, calculating the ratio mapping for each image, and then computing the variance in the ratio over the ten images. Figure 10 shows the calculated percent error as a function of temperature with a fit to a polynomial function. From Fig. 10, we find that the percent error in the ratio follows a U-shaped curve, with the uncertainty in the ratio being greatest at low and high temperatures, while the middle temperatures have the smallest errors. This is due to the higher energy band emission being dim at low temperatures (resulting in more intensity fluctuations), increasing in intensity, and then finally decreasing again as thermal quenching begins occurring.
Having measured the systematic uncertainty, we next computed the temperature resolution at each temperature, which is also shown in Fig. 10. From the temperature resolution curve in Fig. 10, we find that starting at room temperature, the resolution is \(\approx 1\) K and increases roughly as \(T^2\) up to around 1050 K. Above 1050 K, the temperature resolution begins increasing faster than \(T^2\) and reaches a peak value of \(\approx 83\) K at 1173 K. This change is the result of the systematic uncertainty increasing due to thermal quenching.
4 Conclusion
In this study, we demonstrated both the TCTI technique and the usefulness of Dy:YAG and Dy:Y(acac)\(_3\)(DPEPO) as TCT phosphors for subsecond heating events using laser heating. For Dy:YAG, we observe a well-defined temperature profile corresponding to the CO\(_2\) laser beam’s intensity profile, while for Dy:Y(acac)\(_3\)(DPEPO), we find that the temperature profile is noisy and contains hot spots. This difference is attributed to the Dy:Y(acac)\(_3\)(DPEPO) sample being significantly more inhomogeneous than the Dy:YAG samples. However, while Dy:Y(acac)\(_3\)(DPEPO)’s profile is more variable, we find that the average temperature of the “hot spots” in Dy:Y(acac)\(_3\)(DPEPO)’s profile matches within experimental uncertainty the temperatures measured by Dy:YAG. This favorable comparison between materials suggests that Dy:Y(acac)\(_3\)(DPEPO) is a good material for TCTI in heterogeneous samples.
References
M. Chaudhri, J. Field, The role of rapidly compressed gas pockets in the initiation of condensed explosives. Proc. R. Soc. Lond. 340, 113–128 (1974)
R. Pokharel, J. Lind, A. Kanjarla, R. Lebensohn, S. Li, P. Kenesei, R. Suter, A. Rollett, Polycrystal plasticity: comparison between grain-scale observations of deformation and simulations. Annu. Rev. Condens. Matter Phys. 5, 317–346 (2014)
J. Field, G. Swallowe, S. Heavens, Ignition mechanisms of explosives during mechanical deformation. Proc. R. Soc. Lond. Ser. A 382, 231 (1982)
R. Winter, E. Faber, The role of localized plastic flow in the impact initiation of explosives. Proc. R. Soc. Lond. Ser. A 343, 399–413 (1975)
M. Chen, S. You, K. Suslick, D. Dlott, Hot spots in energetic materials generated by infared and ultrasound, detected by thermal imaging microscopy. Rev. Sci. Instrum. 85, 023705 (2014)
M. Chen, S. You, K. Suslick, D. Dlott, Hot spot generation in energetic materials created by long-wavelength infared radiation. Appl. Phys. Lett. 104, 061907 (2014)
Q. An, W. Goddard, S. Zybin, A. Jaramillo-Botero, T. Zhou, Highly shocked polymer bonded explosives at a nonplanar interface: hot-spot formation leading to detonation. J. Phys. Chem. C 117, 26551–26561 (2013)
Y. Wu, F. Huang, A microscopic model for predicting hot-spot ignition of granular energetic crystals in response to drop-weight impacts. Mech. Mater. 43, 835–852 (2011)
P. Rae, H. Goldrein, S. Palmer, J. Field, A. Lewis, Quasi-static studies of the deformation and failure of beta-HMX based polymer bonded explosives. Proc. R. Soc. A Math. Phys. Eng. Sci. 458, 743–762 (2002)
Y. Gruzdkov, Y. Gupta, Vibrational properties and structure of pentaerythritol tetranitrate. J. Phys. Chem. A 105, 6197–6202 (2001)
Y. Gupta, Recent developments to understand molecular-changes in schocked energetic materials. J. Phys. IV 5, 345–356 (1995)
A. Mellor, D. Wiegand, K. Isom, Hot-spot histories in energetic materials. Struct. Prop. Energ. Mater. 296, 293–298 (1993)
J. Field, Hot-spot ignition mechanisms for explosives. Acc. Chem. Res. 25, 489–496 (1992)
J. Field, N. Bourne, S. Palmer, S. Walley, J. Smallwood, Hot-spot ignition mechanisms for explosives and propellants. Philos. Trans. R. Soc. Lond. Math. Phys. Eng. Sci. 339, 269–283 (1992)
S.N. Luo, B.J. Jensen, D.E. Hooks, K.J. Ramos, J.D. Yeager, K. Kwiatkowski, T. Shimada, D.A. Fredenburg, K. Fezzaa, Ultrafast, high resolution, phase contrast imaging of shock response with synchrotron radiation: opportunities and challenges, in APS Meeting Abstracts (2012)
B.J. Jensen, K.J. Ramos, A.J. Iverson, J. Bernier, C.A. Carlson, J.D. Yeager, K. Fezzaa, D.E. Hooks, Dynamic experiment using impulse at the advanced photon source. J. Phys. Conf. Ser. 500, 042001 (2014)
B.J. Jensen, S.N. Luo, D.E. Hooks, K. Fezzaa, K.J. Ramos, J.D. Yeager, K. Kwiatkowski, T. Shimada, D.M. Dattelbaum, Ultrafast, high resolution, phase contrast imaging of impact response with synchrotron radiation. AIP Adv. 2, 012170 (2012)
J. Yeager, S. Luo, B. Jensen, K. Fezzaa, D. Montgomery, D. Hooks, High-speed synchrotron X-ray phase contrast imaging for analysis of low-z composite microstructure. Compos. A Appl. Sci. Manuf. 43, 885–892 (2012)
S.N. Luo, B.J. Jensen, D.E. Hooks, K. Fezzaa, K.J. Ramos, J.D. Yeager, K. Kwiatkowski, T. Shimada, Gas gun shock experiments with single-pulse X-ray phase contrast imaging and diffraction at the advanced photon source. Rev. Sci. Instrum. 83, 073903 (2012)
DCS facility. http://www.dcs-aps.wsu.edu/
New research opportunities in dynamic compression science, Technical report, Institute for Shock Physics, Washington State University (2012)
J. Feist, A. Heyes, K. Choy, B. Su, Phosphor thermometry for high temperature gas turbine applications, in ICIASF 99, in 18th International Congress on Instrumentation in Aerospace Simulation Facilities. Record (Cat. No.99CH37025) (1999), p. 6/1
J. Feist, A. Heyes, S. Seefeldt, Thermographic phosphors for gas turbines: instrumentation development and measurement uncertainties, in 11th International symposium on Application of Laser Techniques to Fluid Mechanics, (Lisbon, Portugal, 2002)
A. Heyes, S. Seefeldt, J. Feist, Two-colour phosphor thermometry for surface temperature measurement. Opt. Laser Technol. 38, 257–265 (2006)
K. Choy, A. Heyes, J. Feist, Thermal Barrier Coating with Thermoluminescent Indicator Material Embedded Therein, United States Patent US8173266B2 (1998)
M. Lawrence, H. Zhao, L. Ganippa, Gas phase thermometry of hot turbulent jets using laser induced phosphorescence. Opt. Express 21, 12260 (2013)
A. Jaber, L. Zigan, A. Sakhrieh, A. Leipertz, Surface temperature measurements in a porous media burner using a new laser-induced phosphorescence intensity ratio technique. Meas. Sci. Technol. 24, 075202 (2013)
H. Seyfried, G. Särner, A. Omrane, M. Richter, H. Schmidt, M. Alden, Optical diagnostics for characterization of a full-size fighter-jet afterburner, in Volume 1: Turbo Expo 2005 (2005), pp. 813–819
A.Z.M. Al-Juboori, Rare earth (Sm\(^{3+}\) and Dy\(^{3+}\))-doped gadolinium oxide nanomaterials for luminescence thermometry. Phys. Scr. T157, 014004 (2013)
A. Khalid, K. Kontis, Thermographic phosphors for high temperature measurements: principles, current state of the art and recent applications. Sensors 8, 5673–5744 (2008)
K. Kontis, A review of some current research on pressure sensitive and thermographic phosphor techniques. Aeronaut. J. 3162, 495–508 (2007)
S.W. Allison, G. Gillies, Remote thermometry with thermographic phosphors: instrumentation and applications. Rev. Sci. Instrum. 68, 2615–2650 (1997)
M. Cates, S. Allison, S. Jaiswal, D. Beshears, YAG: Dy and YAG: Tm fluorescence to 1700 \(^\circ\)C, in The 49th International Instrumentation Symposium—The Instrumentation, Systems, and Automation Society (Orlando, FL, 2003)
G. Gross, A. Smith, M. Post, Surface thermometry by laser induced fluorescence. Rev. Sci. Instrum. 60, 12 (1989)
K. Kontis, Y. Syogenji, N. Yoshikawa, Surface thermometry by laser induced fluorescence of Dy\(^{3+}\):YAG. Aeronaut. J. 106, 453–457 (2002)
K. Kontis, Surface heat transfer measurements inside a supersonic combustor by laser induced fluorescence. J. Thermophys. Heat Transf. 17, 320–325 (2003)
R. Hasegawa, I. Sakata, H. Yanagihara, B. Johansson, A. Omrane, M. Alden, Two-dimensional gas phase temperature measurements using phosphor thermometry. Appl. Phys. B 88, 291–296 (2007)
G.Särner, A. Omrane, H. Seyfried, M. Richter, H. Schmidt, M. Aldén, Laser diagnostics applied to a full-size fighter jet afterburner, in European Combustion Meeting (2005)
J.P. Feist, A.L. Heyes, J.R. Nicholls, Phosphor thermometry in an electron beam physical vapour deposition produced thermal barrier coating doped with dysprosium. Proc. Inst. Mech. Eng. 215, 333–341 (2001)
B.R. Anderson, R. Gunawidjaja, H. Eilers, Two-color thermosensors based on [Y\(_{1-x}\)Dy\(_x\)(acetylacetonate)\(_3\)(1,10-phenanthroline)] molecular crystals. Appl. Phys. B 123, 62 (2017)
B.R. Anderson, R. Gunawidjaja, H. Eilers, Dy\(^{3+}\)-doped yttrium complex molecular crystals for two-color thermometry in heterogeneous materials. J. Lumin. 188, 238–245 (2017)
B.R. Anderson, R. Gunawidjaja, H. Eilers, Soluble Sm-based ternary complexes for non-contact molecular thermometry. J. Lumin. 204, 341–348 (2018)
L. Rosso, S. Tabandeh, G. Beltramino, V. Fernicola, Validation of phosphor thermometry for industrial surface temperature measurements. Meas. Sci. Technol. 31, 034002 (2019)
S.W. Allison, A brief history of phosphor thermometry. Meas. Sci. Technol. 30, 072001 (2019)
P. Du, L. Luo, J.S. Yu, Controlled synthesis and upconversion luminescence of Tm3+-doped NaYbF4 nanoparticles for non-invasion optical thermometry. J. Alloy. Compd. 739, 926–933 (2018)
C.D.S. Brites, P.P. Lima, N.J.O. Silva, A. Millan, V.S. Amaral, F. Palacio, L.D. Carlos, Thermometry at the nanoscale. Nanoscale 4, 4799 (2012)
J. Brübach, T. Kissel, M. Frotscher, M. Euler, B. Albert, A. Dreizler, A survey of phosphors novel for thermography. J. Lumin. 131, 559–564 (2011)
N. Fuhrmann, M. Schneider, C.P. Ding, J. Brübach, A. Dreizler, Two-dimensional surface temperature diagnostics in a full-metal engine using thermographic phosphors. Meas. Sci. Technol. 24, 095203 (2013)
A.L. Heyes, On the design of phosphors for high-temperature thermometry. J. Lumin. 129, 2004–2009 (2009)
E. Hertle, S. Will, L. Zigan, Characterization of YAG:Dy, Er for thermographic particle image velocimetry in a calibration cell. Meas. Sci. Technol. 28, 025013 (2017)
E. Hertle, L. Chepyga, M. Batentschuk, S. Will, L. Zigan, Temperature-dependent luminescence characteristics of Dy3+ doped in various crystalline hosts. J. Lumin. 204, 64–74 (2018)
M. Aldén, A. Omrane, M. Richter, G. Särner, Thermographic phosphors for thermometry: a survey of combustion applications. Prog. Energy Combust. Sci. 37, 422–461 (2011)
L.M. Chepyga, G. Jovicic, A. Vetter, A. Osvet, C.J. Brabec, M. Batentschuk, Photoluminescence properties of thermographic phosphors YAG: Dy and YAG:Dy, Er doped with boron and nitrogen. Appl. Phys. B Lasers Opt. 122, 212 (2016)
G. Jovicic, L. Zigan, S. Will, A. Leipertz, Luminescence properties of the thermographic phosphors Dy3+:YAG and Tm3+:YAG for the application in high temperature systems. Zeitschrift für Physikalische Chemie 229, 977 (2015)
G. Jovicic, L. Zigan, S. Will, A. Leipertz, Phosphor thermometry in turbulent hot gas flows applying Dy:YAG and Dy:Er:YAG particles. Meas. Sci. Technol. 26, 015204 (2015)
L.M. Chepyga, A. Osvet, C.J. Brabec, M. Batentschuk, High-temperature thermographic phosphor mixture YAP/YAG:Dy3+and its photoluminescence properties. J. Lumin. 188, 582–588 (2017)
A. Hashemi, A. Vetter, G. Jovicic, M. Batentschuk, C.J. Brabec, Temperature measurements using YAG:Dy and YAG:Sm under diode laser excitation (405 nm). Meas. Sci. Technol. 26, 075202 (2015)
M. Yu, G. Särner, C.C. Luijten, M. Richter, M. Aldén, R.S. Baert, L.P. De Goey, Survivability of thermographic phosphors (YAG:Dy) in a combustion environment. Meas. Sci. Technol. 21, 037002 (2010)
N. Ishiwada, E. Fujii, T. Yokomori, Evaluation of Dy-doped phosphors (YAG:Dy, Al\(_2\)O\(_3\):Dy, and Y\(_2\)SiO\(_5\):Dy) as thermographic phosphors. J. Lumin. 196, 492–497 (2018)
S.J. Skinner, J.P. Feist, I.J. Brooks, S. Seefeldt, A.L. Heyes, YAG:YSZ composites as potential thermographic phosphors for high temperature sensor applications. Sens. Actuators B Chem. 136, 52–59 (2009)
N.J. Kempema, M.B. Long, Boundary condition thermometry using a thermographic-phosphor-coated thin filament. Appl. Opt. 55, 4691 (2016)
J.P. Feist, A.L. Heyes, S. Seefeldt, Oxygen quenching of phosphorescence from thermographic phosphors. Mea. Sci. Technol. 14, N17 (2003)
A. Hashemi, G. Jovicic, M. Batentschuk, C.J. Brabec, A. Vetter, Contactless temperature determination using dual-channel lock-in phosphor thermometry. Meas. Sci. Technol. 28, 027001 (2017)
J. Brübach, C. Pflitsch, A. Dreizler, B. Atakan, On surface temperature measurements with thermographic phosphors: a review. Prog. Energy Combust. Sci. 39, 37–60 (2013)
E. Hertle, L. Chepyga, M. Batentschuk, L. Zigan, Influence of codoping on the luminescence properties of YAG: Dy for high temperature phosphor thermometry. J. Lumin. 182, 200–207 (2017)
C. Abram, B. Fond, F. Beyrau, Temperature measurement techniques for gas and liquid flows using thermographic phosphor tracer particles. Prog. Energy Combust. Sci. 64, 93–156 (2018)
L. Chepyga, E. Hertle, A. Ali, L. Zigan, A. Osvet, C. Brabec, M. Batentschuk, Synthesis and photoluminescent properties of the Dy\(^{3+}\)-doped YSO as a high-temperature thermographic phosphor. J. Lumin. 197, 23–30 (2018)
L.M. Chepyga, A. Osvet, I. Levchuk, A. Ali, Y. Zorenko, V. Gorbenko, T. Zorenko, A. Fedorov, C.J. Brabec, M. Batentschuk, New silicate based thermographic phosphors Ca\(_3\)Sc\(_2\)Si\(_3\)O\(_{12}\):Dy, Ca\(_3\)Sc\(_2\)Si\(_3\)O\(_{12}\):Dy, Ce and their photoluminescence properties. J. Lumin. 202, 13–19 (2018)
Z. Cao, S. Zhou, G. Jiang, Y. Chen, C. Duan, M. Yin, Temperature dependent luminescence of Dy3+ doped BaYF5 nanoparticles for optical thermometry. Curr. Appl. Phys. 14, 1067–1071 (2014)
J.Y. Chong, Y. Zhang, B.K. Wagner, Z. Kang, Co-precipitation synthesis of YAG: Dy nanophosphor and its thermometric properties. J. Alloy. Compd. 581, 484–487 (2013)
N. Ishiwada, K. Tsuchiya, T. Yokomori, Applicability of Dy-doped yttrium aluminum garnet (YAG:Dy) in phosphor thermometry at different oxygen concentrations. J. Lumin. 208, 82–88 (2019)
J.-C.G. Bünzli, S.V. Eliseeva, Basics of lathanide photophysics, in Lanthanide Luminescence: Photophysical, Analytical and Biological Aspects, ed. by P. Hanninen, H. Harma (Springer, Berlin, 2010)
J. Li, T. Ikegami, J. Lee, T. Mori, Y. Yajima, Co-precipitation synthesis and sintering of yttrium aluminum garnet (YAG) powders: The effect of precipitant. J. Eur. Ceram. Soc. 20, 2395–2405 (2000)
B.R. Anderson, S. Livers, R. Gunawidjaja, H. Eilers, Fiber-based optical thermocouples for fast temperature sensing in extreme environments. Opt. Eng. 58, 097105 (2019)
K. M. McCabe, E. J. Lacherndo, I. Albino-Flores, E. Sheehan, M. Hernandez, LacI(Ts)-Regulated Expression as an In Situ Intracellular Biomolecular Thermometer. Appl Environ Microbiol 77, 2863-2868 (2011)
Acknowledgements
This work was supported by the Air Force Office of Scientific Research, Award # FA9550-15-1-0309 to Washington State University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Anderson, B.R., Gunawidjaja, R., Gese, N. et al. Two-color thermometric imaging of heterogeneous materials during pulsed laser heating. Appl. Phys. B 126, 158 (2020). https://doi.org/10.1007/s00340-020-07507-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00340-020-07507-3