Abstract
Ultra-pure silicon nanoparticles were synthesized through pulsed laser ablation (PLA) in ethanol medium using a Q-switched Nd:YAG nanosecond pulsed laser. Concentration dependence of thermal diffusivity of the silicon nanofluid was studied using dual beam thermal lens technique at room temperature. The thermal diffusivity was found to be decreased with increase in concentration of silicon nanoparticles. This property of nanofluids can be used for thermal trapping and insulating applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
There has been a large interest in nanoparticle research for the last few decades. Semiconductor nanoparticles are one of the key areas of nanotechnologists for light-emitting devices, energy and biomedical applications due to their extensive optoelectronic properties [1, 2]. Silicon-based nanomaterials got wide attention because of their luminescent properties, stability [3,4,5,6,7] and emission tuning with surface capping [8,9,10]. There have been wide applications on silicon quantum dots (Si QDs) as agents for measuring contrast [11], bio-imaging [12], photovoltaics [13] and LED applications [14,15,16,17,18,19]. Further, the studies show that the use of silicon nanoparticles in biological samples has an advantage of low toxicity up to the concentration of 200 mg/ml even after 24 h [20].
Different synthesis methods adopted for Si QDs including that of top-down or bottom-up approaches. In the top-down approach, bulk silicon is fragmented into nanostructures using physical, chemical and their combinations such as pulsed laser ablation [21, 22], electrochemical etching [23], magnetron sputtering [24] etc. In bottom-up approach, solution-phase oxidation/reduction [25,26,27] or laser pyrolysis [28] is utilised to form Si-nanoparticles. Pulsed laser ablation (PLA) is one of the easiest and cleanest method for nanoscale fabrication of materials [29, 30]. The first synthesis of nanoparticles using laser ablation was reported by Okada in 1991 [31]. Out of the two approaches for preparing the nanomaterials through the laser ablation, that is liquid [32, 33] or gas phase [12]; the former provides pure nanoparticle colloidal solution [34] and the latter produces thin nano films (PLD) or nanoparticles itself. The nanoparticles prepared are of almost spherical shapes and free from the contaminations [25]. The size and photoluminescence properties depend upon the laser parameters (wavelength, power and pulse duration), time of ablation and solvents used [21]. However, the lack of production efficiency is the demerit of PLA [35].
Studies about the thermal transport properties of nanoparticles dispersed in liquids got attention in the last few decades [36]. Addition of small amount of nanoparticles of metals or metal oxides into the liquids gives enhancement in the thermal diffusivity [37,38,39]. These can be used for the coolant applications in industries, automobiles, biomedical applications etc. [40]. In some semiconductor nanofluids, when the particle size is much low compared to the phonon mean free path, there is a decrease in the heat transfer properties [41]. This negative thermal transport is also useful for steering thermal agents in medical therapies with below-cellular dimensions as well as in some thermal insulating applications [42].
Thermal diffusivity is an important thermo-physical property that specifies heat transport in a medium through diffusion. Nanoscale effects of thermal diffusion in the target material during laser ablation [43] and thermal diffusivity of nanofluids [44,45,46,47] are widely studied. It is a significant parameter to analyse the thermal performance under flow-based applications. There are several techniques such as thermal lens [48] and photoacoustic techniques [49] to find thermal diffusivity of nanofluids, in which the former technique is mostly preferred as it gives more sensitivity in the measurements [50].
2 Theory
A laser beam absorbed by the liquid sample, produces the local heating effects along the beam waist, and this induces corresponding refractive index changes and a lens forms in that area, which is the principle behind thermal lens (TL) technique. The first study of the TL effect was done by Gordon et al., in 1965 [51]. Mode-matched dual beam thermal lens technique is a well sensitive method to sense small (of the order of 10− 8) refractive index variations across the laser beam due to the temperature changes of ∼10− 5 °C in a liquid sample [52, 53].
The appropriate wavelength for the pump laser selected and focused on the sample. Sample absorbs the pump beam that in turn results non-radiative decay of the sample and generates heat locally. This modifies the refractive index around the heated area, and induces a lens effect. The thermally generated lens may be diverging or converging depending on the temperature coefficient of the refractive index (dn/dT). Most of the materials show a negative temperature coefficient and thus expands on heating and make a diverging lens. The transient effects of thermal effect are studied by switching the pump on and off, and it is done by chopping the pump beam. The probe beam was allowed to pass through the sample collinearly with the pump beam. Thermal lens effect and thus thermal blooming occurs when the pump is on and is revoked when the pump is off. Pump beam was filtered after passing through the sample. The transient signal of the pump-on–pump-off is detected by collecting the probe signal.
Effective lens induced in the liquid is governed by [54],
where F is focal length, dn/dT is the temperature dependence in the refractive index, Pabs is the power absorbed by the liquid, k is the thermal conductivity of the liquid sample and ω is the beam radius. The characteristic time constant tc for thermal diffusion is given by,
where ρ is the density and c is the specific heat of the liquid. A good probe signal can be achieved with proper selection of pump beam power. Then the time-dependent probe beam intensity I(t) follows the expression
where, θ related to the thermo-optic parameters of the sample.
where, (Pth) is thermal power radiated as heat and λp is the laser wavelength. θ and tc can be found by curve-fitting the thermal lens signal to the Eq. (3). The characteristic time constant value provides the diffusivity D through the equation [55].
3 Experimental
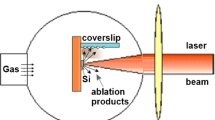
Si nanofluid was prepared by pulsed laser ablation of silicon wafer in ethanol medium. A piece of AR-grade Si wafer was ultrasonically cleaned in ethanol, subsequently in acetone for 10 min and dried. The schematic set up for the synthesis is given in Fig. 1. The wafer was dipped in 10 ml ethanol in a 50 ml beaker placed on the three-dimensional translational stage which was capable of moving the target back and forth. The wafer was irradiated by the beam of pulsed Nd-YAG laser source with the fundamental wavelength of 1064 nm, and its harmonics such as 532 nm and 355 nm. The experiments were extended further by fixing the wavelength at 1064 nm as this has resulted in good distribution and yield of silicon nanoparticles. The pulsed Nd: YAG laser (Spectra physics Quanta-Ray, Lab 190-10, repetition rate 10 Hz, pulse width 9 ns) was used for irradiation. The beam was focused with a convex lens of focal length of 100 mm. The spot radius of the focused beam on the target was calculated as 21 µm and peak intensity was 3.2 × 1011W/cm2. The ablation is carried out for 3 h of duration for which the weight loss from the wafer was found 0.0009 g.
From the prepared sample of stock solution of concentration of 9 mg/ml, various samples were diluted with the different concentrations of 0.75 mg/ml, 1.5 mg/ml, 2.25 mg/ml, 3 mg/ml, 3.75 mg/ml and 4.5 mg/ml and labelled each as R1, R2, R3, R4, R5 and R6.
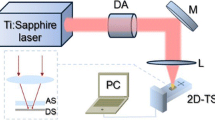
The schematic diagram of the thermal lens technique is presented in Fig. 2. A vertically polarised 403 nm continuous wave DPSS (Diode Pump Solid State) laser (VORTRAN stradusTM 405) of power 100 mW was used as the pump source and a continuous wave JDS Uniphase 633 nm He-Ne laser of 4 mW as the probe was used for the experiment. The pump power was adjusted by changing neutral density filters.
4 Results and discussions
The absorption spectra of the Si nanocrystals were taken using (Jasco-V-570) spectrophotometer. Figure 3 shows the absorption spectra for various concentrations of silicon nanoparticles in ethanol. As the concentration increases, the spectra show an increase in the peaks.
Energy-dispersive spectroscopy (EDS) of the sample (Fig. 4) was taken to confirm that the sample contains silicon nanoparticles of high purity. A trace amount of carbon and oxygen in the EDS data is from the carbon tape used to place the silicon nanoparticles.
The TEM image of silicon nanoparticles (Fig. 5a) was taken using Transmission Electron Microscope Jeol/JEM 2100. From the histogram graph plot (Fig. 5b) of the TEM image, the average size of the silicon nanoparticles were calculated as 6 nm.
Photo-thermal studies of the synthesized silicon nanofluids for various concentrations in ethanol were carried out. Thermal blooming at different pump power for the sample R1–R6 has been recorded. Photographs of thermal blooming at various pump powers for the sample R3 with concentration of 2.25 mg/ml is presented in Fig. 6. As the pump power was increased for the experiment, the blooming exhibits fringe patterns which are due to the effects of aberration in the thermal lens formed.
The Fig. 7a shows the TL signal obtained in the oscilloscope for the sample R3 for the pump power 3.16 mw. The normalised transient curve for pump-on–pump-off also was extracted from the TL signal for each sample and presented in the Fig. 7b. It was observed that, as the concentration of the samples becomes higher, the time delay of the transient curve was found to increase. This indicates that more time was taken to diffuse the heat in the sample, and hence more time to revoke the thermal blooming in the higher concentrated samples.
To find the time constant, tc, the transient curve for the pump-on–pump-off signal was fitted with Eq. (3). The decay curve and the theoretical fit using Eq. (3) for the sample R3 is shown in Fig. 8. The focused spot size (ω) at the sample was calculated as 1.209 × 10− 8 m2 using the well-known method [56]. From the time constant tc and the focused spot size (ω), the thermal diffusivity, D was calculated using the Eq. (5). It has been noted that thermal diffusivity variations for increasing concentration is decreasing and the results are depicted in Table 1 and Fig. 9.
The transient curve for the sample R3 and its theoretical fit using Eq. (3)
The decrease in thermal diffusivity with the increase in the concentration of Si nanofluid is very evident from the graph (Fig. 9).
The highest thermal diffusivity obtained for the sample R1 with the least concentration was 6.23 × 10− 8 m2/s, lower than that of ethanol for which the thermal diffusivity is 9.33 × 10− 8m2/s [57, 58]. The heat energy gets trapped more in the nanofluid containing silicon nanoparticles. Reduction in the Brownian motion of the base fluid molecule is the prime factor for the reduction of diffusivity of nanofluid. As the concentration of Si nanoparticle increases, the Brownian motion of ethanol molecules further reduced.Though collision of silicon nanoparticles occurs in the nanofluid due to the Brownian motion, it contributes less effect in the thermal diffusivity [59]. The thermal conductivity of the fluid caused by collision and the mean speed of the phonon contribute to the heat-transfer mechanisms [59].
The reduction in the thermal diffusivity on concentration can also be explained based on the density and specific heat capacity in comparison with the base fluid [41]. In the present case, there is a slight increase in the thermal conductivity of nanofluid compared to that of ethanol. The thermal conductivity of base fluid ethanol is 0.168 W/mK and that of Si nanoparticle is in the range (2–4 W/mK) [60, 61]. However, the specific heat capacity of nanofluid is slightly higher than that of ethanol. Here the effect of increased thermal conductivity of nanofluid overcomes the overall increase in the density and specific heat capacity of the total fluid. Thus the thermal diffusivity of the silicon nanofluid reduces effectively as concentration increases.
5 Conclusion
Ultra-pure silicon nanofluid was synthesized through pulsed laser ablation in ethanol medium using nanosecond 1064 nm Nd-YAG laser source. The size distribution and purity were analysed through TEM and EDS. The concentration-dependent variation of absorption spectra and thermal diffusivity studies were carried out. Thermal diffusivity was found to be varied inversely with the concentration of silicon nanoparticles in the nanofluid. This is due to the reduction of the Brownian motion of the base fluid molecules in the nanofluids. The results can be explored to utilise the silicon nanofluids for the thermal insulating applications.
References
A. Tartakovskii, Quantum dots: optics, electron transport and future applications. Cambridge University Press, Cambridge (2012)
B. Yan, H. Zhou, J.L. Gardea-torresdey, Bioactivity of Engineered Nanoparticles (Springer, Singapore, 2017)
A. Shiohara, S. Prabakar, A. Faramus, C.-Y. Hsu, P.-S. Lai, P.T. Northcote, R.D. Tilley, Nanoscale 3, 3364 (2011)
T. Nakamura, Z. Yuan, S. Adachi, Nanotechnology 25, 275602 (2014)
Z. Yuan, T. Nakamura, S. Adachi, K. Matsuishi, Nanoscale 9, 1193 (2017)
T. Nakamura, Z. Yuan, K. Watanabe, S. Adachi, Appl. Phys. Lett. 108, 023105 (2016)
G. Lin, C. Lin, C. Lin, L. Chou, Y. Chueh, J. Appl. Phys. 97, 094306 (2005)
M. Dasog, K. Bader, J.G.C. Veinot, Chem. Mater. 27, 1153 (2015)
Y. Zhai, M. Dasog, R.B. Snitynsky, T.K. Purkait, M. Aghajamali, A.H. Hahn, C.B. Sturdy, T.L. Lowary, J.G.C. Veinot, J. Mater. Chem. B 2, 8427 (2014)
M. Dasog, G.B.D.L. Reyes, L.V. Titova, F.A. Hegmann, J.G.C. Veinot, ACS Nano 8, 9636 (2014)
S.V. Zabotnov, F.V. Kashaev, D.V. Shuleiko, M.B. Gongalsky, L.A. Golovan, P.K. Kashkarov, D.A. Loginova, P.D. Agrba, E.A. Sergeeva, M.Y. Kirillin, Quantum Electron. 47, 638 (2017)
M.B. Gongalsky, L.A. Osminkina, A. Pereira, A.A. Manankov, A.A. Fedorenko, A.N. Vasiliev, V.V. Solovyev, A.A. Kudryavtsev, M. Sentis, A.V. Kabashin, V.Y. Timoshenko, Sci. Rep. 6, 1 (2016)
H.R. Rasouli, A. Ghobadi, T.G.U. Ghobadi, H. Ates, K. Topalli, A.K. Okyay, J. Opt. 19, 105902 (2017)
Y. Xin, K. Nishio, K.I. Saitow, Appl. Phys. Lett. 106, 201102 (2015)
G. Lin, C. Lin, H. Kuo, G. Lin, C. Lin, Appl. Phys. Lett. 91, 093122 (2007)
G. Lin, Y. Pai, C. Lin, C. Chen, G. Lin, Y. Pai, C. Lin, C. Chen, Appl. Phys. Lett. 96, 263514 (2010)
G. Lin, C. Lin, C. Lin, Opt. Express 15, 2555 (2007)
C. Cheng, Y. Lien, C. Wu, G. Lin, Opt. Express 21, 391 (2013)
C. Cheng, C. Wu, C. Chen, L. Tsai, Y. Lin, G. Lin, S. Member, C. Cheng, C. Wu, C. Chen, L. Tsai, IEEE Photonics Journal. 4, 1762 (2012)
M.S. Stan, I. Memet, C. Sima, T. Popescu, V.S. Teodorescu, A. Hermenean, A. Dinischiotu, Chem. Biol. Interact. 220, 102 (2014)
M. Stafe, A. Marcu, N.N. Puscas, Pulsed Laser Ablation of Solids (Springer-Verlag, Berlin, Heidelberg, 2014)
L. Vaccaro, L. Sciortino, F. Messina, G. Buscarino, S. Agnello, M. Cannas, Appl. Surf. Sci. 302, 62 (2014)
C.-C. Tu, L. Tang, J. Huang, A. Voutsas, L.Y. Lin, Opt. Express 18, 21622 (2010)
W. Tang, J.J. Eilers, M.A. Van Huis, D. Wang, R.E.I. Schropp, M. Di Vece, J. Phys. Chem. C 119, 11042 (2015)
A. Marinins, R. Zandi Shafagh, W. Van Der Wijngaart, T. Haraldsson, J. Linnros, J.G.C. Veinot, S. Popov, I. Sychugov, ACS Appl. Mater. Interfaces 9, 30267 (2017)
T. Helbich, M.J. Kloberg, R. Sinelnikov, A. Lyuleeva, J.G.C. Veinot, B. Rieger, Nanoscale 9, 7739 (2017)
Q. Li, T.Y. Luo, M. Zhou, H. Abroshan, J. Huang, H.J. Kim, N.L. Rosi, Z. Shao, R. Jin, ACS Nano 10, 8385 (2016)
F. Huisken, G. Ledoux, O. Guillois, C. Reynaud, Adv. Mater. 14, 1861 (2002)
C. Phipps (ed.), Laser Ablation and Its Applications (Springer, US, 2007)
K. Ratautas, M. Gedvilas, G. Račiukaitis, A. Grigonis, J. Appl. Phys. 112, (2012)
R. Okada, S. Iijima, Appl. Phys. Lett. 58, 1662 (1991)
Y. Xin, T. Kitasako, M. Maeda, K. ichi Saitow, Chem. Phys. Lett. 674, 90 (2017)
D. Tan, S. Zhou, J. Qiu, N. Khusro, J. Photochem. Photobiol. C Photochem. Rev. 17, 50 (2013)
K. Bagga, A. Barchanski, R. Intartaglia, S. Dante, R. Marotta, A. Diaspro, C.L. Sajti, F. Brandi, Laser Phys. Lett. 10, (2013)
H. Chang, S.-Q. Sun, Chin. Phys. B 23, 088102 (2014)
L. Wang, X. Wei, Chaos Solitons Fractals 39, 2211 (2009)
J.F.S. Ramírez, J.L.J. Pérez, R.C. Valdez, A.C. Orea, R.G. Fuentes, J.L. Herrera-Pérez, Int. J. Thermophys. 27, 1181 (2006)
J.A. Eastman, S.U.S. Choi, S. Li, W. Yu, L.J. Thompson, Appl. Phys. Lett. 78, 718 (2001)
S. Kakaç, A. Pramuanjaroenkij, Int. J. Heat Mass Transf. 52, 3187 (2009)
S.K. Das, S.U.S. Choi, H.E. Patel, Heat Transfer Engineering. 27, 3 (2006)
J.F. Sánchez Ramírez, S.F. Arvizu Amador, J.L. Jiménez Pérez, A. Bautista Hernández, R.J. Delgado Macuil, J. Díaz, Reyes, E. Chigo Anota, J. Therm. Anal. Calorim. 120, 1563 (2015)
B.A. Kairdolf, A.M. Smith, T.H. Stokes, M.D. Wang, A.N. Young, S. Nie, Annu. Rev. Anal. Chem. 6, 143 (2013)
M. Gedvilas, S. Indrišiunas, B. Voisiat, E. Stankevičius, A. Selskis, G. Račiukaitis, Phys. Chem. Chem. Phys. 20, 12166 (2018)
M. Potenza, A. Cataldo, G. Bovesecchi, S. Corasaniti, P. Coppa, S. Bellucci, AIP Advances. 7, 075214 (2017)
S.M.S. Murshed, Heat Transf. Eng. 33, 722 (2012)
S.M.S. Murshed, K.C. Leong, C. Yang, Appl. Therm. Eng. 28, 2109 (2008)
X. Zhang, H. Gu, M. Fujii, J. Appl. Phys. 100, 044325 (2006)
E. Shahriari, M.G. Varnamkhasti, R.R. Zamiri, Optik (Stuttg). 126, 2104 (2015)
S.E. Braslavsky, G.E. Helbel, Chem. Rev. 92, 1381 (1992)
A. Santhi, M. Umadevi, V. Ramakrishnan, P. Radhakrishnan, V.P.N. Nampoori, Spectrochim. Acta - Part A Mol. Biomol. Spectrosc. 60, 1077 (2004)
J.P. Gordon, R.C.C. Leite, R.S. Moore, S.P.S. Porto, J.R. Whinnery, J. Appl. Phys. 36, 3 (1965)
S.A. Joseph, M. Hari, S. Mathew, G. Sharma, V.M. Soumya, P. Hadiya, Radhakrishnan, V.P.N. Nampoori, Opt. Commun. 283, 313 (2010)
C. Hu, J.R. Whinnery, Appl. Opt. 12, 72 (1973)
J.H. Brannon, D. Magde, J. Phys. Chem. 82, 705 (1978)
J.R. Whinnery, Acc. Chem. Res. 7, 225 (1974)
Y.C. Kiang, R.W. Lang, Appl. Opt. 22, 1296 (1983)
C.V. Bindhu, S.S. Harilal, V.P.N. Nampoori, C.P.G. Vallabhan, L. Division, Opt. Eng. 37, 2791 (1998)
K. Raznjevic, Handbook of Thermodynamic Tables Charts (Hemisphere Publishing Corp, US, 1976)
S.P. Jang, S.U.S. Choi, Appl. Phys. Lett. 84, 4316 (2004)
J. Petravic, J. Chem. Phys. 123, 174503 (2005)
K.-C. Fang, C.-I. Weng, S.-P. Ju, Nanotechnology 17, 3909 (2006)
Acknowledgements
Authors acknowledges UGC,Govt. Of India for the financial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raj Sha, M.M., Mathew, S., Udayan, S. et al. Ultra-pure silicon nanofluid by laser ablation: thermal diffusivity studies using thermal lens technique. Appl. Phys. B 124, 213 (2018). https://doi.org/10.1007/s00340-018-7081-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00340-018-7081-z