Abstract
A mid-infrared laser-based sensor for the detection of gas-phase benzene (C6H6) in ambient air is presented. It is based on the principle of tunable laser absorption spectroscopy utilizing unique fine spectral features of benzene near 3.4 µm, along with the robust design of an optical multi-pass cell. The sensor is capable of providing real-time measurement of benzene as low as 200 ppb (12 ppm m) at 2 Hz, while still being portable enough to fit in the trunk of a small car. A demonstration measurement in a moving vehicle is presented based on the data collected along Highway 101, El Camino Real, and around Stanford University campus in California, showing elevated benzene emission in several spatial regions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Benzene (C6H6) is a common naturally occurring and anthropogenic chemical associated with a range of acute and long-term adverse health effects and diseases, including cancer and aplastic anemia [1]. Exposure to benzene can occur occupationally and domestically as a consequence of using petroleum products, including motor fuels, paints and solvents [2, 3]. Active and passive exposure to tobacco smoke is also a significant source of benzene [4]. It ranks in the top 20 chemicals manufactured in production output (5.8 million tonnes/year) in the USA. Some industries use benzene to make other chemicals that are converted to plastics, resins, and nylon and synthetic fibers. Benzene is also used to make a number of lubricants, rubbers, dyes, detergents, drugs, and pesticides. Natural sources of benzene include volcanoes and forest fires [5].
Because of benzene’s strong effects on human health, efforts are needed to reduce the exposure of workers and the general population to benzene. The Environmental Protection Agency (EPA) defines the maximum contaminant level (MCL) for benzene in an aqueous environment to be 0.005 mg/L (5 ppb). In air, the short-term exposure limit for benzene is 5 ppm for 15 min; the maximum exposure standard for 8 h is 1 ppm (Occupational Safety and Hazard Administration), with 0.1 ppm being the recommended 8-h standard. However, benzene is difficult and expensive to monitor in real time. To measure people’s exposures in their environments, homes, and workplaces, sensors are needed to detect trace quantities of benzene. Currently, to our knowledge, no such portable real-time benzene sensor exists in the market, particularly one with suitable lower detection limit, reasonable detection bandwidth, and portability. For example, the “benzene-specific” PID sensors (https://www.raesystems.com/sites/default/files/content/resources/eBook-gas-detection-tube-and-sampling-handbook.pdf) with standard range from RAE Systems™ have 80% cross-sensitivity from CO, 40% from H2S, 40% from NO, > 200% from hexane and 20% from toluene, etc. Only a handful of devices to our knowledge can measure benzene and none of them appears able to measure it without interference from similar hydrocarbons such as toluene, ethylbenzene and xylenes and sometimes even from common atmospheric gases.
The lack of a portable benzene sensor results in limited benzene monitoring in communities. Macey et al. [6] collected atmospheric samples using Tedlar bags and analyzed the samples in the laboratory. The drawback of this approach is the lack of continuous data collection in the field and the limited spatial and temporal extent of available samples. Variations in gas concentrations, for instance, depend on dynamic factors that include wind speed and direction, local air circulation, and many additional factors. A GPS-enabled benzene-specific sensor that is field deployable is strongly needed, which is the goal of the new instrument we describe here.
Our benzene sensor is based on the principle of tunable laser absorption spectroscopy [7]. Several types of absorption spectroscopy techniques for gas detection in ambient conditions have been demonstrated, such as tunable diode laser absorption spectroscopy (TD-LAS) [8], external-cavity quantum-cascade laser (EC-QCL) spectroscopy [9], interband cascade laser (ICL) spectroscopy [10], cavity ring-down spectroscopy (CRDS) [11] and cavity-enhanced absorption spectroscopy (CEAS) [12]. Previous research by Hanson et al. has pioneered the development of several key variations of such techniques over the last few decades [13] with a primary focus on applications in combustion-related harsh environments, including shock tubes [14], scramjet combustors [15, 16], coal gasifiers [17] and even in the confined volumes of fuel cells [18].
For our benzene sensor, we identified a spectral region near 3.4 µm in wavelength with a number of unique attractive spectral properties of benzene, as discussed below. This current sensor showcases the utility of narrow spectral features in the design of a real-time benzene sensor that can be deployed in the field.
2 Molecular spectrum of benzene and occurrence of narrowband spectral features
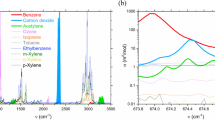
A broad survey of benzene absorption spectra was obtained from the Pacific Northwest National Laboratory (PNNL) infrared database [19] and is presented in Fig. 1. The two strongest bands (Band #1 in Fig. 1, a fundamental band at 675 cm−1 or 14.8 µm as a result of out-of-plane C–H deformations, and Band #6: 3096 cm−1 or 3.22 µm due to C–H stretching vibrations [20]), are investigated here because of their absorption strength for benzene and the absence of water interference. Based on a review of the infrared spectra of other hydrocarbons in the PNNL IR database, other species absorb in the 3 µm region because of the fundamental C–H stretch that resonates at those wavelengths. Therefore, spectral features are needed that are unique to benzene and that can be utilized to make a specific sensor for it. However, until now, such unique structures in the benzene transitions were not to our knowledge discovered other than the 674 cm−1 Q-branch of the fundamental band (Fig. 1b), which is still outside the accessible range for tunable lasers and hence cannot currently be used.
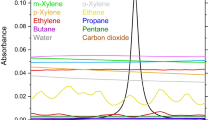
Here for the first time, we have identified and characterized high-resolution narrowband spectral features of benzene to form the basis of a rapid and portable atmospheric sensor based on tunable laser spectroscopy. A set of isolated narrowband structures of benzene was observed at sub-atmospheric pressure conditions around 3090 cm−1 as shown in Fig. 2a. The absorption spectrum of benzene at target testing conditions (296 K, 0.26 atm) is plotted in Fig. 2b, along with the local water vapor spectra [21] at 2% mole fraction (72% RH at 296 K). It can be immediately observed that there are flat regions of water vapor spectra where these benzene transitions can facilitate its interference-free measurement. At ambient pressures, the absorption spectrum becomes more blended and water interference becomes more prominent due to spectral broadening. These spectrally narrow features were previously unobservable due to the fact that the PNNL database reports spectral data at 1 atm, and no previous high-resolution data were taken at lower pressures at room temperature in the 3 µm region, where these features become apparent. In our work, a spectral database focused on these features was created for benzene in air as a function of pressure. This database was then utilized to provide the fundamental spectroscopic parameters for the portable sensor design as described in the next section.
3 Sensor theory
The benzene sensor is based on the principle of tunable laser absorption spectroscopy. Although the fundamentals of tunable laser absorption spectroscopy have been published many times, these principles are reviewed briefly in the next section to define units and to put the demonstration measurements in context.
The theory of laser absorption spectroscopy is well documented in the literature (e.g., [7, 13]). The spectral transmissivity, Tν, of monochromatic radiation at frequency ν through a gaseous absorbing medium is given by the Beer–Lambert relation:
where I0 is the incident beam intensity, I is the transmitted beam intensity, and αν is the spectral absorbance given by
where σν is the absorption cross section in m2/mol at frequency ν, L is the path length in m, P is the total pressure in Pascal, R is the gas constant as 8.314 J/K/mol, T is the gas temperature in K and \(x_{{{\text{C}}_{6} {\text{H}}_{6} }}\) is the mole fraction of benzene. The absorption cross section was measured for benzene at various pressures in a certified mixture with air and used as a basis for the sensor operation.
4 Portable laser-based benzene sensor prototype
A portable sensor based on the above spectral features near 3090 cm−1 was created for measurement of atmospheric benzene as shown in Fig. 3, with its 16 components listed in Table 1. The sensor is built around the 63-m aluminum multipass cell (Component 0) in the center. The interband cascade laser from Nanoplus (7) is mounted on the top of the cell. The silicon beamsplitter (8) is used to co-align a fiber-coupled visible laser pitched using a collimator (11) for alignment. The pitch mirrors (3) are used to launch the laser into the multipass cell. The output beam is captured and focused by the catch mirrors (2) onto the Vigo PVI-4TE-4 MCT detector (1). The laser current and temperature are controlled using a Wavelength Electronics LDTC 0520 laser diode and temperature controller (13). The laser modulation current is controlled using arbitrary signal generation by the Picoscope 5442 data acquisition system (15). The detector voltage is fed into the picoscope input channel. A portable pump is used to draw the air sample into the multipass cell. The sample pressure is measured using an Omega PX309-050A5V pressure transducer (6). The various temperature and pressure data require lower sampling rate and were hence recorded using an Arduino Due (14). Ventilation fans (4 and 9) were used to circulate air and enable efficient cooling of the sensor package.
5 Sensor validation
We validated the sensor using a certified 2000 ppm benzene bottle from Praxair, diluted with air in different ratios, using differential flow rates produced by two Alicat scientific mass flow controllers FC1 and FC2, as shown in Fig. 4. The pressure in the sensor is controlled using the metering valve V2. A range of benzene concentrations from 200 ppb to 333 ppm was created using this differential flow mixing technique, with a constant total volumetric flow rate of 3.5 standard liters per minute (SLPM). A comparison between expected concentration from flow rate-based estimates and laser sensor-based measured concentrations is shown in Fig. 5a. The results show ± 2% agreement above 10 ppm and < ± 200 ppb agreement below 10 ppm. Figure 5b shows a staircase plot of time-varying concentration measurements of benzene. The oscillations around the lowest detected concentration of 200 ppb are partially attributable to the oscillations in the mass flow controller FC2, at around 0.2% full scale values. A noise floor was observed around 200 ppb for an averaging time of 0.5 s. Better detection limits can be achieved with longer averaging times. The ultimate detection limit can be improved significantly with a higher optical path length without much concern from water interference. However, this change would require a larger volume multipass cell that can still be mounted on a car, but difficult to carry on foot.
6 Field demonstration with car-based measurements
Figure 6 shows the benzene sensor loaded into a car. The sensor was kept on four vibration isolation blocks to reduce noise from the motion of the car on uneven roads. The sampled air was drawn into the sensor by the pumps and real-time measurements were displayed on a laptop computer. A GPS unit, magnetically attached on top of the car, provided real-time GPS coordinate measurements logged with the measured benzene concentrations.
Measurements were taken with this prototype around the greater San Francisco area on two occasions. Figures 7, 8, 9, 10 and 11 show some results from these measurements. The results obtained from day 1 (17 °C, 67% RH, cloudy to partially cloudy) of tests are summarized in Fig. 7. The main observations of elevated benzene levels were observed due to (1) traffic congestion at intersections and along Highway 101, (2) gas stations, and (3) underground parking lots. These individual sources are discussed separately below.
6.1 Elevated benzene levels due to traffic congestion in rush hour and at intersections
Numerous measurements of ~ 1 ppm benzene were observed (Fig. 8) attributable to traffic congestion in rush hours on Highway 101. The vehicle was moving at an average speed of 5 miles per hour during the traffic jam, at the locations marked by the yellow arrows in the figure below. This result is not surprising because car engines run more inefficiently under such conditions, with frequent braking and acceleration leading to relatively high amounts of unburnt gasoline in the exhaust.
6.2 Gas stations
Different blends of gasoline contain different levels of benzene. In 2007, EPA updated the Control of Hazardous Air Pollutants from Mobile Sources (MSAT2), which caps the total content of benzene in gasoline at 0.62 percent. Not surprisingly, then, benzene was found in significantly higher levels in the near vicinity of gas pumps, especially while filling the tank, than in surrounding areas. The following measurements were conducted on day 2 (17 °C, 63% RH, cloudy), especially focused on the levels of benzene found around gas stations on El Camino Real between Palo Alto and Mountain View, California. Static measurements were taken near the nozzles of the nine gas stations with the car engine shut off. Those results are shown as the spikes 1–9 in Figs. 9 and 10. Different gas stations had slightly different readings, and the amount of benzene could be correlated with the specific gas provider.
6.3 Underground parking structure
Elevated levels of benzene in an underground parking structure had sources similar to the ones observed in congested traffic. However, a key difference is observed attributable to low air ventilation and the fact that benzene (78.11 g/mol) is about 2.7 times heavier than air (~ 29 g/mol) and, therefore, tends to settle by density to lower levels of the parking garage. A plot of measured benzene levels in an underground parking garage is shown in Fig. 11. A maximum reading of 2 ppm was observed. The GPS connection was typically poor underground and merely showed the locations near the garage.
7 Conclusion
We identified a previously unrecorded occurrence of narrowband absorption transitions of benzene and, after characterizing these features, we used them as the basis for a new field portable sensor. The sensor was validated under controlled environments in the laboratory, and a low detection limit of ~ 200 ppb was achieved with 0.5 s averaging time. This sensor was also successfully operated in a moving vehicle, with elevated benzene concentrations observed in highway traffic and intersections, at gasoline stations, and in an underground parking structure.
References
W. Abplanalp et al., Benzene exposure is associated with cardiovascular disease risk. PLoS One 12(9), 1–15 (2017)
H.H. Hamid, N.S. Jumah, M. Talib Latif, N. Kannan, BTEXs in indoor and outdoor air samples: source apportionment and health risk assessment of benzene. J. Environ. Sci. Public Heal. 1(1), 49–56 (2017)
A.M. Moro et al., Biomonitoring of gasoline station attendants exposed to benzene: effect of gender. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 813, 1–9 (2017)
B.R. Appel et al., Benzene, benzo(a)pyrene, and lead in smoke from tobacco products other than cigarettes. Am. J. Public Health 80(5), 560–564 (1990)
C.C. Austin, D. Wang, D.J. Ecobichon, G. Dussault, Characterization of volatile organic compounds in smoke at experimental fires. J. Toxicol. Environ. Heal. Part A 63(3), 191–206 (2001)
G.P. Macey et al., Air concentrations of volatile compounds near oil and gas production: a community-based exploratory study. Environ. Health 13(1), 82 (2014)
R.K. Hanson, R.M. Spearrin, C.S. Goldenstein, Spectroscopy and Optical Diagnostics for Gases, 1st edn. (Springer, New York, 2016)
P.O. Wennberg et al., On the sources of methane to the Los Angeles atmosphere. Environ. Sci. Technol. 46(17), 9282–9289 (2012)
R. Lewicki et al., Ammonia sensor for environmental monitoring based on a 10.4 µm external-cavity quantum cascade laser. in Lasers, Sources and Related Photonic Devices, OSA Technical Digest Series (CD) (2010). https://doi.org/10.1364/LACSEA.2010.LTuD2
R. Ghorbani, F.M. Schmidt, ICL-based TDLAS sensor for real-time breath gas analysis of carbon monoxide isotopes. Opt. Express 25(11), 12743 (2017)
S. Affolter, D. Fleitmann, M. Leuenberger, New online method for water isotope analysis of speleothem fluid inclusions using laser absorption spectroscopy (WS-CRDS). Clim. Past 10(4), 1291–1304 (2014)
M. Gupta, Highly-precise measurements of ambient oxygen using near-infrared cavity-enhanced laser absorption spectrometry. Anal. Chem. 84(18), 7987–7991 (2012)
R.K. Hanson, Applications of quantitative laser sensors to kinetics, propulsion and practical energy systems. Proc. Combust. Inst. 33(1), 1–40 (2011)
Y. Ding, C.L. Strand, R.K. Hanson, High-temperature mid-infrared absorption spectra of methanol (CH3OH) and ethanol (C2H5OH) between 930 and 1170 cm−1. J. Quant. Spectrosc. Radiat. Transf. 224, 396–402 (2019)
I.A. Schultz et al., Multispecies midinfrared absorption measurements in a hydrocarbon-fueled scramjet combustor. J. Propuls. Power 30(6), 1595–1604 (2014)
G.B. Rieker, J.B. Jeffries, R.K. Hanson, Calibration-free wavelength-modulation spectroscopy for measurements of gas temperature and concentration in harsh environments. Appl. Opt. 48(29), 5546–5560 (2009)
R. Sur, K. Sun, J.B. Jeffries, J.G. Socha, R.K. Hanson, Scanned-wavelength-modulation-spectroscopy sensor for CO, CO2, CH4 and H2O in a high-pressure engineering-scale transport-reactor coal gasifier. Fuel 150, 102–111 (2015)
R. Sur, T.J. Boucher, M.W. Renfro, B.M. Cetegen, In situ measurements of water vapor partial pressure and temperature dynamics in a PEM fuel cell. J. Electrochem. Soc. 157(1), B45 (2010)
S.N. Sharpe, T.J. Johnson, R.L. James, P.M. Chu, G.C. Rhoderick, P.A. Johnson, Gas phase database for quantitative infrared spectroscopy. Appl. Spectrosc. 58(12), 1452–1461 (2004)
W. Kemp, Organic Spectroscopy, 3rd edn. (Wacmillan Education, Ltd., London, 1991)
I.E. Gordon et al., The HITRAN2016 molecular spectroscopic database. J. Quant. Spectrosc. Radiat. Transf. 203, 3–69 (2017)
Acknowledgements
We acknowledge support from the Stanford Woods Institute for the Environment through the Environmental Ventures Program (EVP).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sur, R., Ding, Y., Jackson, R.B. et al. Tunable laser-based detection of benzene using spectrally narrow absorption features. Appl. Phys. B 125, 195 (2019). https://doi.org/10.1007/s00340-019-7311-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00340-019-7311-z