Abstract
Single crystals of pure and crystal violet (CV) dye-doped anthracene single crystals have been synthesized by solvent evaporation technique utilizing chloroform as the solvent. There is a significant enhancement in optical, mechanical, photoacoustical and third-order non-linear properties have been attained due to the incorporation of dye in the host material. The unit cell parameters and crystallinity of the grown crystals were examined using single crystal and powder crystal XRD analysis. The presence of various functional groups and mode of vibrational frequencies present in the grown crystal is determined using FTIR spectra. The doped crystal exhibits high optical transparency than pure which is evident in UV–Visible spectrum. Using Knoop hardness study the Young’s modulus value of pure and doped crystal is found to be 1.154 × 1010 Nm−2 and 1.394 × 1010 Nm−2. Doping of CV dye results in the enhancement of hardness number and various hardness parameters such as brittleness index, elastic stiffness constant and fracture toughness are calculated using Vicker’s microhardness study. Photoacoustic study depicts the thermal diffusivity value for the grown crystals and it is found to be 1.2505 × 10–6 and 3.128 × 10–6 for undoped and doped crystal respectively. The real, imaginary and third-order susceptibility of the doped crystal is prominent than undoped crystal were elucidated using Z-scan study.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In the modern scientific era, organic nonlinear crystal has grabbed a noteworthy attention among the scientific community due to its extensive applications in the field of photonics and opto-electronics. The third-order nonlinear optical materials play a viable role in the field of optical data storage, optical communications and all optical limiting devices [1,2,3]. Anthracene is an archetype of molecular crystals, which possesses optical, mechanical, electrical advantages, includes high fluorescence quantum yield and fluroescence anisotrpy for large scale opto-electronic devices and it was reported first in 1948. The growth methodology of anthracene single crystals were mainly focused on Bridgmann–Stockbarger technique and the crystal obtained by Bridgmann method was found to be highly defective and several reports on single and bulk growth of anthracene crystals requires well equipped laboratory [4,5,6,7,8]. Few reports are available in the literature for anthracene bonded with metals such as zinc chloride, nickel, cobalt, ferric oxide and iron [9, 10]. Optical and electronic nature of anthracene crystal is soley rely on many factors, mainly their structural defects, doping and its morphology. Currently, researchers shows considerable interest on introduction of dye molecule as a dopants into the crystals which become a promising candidate for solid state lasers and photonic applications. Dye is an organic aromatic chemical group which is soluble in water and strongly absorb or emit visible light that affects the optical, mechanical and electrical properties of a material which is essential in fabrication of devices. In the present investigation, crystal violet (C25H30N3Cl) CV dye is selected as a dopant and it belongs triarylmethane class dye contain three aryl groups attached to a central carbon atom [11, 12]. The inclusion of dye in crystals offers rich stereochemistry and make the material suitable for spectroscopic and photonic device applications. Pengqiang Zhang et al., Madhurambal et al., has already grown anthracene single crystals using slow evaporation technique with various solvents namely acetone (C3H6O), benzene (C6H6), carbon tetrachloride (CCl4), carbon bisulfide (CS2) [13, 14]. Following the literature, in our present investigation we made an attempt to grow pure and doped anthracene single crystals by adopting solvent evaporation technique using chloroform as a solvent instead of aforesaid solvents. Dye incorporated crystals is superior to pure anthracene and the outcome appears to infer that there is significant enhancement in their optical, mechanical, photo-acoustic and third-order nonlinear properties and it consequently turned out to be gainful for the utilization of light emitting devices and other optoelectronic systems.
2 Material synthesis
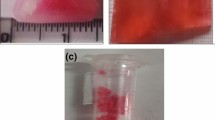
Analytical Reagent grade anthracene and CV dye were utilized for the crystal growth. 50 ml of chloroform is poured in a beaker in which anthracene was added so as to prepare saturated solution at normal atmosphere temperature. In addition to the above mentioned solution, 0.2 mol% of CV dye is added as a dopant and the solution was vigorously stirred for 6 h to yield homogenous solution and the solutions prepared was filtered using Whatman filter paper and kept undisturbed for solvent evaporation. By repeated re-crystallization process a good quality, transparent single crystals of pure and dye-doped anthracene has been harvested within a period of 30 days and the photograph of as-grown crystal is shown in Fig. 1.
3 Results and discussion
3.1 Single crystal XRD
Single crystal X-ray diffraction (XRD) analysis of pure and doped anthracene single crystals was carried out using ENRAF NONIUS CAD-4 diffractometer with MoKα (λ = 0.7170 Å) radiation. The XRD analysis reveals that doping with CV dye induces lattice strain in the crystal system, due to which unit cell parameters slightly varied but the crystal system (monoclinic) and space group (P21/a) of the doped crystal remains the same as that of pure crystal. The data are tabulated in Table 1 seems to be matched well with the reported values.
3.2 Powder X-ray analysis
The powder form of the grown crystal was subjected to powder X-ray diffraction analysis using PAN analytical X-pert pro diffractometer with CuKα (λ = 1.5404 Å) radiation. The sharp intensity peak signifies the purity of pure and doped crystals and the observed XRD pattern and peak values were in good agreement with the JCPDS XRD data (39–1848) of anthracene. From Fig. 2, there is a marked variation of reduction of peak intensity in the dye-doped crystals compared to pure crystals that confirms the incorporation of CV into the anthracene lattices. The absorbed prominent peaks for pure and doped anthracene are (100), (011), (200), (002), (102), (012), (300), (311), (222), (500), respectively. The internal lattice strain arises from the large fraction of surface atom and unsaturated dangling bonds which are responsible for excess peak smears in between (311) and (222) peaks. The ionic radii of anthracene (d001 = 9.23 Å) have good correlation with lattice strain of the crystal [15, 16].
3.3 FTIR analysis
Fourier transform infrared spectrum (FT-IR) of pure and dye-doped crystals were recorded in the wavelength range of 400–4000 cm−1 by Shiraz spectrometer using KBR pellet technique. The combined IR-absorption spectrum of pure and doped crystal shown in Fig. 3 consists of very sharp peak at 3047 cm−1 that is due to aromatic C-H stretching. Furthermore, the peak at 723 cm−1 is due to C–H out of plane bending vibrations which confirms the presence of two sets of four adjacent hydrogen atoms. C–H out of plane bending vibrations, which corresponds to two isolated hydrogen atoms, is witnessed by peak at 881 cm−1. The out of plane (carbon carbon double bond) can be evidenced at 469 cm−1. The peaks at 1620, 1528, 1445 cm−1 is due to skeletal vibrations of carbon carbon double bond. The additional peaks present in the dye-doped anthracene at 1582, 1344, 998 cm−1 are due to C=C stretching, CH3 bending, C–H bending vibrations, respectively. These extra peaks authenticate the inclusion of CV dye into the anthracene crystals.
3.4 Optical study
UV–vis-NIR spectral analysis was performed using SHIMADZU spectrophotometer in the region of 200–1200 nm. Figure 4 depicts the significant enhancement in optical transparency from 58% (pure) to 78% (CV doped) crystals, respectively. The optical transparency is increased due to dye incorporation that indicates the improvement in crystalline perfection and it occur because of the vacancies which were present in pure anthracene crystals are filled now by dye inclusion that results in less absorbance of the incident light fall on it. Reduction in scattering centers in dye-doped anthracene and the better crystalline perfection makes the enrichment in optical transparency of CV-doped crystal and several researchers are also reported by adding small quantity of dye in the host material results in similar enhancement in optical transparency for dye-doped crystals [17, 18].
Absorption coefficient (α) was calculated using the equation:
where d denotes sample thickness and T denotes the light pass through the crystal. Using Tauc’s relation [19] the bandgap (Eg) was determined by plotting (αhυ)2 vs photon energy is illustrate in Fig. 5. The optical bandgap (Eg) for pure and dye incorporated anthracene crystal is 4.0 eV and 4.7 eV, respectively. The increment in the optical band gap of doped crystal suggests its prominence for optoelectronic applications [20,21,22].
3.5 Knoop hardness study
Knoop hardness study was carried out using Futuretech FM 800 type E series by varying weights at 303 K. The applied load varies from 1 to 25 g for constant indentation period of 7 s using Knoop indenter fitted to microscope. The impression produce by Knoop indenter were almost rhombohedral in shape. The value of (Hk) is calculated using the expression:
Eventually, crack occurs in the surface of the crystal when the load was increased beyond 25 g. Knoop hardness test for pure and dye-doped crystal could not extend out above this load. The graph drawn against Knoop hardness number (Hk) vs load P is shown in Fig. 6. When the load extends up to 10 g the crack occurs in pure crystal, while in doped crystal, it is observed at 25 g. When the applied load increases beyond 25 g the Knoop hardness number dwindles [23].
Young’s modulus was found using Knoop hardness measurements:
Hk is knoop hardness for a load P applied, a, b are shorter knoop indentation diagonal and the long depression diagonal [24]. The determined Young’s modulus for the pure and doped crystal was found to be 1.154 × 1010 Nm−2 and 1.394 × 1010 Nm−2 respectively.
3.6 Vickers microhardness study
Mechanical stability of the crystal plays a pivotal role in the various optoelectronic applications and the materials’ stability is rely on various parameters notably its structure, lattice energy and Debye temperature [25]. The mechanical nature of the grown crystal was examined using Fututretech FM 800 type E series Vicker’s microhardness tester at varying loads for the constant indentation period of 7 s for all applied loads. When low loads were applied, only the top layer of the crystal was penetrated using diamond indenter results in increase of Hv value. When the load was attained 50 g cracks observed in pure anthracene, whereas in doped crystal crack observe when the load increase beyond 100 g and the Hv was determined using the expression:
From Fig. 7, it is evident that hardness value increases with increasing applied load and the grown crystal exhibits reverse indentation size effect and inclusion of dye enhances its mechanical hardness and great stress is required to form dislocation in the crystal [26]. Figure 8 reveals the plot of log P vs log d which gives a straight line and the slope gives work hardening coefficient ‘n’. The obtained ‘n’ values for pure and doped crystal is 2.62 for pure and 3.18 for the doped crystal. According to Onitsch, the ‘n’ value lies at 1 and 1.6 for harder materials and it is above 1.6, it comes under soft material [27] category and it concludes both the material fall under soft material category.
Using Mayer’s law the Mayer’s index number was calculated which connects the indentation diagonal length ‘d’ and load P is given by relation:
where K1 is termed a standard hardness value which can be obtained from the plot of load P vs (diagonal length)n. As the material require some time to turn back to elastic mode after certain indentation, correction is applied to the diagonal value:
The slope of (diagonal length)n/2 vs diagonal length give (K2/K1)1/2 and intercept is a measure of ‘x’. Brittleness index ‘Bi’ is calculated using the expression [28]:
Using Wooster empirical relation, the elastic stiffness constant (C11) was calculated using the expression [29]:
Fracture toughness (Kc) explicate that how much strain is enforced constituent load with well-determined crack, resilience to break denote material toughness. Measurement taken from the depression centre point to its end gives the crack length C and its mean length for every depression C ≥ d/2 and it’s calculate using [30]
P is the load applied, β is coined as geometrical constant and it’s equal to 7 for Vicker’s indenter. Table 2 shows the calculated hardness parameters of pure and doped crystal.
3.7 Photoacoustic study
It is an effectual study to determine the thermal properties of the grown crystal. To construct high-efficiency laser system, it is necessary to know about the material’s thermal effusivity, thermal diffusivity and thermal conductivity of the materials. In the heat transmission processes an open photoacoustic cell or OPC, is utilized in the present study. The sample is placed directly on top of the microphone; evacuate small volume of air between the microphone and the sample. The crystal was illuminate on the front side by modulate monochromatic light and 500 W tungsten filament was used as a source and the constructed spectrophotometer is calibrated using the standard materials such as quartz glass, potassium dihydrogen phosphate (KDP) and BK7 glass. The photoacoustic signal produced in the sample cell is recognize by microphone controlled using computer associated with sound recording software [31, 32]. The schematic diagram of photoacosutic spectrometer is shown in Fig. 9. The measurement was done for both pure and doped crystal with function of chopping frequency ranges from 30 to 125 Hz. The normalized photoacoustic (PA) signal against square root of chopping frequency for the grown crystal is shown in Fig. 10. From the obtained graph, the thermal diffusivity was calculated by adopting Fariea and Barros Mela [33]. The thermal diffusivity value for standard KDP is 0.9500 × 10–6 m2/s [34]. The thermal diffusivity for pure and doped crystal is 1.2505 × 10–6 and 3.128 × 10–6, respectively. The doped crystal is 1.8 times higher than the pure crystal and 3.1 times higher than the standard KDP crystal, which is favoring nature of nonlinear optical materials in real-time applications.
3.8 Estimation of solid state parameters
Theoretical computation shows that dielectric constant ɛr at higher frequencies are particularly depend on its average energy and valence electrons plasma energy. Plasma energy is directly calculated by taking their total number of valence electrons present in it [35]. Valence electron plasma energy (ħ \(\omega_{{\text{p}}} )\) is calculated using the expression:
The value of \((\hbar \omega_{{\text{p}}} )\) is very large, since the organic crystals shows short range order. Hence theoretical Penn model is utilized to measure the electronic transition. Penn energy plays a prominent role in the magnitude of dielectric constant. Fermi energy (EF) and Penn gap (EP) explained in the terms of plasma energy is given by [36, 37]
Electronic polarizability (α) of the grown crystal is calculated using the below equation:
where \({\text{S}}_{{\text{o}}}\) is a constant for a particular material which is given by
The value obtains by α agrees with Clausius–Mossotti equation and expressed in [38]
where ρ and M be the density and molecular weight of the material and the calculated parameters are shown in Table 3.
3.9 Third-order NLO study (Z-scan)
To determine the nonlinear absorption coefficient [β], nonlinear susceptibility \(\left[ {\chi^{\left( 3 \right)} } \right]\) and nonlinear refractive index [n2] for the grown crystal Z-scan technique is used with Helium–Neon laser of wavelength λ = 632.8 nm as a light source, focused by lens of 30 mm in focal length. The light emit from the laser source is pass through an aperture which is kept far field and collect using photo detectors. The laser beam input power is noted by digital power meter [39, 40]. In open aperture, instead of aperture lens was kept to accumulate the whole laser source that passes through the crystal. Figure 11a, b reveals the plot of Z in mm against normalized transmittance for both open and close aperture.
The difference in peak and valley (∆Tp–v) in terms of the on axis phase shift at the focus as [41, 42]
where S is denoted as linear aperture transmittance and calculated using the relation:
where \(r_{a}\) is indicated as aperture radius and \(\omega_{a}\) is the beam radius. The n2 value was found using relations:
where k = \(\frac{2\pi }{{\lambda }}\), (λ is defined as wavelength of the laser), I0 is the intensity of the laser beam at (Z = 0), \(L_{{{\text{eff}}}} = \left[ {1 - \exp^{{\left( {\alpha L} \right)}} } \right]/\alpha\) is sample’s effective thickness, L is the thickness of the sample and α is the linear absorption [43].
From the open aperture Z-scan plot, the β value is calculated by the relation:
The real and imaginary parts of susceptibility \({\upchi }^{\left( 3 \right)}\) are defined as [44, 45]
All the calculated values for (n2), (β), real and imaginary parts of susceptibilities \(\chi^{\left( 3 \right)}\) is given in Table 4 and the enhancement in third-order susceptibility for doped crystal makes it as a noteworthy material for nonlinear optical applications.
4 Conclusion
Pure and CV dyed-doped anthracene crystals were grown by utilizing slow solvent evaporation technique using chloroform as a solvent and the harvested crystals were subjected to various characterization studies. The lattice parameters and space group of the grown crystal is found using single crystal XRD analysis. From powder XRD, it is evident that CV dye incorporated crystal has the same structure as that of pure anthracene. The presence of various functional groups present in the grown crystal is evaluated using FTIR spectra. UV-vis-NIR analysis depicts that the doped crystal possesses high transparency than pure which ratifies the dye incorporation in the grown crystal and make it as suitable for optoelectronic applications. Using Knoop hardness study, the Youngs modulus of the material is calculated. Thermal diffusivity of the material is determined using Photoacoustic spectroscopy. The thermal diffusivity for pure and doped crystal is 1.2505 × 10–6 and 3.128 × 10–6, respectively. The doped crystal is 1.8 times higher than the pure crystal and 3.1 times higher than the standard KDP crystal, which is favoring nature of nonlinear optical materials in real-time applications. Vicker’s microhardness study portrays that both crystals come under soft material category and the various hardness parameters such as brittleness index (Bi), fracture toughness (Kc) and elastic stiffness constant (C11) were also enhanced due to dye inclusion. The real, imaginary and third-order susceptibility of pure and doped crystal is enhanced due to dye doping which is found using Z-scan study. Thus with numerous attractive optical, mechanical, photo-acosutic and third harmonic generation studies manifest that doped crystal is superior to pure and makes the doped anthracene is a benchmark crystal for nonlinear optical applications.
References
D.S. Chemla (ed.), Nonlinear Optical Properties of Organic Molecules and Crystals, vol. 1 (Elsevier, 2012)
Ch. Bosshard, J. Hulliger, M. Florsheimer, P. Gunter, Organic Nonlinear Optical Materials (CRC Press, Taylor & Francis Group, Boca Roton, FL, 2001), p. 256
M. Shkir, V. Ganesh, I.S. Yahia, H.S.M. Abd-Rabboh, S. AlFaify, Effects of methyl violet dye on the growth and properties of zinc (tris) thiourea sulfate single crystals. J. Phys. Chem. Solids 123, 336–343 (2018)
C.E. Feazel, C.D. Smith, Production of large crystals of naphthalene and anthracene. Rev. Sci. Instrum. 19(11), 817–818 (1948)
A.N. Aleshin, J.Y. Lee, S.W. Chu, J.S. Kim, Y.W. Park, Mobility studies of field-effect transistor structures basedon anthracene single crystals. Appl. Phys. Lett. 84(26), 5383–5385 (2004)
A. Arulchakkaravarthi, C.K. Laksmanaperumal, P. Santhanaraghavan, K. Sivaji, R. Kumar, S. Muralithar, P. Ramasamy, Preparation of high-quality anthracene crystals using double run selective self-seeding vertical Bridgman technique (DRSSVBT). J. Cryst. Growth 246(1–2), 85–89 (2002)
Qi Wang, Y. Luo, H. Aziz, Evidence of intermolecular species formation with electrical aging in anthracene-based blue organic light-emitting devices. J. Appl. Phys. 107(8), 084506 (2010)
V. Nagarajan, A.A. Ananth, S. Ramaswamy, P. Kavitha, T.A. Doss, Investigations on the growth, optical, mechanical, thermal and nonlinear behavior of transition metals doped anthracene crystals. Mater. Res. Innov. 22(7), 427–433 (2018)
N. Sinha, M.K. Gupta, N. Goel, B. Kumar, Effect of zinc chloride on structural, optical and dielectric behavior of solution grown anthracene crystal. Phys. B Condens, Matter 406(17), 3206–3209 (2011)
A.V. Kukhta, I.N. Kukhta, N.A. Kukhta, O.L. Neyra, E. Meza, DFT study of the electronic structure of anthracene derivatives in their neutral, anion and cation forms. J. Phys. B: At. Mol. Opt. Phys. 41(20), 205701 (2008)
N. Sinha, S. Bhandari, H. Yadav, G. Ray, S. Godara, N. Tyagi, J. Dalal, S. Kumar, B. Kumar, Performance of crystal violet doped triglycine sulfate single crystals for optical and communication applications. Cryst. Eng. Comm. 17(30), 5757–5767 (2015)
P. Rajesh, A. Silambarasan, P. Ramasamy, Effect of crystal violet dye on the optical, dielectric, thermal and mechanical properties of %3c 0 0 1%3e directed KDP single crystal. Mater. Res. Bull. 49, 640–644 (2014)
P. Zhang, J. Deng, X. Zeng, Z. Liu, Y. Qiu, H. Zhong, Y. Fan, J. Huang, J. Zhang, Xu Ke, Growth mechanism of large-size anthracene single crystals grown by a solution technique. J. Cryst. Growth 311(23–24), 4708–4713 (2009)
G. Madhurambal, P.A. Srinivasan, Growth of high quality anthracene crystals by a simple solution technique. Cryst. Res. Technol. J. Exp. Ind. Crystallogr. 41(3), 231–235 (2006)
H. Li, D. Zhang, L. Duan, G. Dong, L. Wang, Y. Qiu, Morphological structure and optical property of anthracene single crystals grown from solution. Jpn. J. Appl. Phys. 46(12R), 7789 (2007)
S. DeHaven, R. Wincheski, S. Albin, Anthracene fibers grown in a microstructured optical fiber for X-ray detection. Materials 7(9), 6291–6303 (2014)
S. Kumar, N. Sinha, S. Goel, B. Kumar, Effect of xylenol orange dye on morphological, optical, piezo-/di-electric and mechanical properties of potassium hydrogen phthalate single crystals. Vacuum 175, 109240 (2020)
M. Shkir, S. AlFaify, V. Ganesh, I.S. Yahia, H. Algarni, H. Shoukry, Brilliant green dye added zinc (tris) thiourea sulphate monocrystal growth with enhanced crystalline perfection, optical, photoluminescence and mechanical properties. J. Mater. Sci. Mater. Electron. 27(10), 10673–10683 (2016)
J. Tauc (ed.), Amorphous and Liquid Semiconductors (Springer Science & Business Media, 2012)
C.N.R. Rao, Ultraviolet and Visible Spectroscopy of Organic Compound (Prentice Hall Pvt. Ltd., New Delhi, 1984), p. 60
M. Shkir, I.S. Yahia, A.M.A. Al-Qahtani, V. Ganesh, S. AlFaify, Investigation on physical properties of L-alanine: an effect of methylene blue dye. J. Mol. Struct. 1131, 43–50 (2017)
G.B. Rao, P. Rajesh, P. Ramasamy, A study on the growth, optical, thermal, mechanical, dielectric and piezoelectric properties of dye doped KAP single crystals. Mater. Res. Bull.n 60, 709–713 (2014)
K. Jagannathan, S. Kalainathan, T. Gnanasekaran, Microhardness studies on 4-dimethylamino-N-methyl 4-stilbazolium tosylate (DAST). Mater. Lett. 61(23–24), 4485–4488 (2007)
G. Murugesan, S. Kalainathan, Experimental and theoretical modelling of Vickers, Knoop microhardness and laser damage threshold studies on Ca0.9Nd0.1Ti0.9Al0.1O3 single crystals grown by optical floating zone technique. J. Alloy. Compd. 677, 121–126 (2016)
S. Chandran, R. Paulraj, P. Ramasamy, Influence of amaranth dye on the growth and properties of KDP single crystal. Mater. Res. Bull. 68, 210–215 (2015)
K. Sangwal, On the reverse indentation size effect and microhardness measurement of solids. Mater. Chem. Phys. 63(2), 145–152 (2000)
E.M. Onitsch, Über die mikrohärte der metalle. Mikroskopie 2, 131 (1947)
B. Lal, K.K. Bamzai, P.N. Kotru, B.M. Wanklyn, Microhardness, fracture mechanism and dielectric behaviour of flux-grown GdFeO3 single crystals. Mater. Chem. Phys. 85(2–3), 353–365 (2004)
W.A. Wooster, Physical properties and atomic arrangements in crystals. Rep. Prog. Phys. 16(1), 62 (1953)
M. Shkir, V. Ganesh, S. AlFaify, A. Black, E. Dieguez, G. Bhagavannarayana, VGF bulk growth, crystalline perfection and mechanical studies of CdZnTe single crystal: a detector grade materials. J. Alloys Compd. 686, 438–446 (2016)
R. Ragu, M. Akilan, J.P. Angelena, P.S.L. Mageshwari, J.S. Das, Growth, optical, mechanical, thermo-physical, laser damage threshold (LDT) and Z-scan studies on dilithium succinate single crystal for optical limiting applications. J. Mater. Sci. Mater. Electron. 30(6), 6287–6299 (2019)
T. Jayapalan, S.J.D. Sathiyadhas, J. Michael, B. Settu, M.B.D.S. Amalapushpam, Thermophysical and optical properties of L-Tartaric acid crystal. Cryst. Res. Technol. 53(3), 1700267 (2018)
W.L.B. Melo, R.M. Faria, Photoacoustic procedure for measuring thermal parameters of transparent solids. Appl. Phys. Lett. 67(26), 3892–3894 (1995)
R. Ragu, P.S.L. Mageshwari, M. Akilan, J.P. Angelena, S.J. Das, Enrich mechanical, photo-acoustic, SHG and Z-scan studies on pure and crystal violet dye (CV) incorporated sodium acid phthalate crystal for optical applications. J. Mater. Sci. Mater. Electron. 30(2), 1670–1676 (2019)
P.P. Vinaya, A.N. Prabhu, K.V. BhatUpadhyaya, Synthesis, growth and characterization of a long-chain π-conjugation based methoxy chalcone derivative single crystal; a third order nonlinear optical material for optical limiting applications. Opt. Mater. 89, 419–429 (2019)
N.M. Ravindra, V.K. Srivastava, Electronic polarizability as a function of the penn gap in semiconductors. Infrared Phys. 20(1), 67–69 (1980)
D.R. Penn, Wave-number-dependent dielectric function of semiconductors. Phys. Rev. 128(5), 2093 (1962)
P. Van Rysselberghe, Remarks concerning the Clausius–Mossotti law. J. Phys. Chem. 36(4), 1152–1155 (1932)
M. Sheik-Bahae, A.A. Said, E.W.V. STryland, High-sensitivity, single-beam n2 measurements. Opt. Lett. 14(17), 955–957 (1989)
M. Sheik-Bahae, A.A. Said, T.-H. Wei, D.J. Hagan, E.W.V. Stryland, Sensitive measurement of optical nonlinearities using a single beam. IEEE J. Quantum Electron. 26(4), 760–769 (1990)
V. Subhashini, S. Ponnusamy, C. Muthamizhchelvan, B. Dhanalakshmi, Growth and characterization of piperazinium 4-nitrophenolate monohydrate (PNP): A third order nonlinear optical material. Opt. Mater. 35(7), 1327–1334 (2013)
M. Akilan, R. Ragu, J.P. Angelena, S.J. Das, Enhancement in mechanical, optical, SHG, photoacoustic and Z-scan studies on pure and crystal violet dye doped L-proline cadmium chloride single crystal for nonlinear optical applications. J. Mater. Sci. Mater. Electron. 30(4), 3655–3662 (2019)
M. Shkir, M. Anis, S.S. Shaikh, S. AlFaify, An investigation on structural, morphological, optical and third order nonlinear properties of facilely spray pyrolysis fabricated In:CdS thin films. Superlattices Microstruct. 133, 106202 (2019)
M.K. Krishna, S. Sudhahar, P. Pandi, G. Bhagavannarayana, M.R. Kumar, Studies of the structural and third-order nonlinear optical properties of solution grown 4-hydroxy-3-methoxy-4′-N′-methylstilbazolium tosylate monohydrate crystals. Opt. Mater. 36(5), 988–995 (2014)
M. Shkir, A. Irfan, S. AlFaify, P.S. Patil, A.G. Al-Sehemi, Linear, second and third order nonlinear optical properties of novel noncentrosymmetric donor-acceptor configure chalcone derivatives: A dual approach study. Optik 199, 163354 (2019)
Acknowledgements
The authors are thankful to management of Loyola College, Chennai – 34 for granting Times of India Major Research Project (3LCTOI14PHY002). Furthermore, the authors express heartfelt gratitude to Dr. R. Robert, Assistant Professor, Govt. Arts College for Men, Krishnagiri and Dr. M. Jose, Assistant Professor, Sacred Heart College, Tirupattur for fruitful discussions throughout this research work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ragu, R., Mageshwari, P.S.L., Akilan, M. et al. Enhanced optical, mechanical, photoacoustic and third-order nonlinear property of pure and crystal violet (CV) dye incorporated anthracene crystal: an efficacious material for nonlinear optical applications. Appl. Phys. B 126, 95 (2020). https://doi.org/10.1007/s00340-020-07439-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00340-020-07439-y