Abstract
Ratios of the 2nd and 1st harmonics at the line center are very sensitive to absorbance, but not to line profiles when the modulation index is set to 0.94. Based on this characteristic, we proposed a method which uses the 1st and 2nd harmonics to measure the absolute absorbance, and then obtain gas pressure and concentration. Some transitions of CO2 and H2O molecules near 6980 cm−1 are selected to verify this method. The satisfactory agreement between measurement results and theoretical values validates the proposed method.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Since the introduction of wavelength modulation spectroscopy (WMS) to tunable diode laser absorption spectroscopy (TDLAS), scientists have proposed several calibration-free methods to monitor gas parameters, and have achieved valuable results [1,2,3,4,5,6,7,8]. In Ref. [8], we presented a method that uses the 1st, 2nd, and 4th harmonics at the line center to measure line width, and then obtain gas pressure and concentration. This method only uses the line center’s harmonics to infer gas parameters, so it can effectively eliminate the effects of laser intensity fluctuations and particles concentration, which are prevalent in industrial fields. Besides the advantage mentioned above, compared with the former “calibration-free 2f/1f method” [9,10,11,12,13], one of the best advantages of this method is that it uses the measured line width rather the calculated one to determine gas pressure and concentration, so the changes in gas parameters, such as partial and total pressures are easily accounted for in actual measurements. However, Ref. [8] used the second-order Taylor series to approximate the laser transmission. Thus, the derived expression has a high accuracy only when the absorbance is less than 30%, and measurement errors will increase sharply with the increase of absorbance [14]. For example, simulation results show that the relative errors of gas partial pressure reach to 6.5% when the absorbance is 50%. Moreover, for beginners or practitioners, the implementation processes of this method are relatively complicated; especially the computational formulas of the Fourier components and partial pressure are very complex.
Considering the complex data analysis and a narrow scope of application of the above method, in this paper, we found that the ratios of the 2nd and 1st harmonics (R 21) at the line center are very sensitive to absorbance, but not to line profiles when the modulation index is set to 0.94. Inspired by this feature and the technique of direct absorption spectroscopy (DAS) data processing [15, 16], we put forward a simpler method to measure gas pressure and concentration using the 1st, 2nd, and 4th harmonics at the line center. Based on Refs. [7, 8], the proposed method uses the ratios of the 2nd and 4th harmonics (R 24) to determine line width, and then, the modulation index corresponding to each modulation depth is calculated according to the measured line width. However, in succeeding measurements, the proposed method employs the ratio of R 21 at a special modulation index (m = 0.94) to infer absorbance, rather than calculating gas partial pressure using the computational “calibration-free 2f/1f” formulas. Once the line width and absorbance are measured, gas pressure and concentration can be easily determined, similar to DAS data analysis. This method offers all the advantages of WMS (high frequency modulation produces smaller laser and 1/f noises, as well as reduces the influences of infrared radiation and other noises) and DAS (completely calibration-free, simple and accurate data analysis, changes in gas parameters can be accounted for in practical measurements). Compared with Ref. [8], this method is much simpler and more likely to be mastered, and the application scope of the absorbance increased to 50% or even more. To verify its reliability and accuracy, some transitions of CO2 and H2O near 6980 cm−1 are selected to measure gas pressure and concentration in laboratory and industrial fields.
2 Measurement principles
At the line center, the ratios of the 2nd and 1st harmonics can be written as [6, 8]:
where i 0 is the linear intensity modulation amplitude, ψ 1 is the phase shift between the intensity and frequency modulation, i 0 and ψ 1 are the characteristics of the laser, Z = i 0 R 21cosψ 1. A 0 and A 2 are the harmonic coefficients of spectral absorbance and can be written as follows:
where α(ν 0) = PXS(T)Lφ(ν 0) is the absorbance at the line center. ν 0 (cm−1) is the center frequency of transition, P (atm) is the total pressure, X is the gas concentration, S(T) (cm−2 atm−1) is the line strength, L (cm) is the absorption path length, and φ(ν 0) (cm−1) is the line shape at the line center. Here, line shape is described by Voigt profile [17]. And a is the frequency modulation depth.
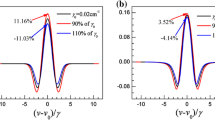
Figure 1 shows the simulation results of Z with different absorbance and line profiles when ψ 1 = 36°, where the Z values monotonously increase and intersect at a fixed point with various line profiles under a certain absorbance. Here, all the line profiles are assumed to be Voigt profiles and the dimensionless parameter d {d = (δv L − δv D)/(δv L + δv D) [17]} ranges from −1 to 1 with a step of 0.2. With the increase of absorbance, the ordinates of the fixed points increase sharply, while the abscissas (m) remain basically unchanged. Calculation results indicate that the abscissas of these fixed points are within the range of 0.94 ± 0.005 when the absorbance is less than 50%. According to this characteristic, when the modulation index is set to 0.94, the values of Z will depend mainly on absorbance, regardless of the line profiles.
To obtain more precise relationship between Z and α(ν 0), Fig. 2 plots Z with different α(ν 0) and line profiles when m = 0.94. As can be seen, the values of Z increase with the increase of absorbance, and are not significantly affected by line profiles. Using the simulation data, the relationships between Z and α(ν 0) can be expressed by the following:
However, as shown in the partial view, the line profiles have some influences on the Z values. For example, if the measured Z is 0.091, the predicted absorbance will remain within the range of 41.996–42.045% with different line profiles. The bottom graph presents the measurement errors with different line profiles when Eq. (3) is used to determine absorbance, where the maximum relative error of absorbance is less than 0.30% so that it can be ignored in practical measurements.
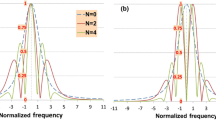
Theoretically, the 2nd harmonic reaches its maximum value for all line profile when m is 2.2. The high sensitivity and signal-to-noise ratio (SNR) are the reasons why m ~ 2.2 is widely used to monitor gas parameters [18, 19, 20]. Figure 3 shows the comparison of the 2nd harmonic signals for m = 0.94 and 2.2. It is clear that the peak value of the 2nd harmonic signals for m = 0.94 is more than half (0.57) of that with m = 2.2. This reflects that the sensitivity and SNR when m = 0.94 is still very high. More importantly, the shape of the harmonic signal depends highly on the modulation index and it becomes narrower when the modulation index decreases. This is clear, as shown in Fig. 3, that the amplitude of B 1 (4 × γ) is about 8.63% of its peak value when m = 2.2, while it is only 1.37% for B 2 when m = 0.94. This indicates that the interference of the adjacent line on the harmonic signal is diminished when modulation index m reduces to 0.94. This is a compensation of the reduction in peak value for the SNR when m = 0.94 compared with 2.2. That is to say, when the modulation index is set to 0.94, the 2nd harmonic also has a high SNR, and the interference between adjacent lines can be effectively reduced.
Based on the above analysis, we know that the accurate measurement of line width and absorbance is a prerequisite for measuring gas pressure and concentration. Based on Ref. [8], the line width is determined by the ratios of R 24 at the line center. In this paper, the application scope of absorbance is extended to 50%. Figure 4 is the simulation results of R 24 values with different absorbance and line profiles, where the abscissas (m) and ordinates (R 24) of these fixed points increase as the absorbance increases, and the relationships between the fixed points and absorbance can be written as:
According to Fig. 4, the approximate point O 1 (2.504, 2.300) can firstly be used to estimate the line width and line shape, then, the ratios of R 21 at m = 0.94 are employed to infer gas absorbance. Finally, the partial pressure, P i = PX [atm], can be calculated based on the principle of DAS [21, 22]:
Once the partial pressure is determined, the total pressure and concentration can be obtained through the data processing similar as Ref. [8], the detail is also presented in Sect. 3.
3 Laboratory experiments
The transition of CO2 molecule at 6982.0678 cm−1 is selected to assess the proposed method in laboratory conditions. The spectroscopy constants and experimental setup are the same as Ref. [8] (Table 1 and Fig. 4). A high frequency sinusoidal modulation signal (as shown in Fig. 5a) is added on the DC injection current to produce harmonics. The modulation current expression can be written as follows:
where T (s) is the amplitude modulation cycle (low frequency), x is the modulation level, x∈[0, 1], η 0 is the modulation current at T/4, and f (Hz) is the sinusoidal modulation frequency (high frequency).
A typical experiment is used to illustrate the measurement processes of the proposed method. Before the experiment, the gas cell is evacuated by a vacuum pump to an ultimate pressure of 1.0 Pa, and then filled with a CO2/N2 mixture controlled by two mass flow controllers. The CO2 partial and total pressures are 7.64 ± 0.20 and 29.91 ± 0.10 kPa, respectively, and the gas temperature and absorbing path length are 296.0 ± 0.2 K and 120 cm, respectively. In addition, modulation parameters such as T, x, f, and η 0 are 0.2 s, 0.7, 2500 Hz, and 3.75 mA, respectively.
Figure 5a gives the modulation current in a cycle, where the modulation frequency is reduced to 50 Hz to show the sinusoidal signals. Once the modulation coefficient (ε = 1.18 × 10−2 cm−1 mA−1) and current are known, the frequency modulation depth a can be obtained as shown in Fig. 5b. Meanwhile, Fig. 5c indicates the laser intensities, where the black and blue curves represent the incident (no absorption) and transmitted (with absorption) intensities, respectively. According to the incident intensity, the linear intensity modulation amplitude i 0 can be determined, and the calibration results of i 0cosψ 1 are given in Fig. 5d, where ψ 1 = 36°.
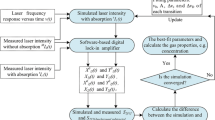
When the frequency modulation depth and linear intensity modulation amplitude are determined, the following processes can be used to measure the line width and absorbance. Detailed measurement processes are provided in Fig. 6.
For a start, the optical power exiting from the gas cell is detected by a Ge photodiode (PDA 50B-EC). Meanwhile, the detected signals are recorded in a high-speed memory data acquisition card, and then are demodulated by digital lock-in software (LabVIEW). Figure 7a shows the amplitudes of the 1st, 2nd, and 4th harmonics at the line center with different modulation currents, where the amplitude of the 2nd harmonic increases first and then decreases as the modulation currents increase. Using the experimental data in graph, the values of R 21 = S 2f /S 1f and R 24 = S 2f /S 4f are provided in Fig. 7b.
According to the relationships between scan time and frequency modulation depth (Fig. 6b), Fig. 8 gives the values of Z and R 24 with different modulation depths, where the average of 10 sequential raw data scans (see Fig. 7b) are used to improve the SNR. The modulation depth at the approximate fixed point O 1 (2.504, 2.300) can be easily determined using the R 24 curve. Once the fixed point modulation depth is known \((a_{1}^{*}\) = 6.365 × 10−2 cm−1), the line width is computed as: \(\gamma_{1}=a_{1}^{*}/m_{1}^{*}\) = 2.542 × 10−2 cm−1. The m corresponding to each modulation depth is then calculated according to the measured line width, and the calculated results are given in the bottom abscissa. When m is known, the value of Z is determined as 0.03576 when m = 0.94. Submitting the measured Z into Eq. (3), the α(ν 0) is obtained as 15.99%.
Since the approximate fixed point (2.504, 2.300) employed to infer line width will bring in certain measurement errors. The following iteration method is used to obtain the optimal values of line width and absorbance. Figure 9 gives the detailed iteration processes. First, the line width and absorbance are measured according to the approximate fixed point (O 1), as mentioned above. Next, submitting the measured absorbance into Eq. (4), the coordinates of the new fixed point (O 2) are calculated as: \((m_{2}^{*}\) = 2.4995 and \((R_{24-2}^{*}\) = 2.2484. Similar to the above analysis, the modulation depth at the new fixed point can be inferred as \((a_{2}^{*}\) = 6.535 × 10−2 cm−1, and the new line width and absorbance are then computed as: \(\gamma_{2}=a_{2}^{*}/m_{2}^{*}\) = 2.614 × 10−2 cm−1 and 16.41% (Z 2 = 0.03771), respectively. These procedures are performed until the absorbance attains convergence and stability. For example, when carrying out the next iteration, the measurement values of line width and absorbance are 2.612 × 10−2 cm−1, and 16.40%, respectively. Obviously, the relative error between the last twice measured absorbance is only 0.06%, and the next iteration is unnecessary. In general, only two iterations are needed to obtain accurate results in practical measurements.
Once the line width and absorbance are determined, the CO2 partial pressure, total pressure, and volume concentration can be inferred as: 7.53 kPa, 29.71 kPa, and 25.35%, respectively. To assess the precision of the proposed method, a long time measurement results are present in Fig. 10, where gas temperature (296 K), absorption length (120 cm), CO2 partial and total pressures (set values: 7.64 and 29.91 kPa) are the same as these in Figs. 7, 8 and 9. According to the above parameters, the theoretical values of line width, absorbance, and volume concentration are calculated as: 2.631 × 10−2 cm−1, 16.51, and 25.55%, respectively.
Using the experimental data, measured values, such as averages and standard deviations (σ) of these gas parameters are presented in the graphs. As shown in Fig. 10, the parameters are fluctuant at the beginning and then stabilized because the diode laser is not stable during the early operation period. The results from experiments indicate that the proposed method can synchronously measure the line width, absorbance, partial and total pressures, and has high accuracy and sensitivity. This method only uses the harmonic signals at the line center to measure gas parameters, so it can eliminate the effects of particles concentration, laser intensity fluctuations, and low frequency noises in actual measurements.
In the following experiments, we investigated the efficacy of the proposed method in dealing with other conditions. Figure 11a, b gives the results under different absorbance (about 5.4 and 26.5%, respectively), where gas temperature (296 K) and absorption length (120 cm) remain unchanged, while the set values of CO2 partial and total pressures are different. The theoretical values of line width, absorbance, and volume concentration are calculated according to above parameters, and the values are shown in bottom of graphs. The measurement results coincide well with the set values, and the relative errors are less than 2.0%, which ensures the accuracy of the proposed method.
Measurement results of the line width, absorbance, CO2 partial pressure, total pressure, and volume concentration under different conditions: (a1)–(e1) set values: γ = 2.576 × 10−2 cm−1, α(v 0) = 5.42%, P i = 2.45 kPa, P = 30.93 kPa, X = 7.93%; (a2)–(e2) set values: γ = 2.732 × 10−2 cm−1, α(v 0) = 26.52%, P i = 12.75 kPa, P = 29.55 kPa, X = 43.21%
Above experiments are carried out under conditions where gas parameters are unchanged. Nevertheless, in industrial fields, gas parameters, especially the partial and total pressures vary with time. For this reason, we simulated industrial environments by changing total gas pressures. The measurement results are presented in Fig. 12, where the red and black numbers represent the theoretical and measured values of gas parameters, respectively. Initially, the gas cell is filled with CO2/N2 mixture, and CO2 partial and total pressures are set to 4.88 and 22.50 kPa, respectively. During the stage of A, the gas parameters are unchanged, and the experiment results agree well with theoretical values. In subsequent experiments, the gas parameters are changed by exhausting or filling with N2. Meanwhile, the total gas pressure is recorded by a pressure sensor, and line width, absorbance, CO2 partial pressure, and volume concentration are computed according to the changes of total gas pressure. As shown in Fig. 12, gas parameters in the stages of A, C, E, G, I, K, and M are stable, but vary in the B, D, F, H, J, and L stages due to the changes of total gas pressure. For example, during the exhaust stages (B, D, and F), the measured line width, absorbance, and CO2 partial pressure reduce with the decrease of total pressure, while the volume concentration is basically unchanged. These findings are identical with those from the theoretical analysis. Similarly, when the gas cell is filled with N2 (H stage), the measured line width, absorbance, total pressure, and concentration change dramatically, while CO2 partial pressure stays unchanged. The measurement results of the J and L stages also have similar characteristics. Accordingly, we can conclude that during the changing condition, although the changing parameters are not compared with the theoretical values, the perfect coincidence of the unchanged parameter with its theoretical value reflects the validity of the proposed algorithm.
4 Field measurements
In this section, to verify the proposed method for measuring gas parameters in industrial fields, the proposed method is employed to measure water vapor concentration simultaneously with Ref. [8] in exhaust gases in a coal-fired power plant using the transition of H2O at 6979.2995 cm−1. Figure 13 shows the measurement results under half-load working condition (approximately 130 WM), where the measuring conditions are the same as those in Fig. 12 in Ref. [8] (total pressure is maintained at 38 ± 4 kPa). The blue line represents the proposed method in this paper. The load is about 135 MW (coal consumption and primary air are set as 56 and 140 t/h, respectively) at the first hour, and then decreases to 128 MW (coal consumption is reduced to 54 t/h while primary air remains the same). The measurement results of both methods are with a high degree of consistency. The measured vapor concentrations are about 6.0 and 5.7% when the loads are set to 135 and 128 MW, respectively, which are in good accordance with the design value. According to the above analysis, this method can be used to measure gas pressure and concentration in harsh environments, and has the same precision and sensitivity in field measurements when compared with the method in [8].
5 Conclusions
Taking into account the shortcoming (i.e., very complex calculation processes, a narrow scope of application of absorbance) of the method proposed in Ref. [8], based on the DAS data analysis technique, this paper proposed a new method that employs the 1st and 2nd harmonics at the line center to measure the absolute absorbance. According to the R 24 theory, this method also uses the ratio of 2nd and 4th harmonics to infer the line width, and then obtain the absorbance according to the R 21 value at m = 0.94. Subsequently, the gas concentration, partial and total pressures are determined. To assess the reliability and accuracy of the proposed method, the transition of CO2 at 6982.0678 cm−1 is selected to measure gas parameters under laboratory conditions. The satisfactory agreement between measurement results and theoretical values indicates the validity of the proposed method.
The application of the proposed method in industrial fields is also discussed in this paper. To compare the proposed method with the method in Ref. [8], the transition of H2O at 6979.2995 cm−1 is selected to measure the vapor concentration in exhaust gas in a coal-fired power plant. Although the measuring principles of both methods are different, their measurement results with a high degree of consistency. The advantages of the method proposed in the current paper are simpler data processing and wider range of applications (the scope of the absorbance reach to 50% or even more). However, due to the background signals problem, the minimum absorbance of the proposed method in this paper is also 0.1%. In addition, it also must be mentioned that the proposed method also has practical limitations. To avoid the effect of the adjacent line on the harmonic signal, the proposed method is only valid for the isolated absorption line or when the distance between adjacent lines is more than five times larger than the line widths of the selected line.
References
K. Duffin, A.J. McGettrick, W. Johnstone, G. Stewart, D.G. Moodie, Tunable diode laser spectroscopy with wavelength modulation: a calibration-free approach to the recovery of absolute gas absorption line-shapes. J. Lightw. Technol. 25(10), 3114–3125 (2007)
G. Stewart, W. Johnstone, J.R.P. Bain, K. Ruxton, K. Duffin, Recovery of absolute gas absorption line shapes using tunable diode laser spectroscopy with wavelength modulation—part 1: theoretical analysis. J. Lightw. Technol. 29(6), 811–821 (2011)
A. Hangauer, J. Chen, R. Strzoda, M.C. Amann, Multi-harmonic detection in wavelength modulation spectroscopy systems. Appl. Phys. B 110(2), 177–185 (2013)
A.L. Chakraborty, K. Ruxtona, W. Johnstone, Influence of the wavelength-dependence of fiber couplers on the background signal in wavelength modulation spectroscopy with RAM-nulling. Opt. Express 18(1), 267–280 (2010)
K. Ruxtona, A.L. Chakraborty, W. Johnstone, M. Lengden, G. Stewart, K. Duffin, Tunable diode laser spectroscopy with wavelength modulation: elimination of residual amplitude modulation in a phasor decomposition approach. Sens. Actuators, B 150(1), 367–375 (2010)
Z.M. Peng, Y.J. Ding, L. Che, Q.S. Yang, Odd harmonics with wavelength modulation spectroscopy for recovering gas absorbance shape. Opt. Express 20(11), 11976–11985 (2012)
L.J. Lan, Y.J. Ding, Z.M. Peng, Y.J. Du, Y.F. Liu, Z. Li, Multi-harmonic measurements of line shape under low absorption conditions. Appl. Phys. B 117(2), 543–547 (2014)
L.J. Lan, Y.J. Ding, Z.M. Peng, Y.J. Du, Y.F. Liu, Calibration-free wavelength modulation for gas sensing in tunable diode laser absorption spectroscopy. Appl. Phys. B 117(4), 1211–1219 (2014)
H. Li, G.B. Rieker, X. Liu, J.B. Jeffries, R.K. Hanson, Extension of wavelength modulation spectroscopy to large modulation depth for diode laser absorption measurements in high-pressure gases. Appl. Opt. 45(5), 1052–1061 (2006)
H. Li, S.D. Wehe, K.R. McManus, Real-time equivalence ratio measurements in gas turbine combustors with a near-infrared diode laser sensor. Proc. Combust. Inst. 33(1), 717–724 (2011)
A. Farooq, J.B. Jeffries, R.K. Hanson, Sensitive detection of temperature behind reflected shock waves using wavelength modulation spectroscopy of CO2 near 2.7 μm. Appl. Phys. B 96(1), 161–173 (2009)
G.B. Rieker, J.B. Jeffries, R.K. Hanson, Calibration-free wavelength modulation spectroscopy for measurements of gas temperature and concentration in harsh environments. Appl. Opt. 48(29), 5546–5560 (2009)
K. Sun, X. Chao, R. Sur, J.B. Jeffries, R.K. Hanson, Wavelength modulation diode laser absorption spectroscopy for high-pressure gas sensing. Appl. Phys. B 110(4), 497–508 (2013)
Z.M. Peng, Y.J. Ding, L. Che, X.H. Li, K.J. Zheng, Calibration-free wavelength modulated TDLAS under high absorbance conditions. Opt. Express 19(23), 23104–23110 (2011)
S. Hunsmann, K. Wunderle, S. Wagner, U. Rascher, U. Schurr, V. Ebert, Absolute, high resolution water transpiration rate measurements on single plant leaves via tunable diode laser absorption spectroscopy (TDLAS) at 1.37 μm. Appl. Phys. B 92(3), 393–401 (2008)
F. Li, X.L. Yu, H.B. Gu, Z. Li, Y. Zhao, L. Ma, L.H. Chen, X.Y. Zhang, Simultaneous measurements of multiple flow parameters for scramjet characterization using tunable diode-laser sensors. Appl. Opt. 50(36), 6697–6707 (2011)
Y.Y. Liu, J.L. Lin, G.M. Huang, Y.Q. Guo, C.X. Duan, Simple empirical analytical approximation to the Voigt profile. J. Opt. Soc. Am. B. Opt. Phys. 18(5), 666–672 (2001)
J.A. Silver, D.J. Kane, Diode laser measurements of concentration and temperature in microgravity combustion. Meas. Sci. Technol. 10(10), 845–852 (1999)
J.T.C. Liu, J.B. Jeffries, R.K. Hanson, Wavelength modulation absorption spectroscopy with 2f detection using multiplexed diode lasers for rapid temperature measurements in gaseous flows. Appl. Phys. B 78(3–4), 503–511 (2004)
F. Wang, K.F. Cen, N. Li, Q.X. Huang, X. Chao, J.H. Yan, Y. Chi, Simultaneous measurement on gas concentration and particle mass concentration by tunable diode laser. Flow Meas. Instrum. 21(3), 382–387 (2010)
Y. Li, L. Chang, Y. Zhao, X. Meng, Y. Wei, X. Wang, J. Yue, T. Liu, A fiber optic methane sensor based on wavelength adaptive vertical cavity surface emitting laser without thermoelectric cooler. Measurement 79, 211–215 (2016)
Y.J. Ding, X.H. Li, Z.M. Peng, L. Che, Half-width integral method for gas concentration measuring in tunable diode laser absorption spectroscopy. Spectrosc. Lett. 46(7), 465–471 (2013)
Acknowledgements
This work was supported by the National Natural Science Foundation of China under Grant No. 51676105.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Du, Y., Lan, L., Ding, Y. et al. Measurement of the absolute absorbance based on wavelength modulation spectroscopy. Appl. Phys. B 123, 205 (2017). https://doi.org/10.1007/s00340-017-6780-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00340-017-6780-1