Abstract
Laser-induced breakdown spectroscopy (LIBS) is a technique increasingly used to perform fast semi-quantitative multi-elemental analyses of various materials without any complex sample preparation, being also suitable for in situ analyses. Few studies have been performed to understand the influence of laser wavelength on LIBS analytical performance on environmental samples. The main goal of this study was to perform a comparative elemental analysis of a number of soils, citrus leaves, and synthetic solid matrices using two different wavelengths, i.e., 532 and 1064 nm of Nd:YAG lasers, and a spectrometer coupled to a non-intensified charge-coupled device camera as the detection system. The emission lines with higher upper energy level, i.e., C I—193.03 (7.685 eV) and Si I—212.41 nm (6.616 eV), were more intense when using the 532 nm than the 1064 nm laser light, whereas the opposite occurred for elements with lower upper energy level, i.e., Ti I—336.12 nm (3.716 eV) and Fe I—368.75 nm (4.220 eV). The observed increase in LIBS signal between the two wavelengths is about 30–50%. The relationship between the line emission intensities and the used excitation wavelengths were associated to the upper level energy of the element.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Laser-induced breakdown spectroscopy (LIBS) is an elemental analytical technique considered to represent a promising tool for low-cost analyses in situ. Additional advantages offered by this technique are minimal sample preparation, quasi non-destructive and fast measurement, and no generation of chemical residues. LIBS has been successfully applied for elemental analysis of various materials, including metals [1–3], soils [4–9], plant leaves [10–12], and others. However, several limitations still exist in the analytical use of LIBS, including relatively low repeatability, strong matrix dependence, and low sensibility and accuracy. Many of these problems are related to plasma formation that occurs in different modes when using different wavelengths.
The efficiency of a particular wavelength to couple energy into the sample analyzed depends on various effects acting on the resulting LIBS emissions. Although these effects have been studied by some authors [13–18] in analyzing metals such as aluminum, steel, and various liquids and transparent materials, very limited studies exist on the influence of LIBS wavelength on the elemental analysis of environmental materials. In particular, soil and plant samples have been analyzed by LIBS mostly using one excitation wavelength [4–12], although two excitation wavelengths have been used in one study [19]. A suitable choice of excitation wavelengths and other analytical parameters, such as sample homogeneity, light collection, and accumulated pulses [13], is expected to improve the sensitivity of the technique. When evaluating these parameters, the laser–matter interaction mechanisms, such as light absorption, laser ablation, and plasma initiation and formation, also need to be taken into due account [20]. Further, the use of double-pulse (DP) LIBS setups in either collinear or orthogonal configurations have been proved to improve the efficiency of LIBS by enhancing the signals [21–23].
In particular, Fornarini et al. [24] investigated theoretically and experimentally the influence of two laser wavelengths, i.e., 1064 and 355 nm, on the analytical performance of LIBS in the diagnosis of bronze alloys. These authors found that at the wavelength of 1064 nm the model predicted a slower rise of sample surface temperature due to intense plasma absorption. In particular, a relevant plasma shielding effect was also observed with less energy reaching the sample surface. As a consequence, the spectrum obtained at 1064 nm was more intense than that obtained at 355 nm. Further, this study showed that ionic lines of heavy elements were present predominantly in the near-ultraviolet (UV) region (355 nm), whereas atomic emissions from light elements appeared prevalently in the near- infrared (IR) region (1064 nm). Differently, at the wavelength of 355 nm and using shorter laser pulse durations a reduced plasma shielding and a higher intensity in the near-UV region occurred.
Barnett et al. [13] found that, although at the wavelength of 266 nm a greater mass removal occurred, at the wavelength of 532 nm emission intensities were obtained from 6 to 13 times higher than those at 266 nm. The authors concluded that the 266 nm wavelength was preferable for LIBS analysis of the elements Al, Ba, and Sr in transparent glass samples due to higher precision, low laser fluence, and better laser–target coupling.
In comparing the characteristics of plasmas produced by IR and UV laser wavelengths, Sdorra et al. [25] found that the LIBS emission signals of Al and Mn obtained using the UV radiation were not linearly correlated with the analyte concentrations, even after comparing the LIBS signals with reference lines. The authors ascribed this result to the incomplete atomization of ablated material.
In comparing the fundamental (1064 nm) neodymium-doped yttrium aluminum garnet (Nd:YAG) laser with the UV third harmonic for the detection of Ni in water, Berman and Wolf [26] observed that the UV light generated a lower continuum intensity leading to a better signal-to-noise ratio. The analytical efficiency of the LIBS technique was confirmed by the high discrimination power of both visible and IR LIBS observed in analyzing black gel inks [27].
The studies mentioned above investigated the influence of the wavelength using high-resolution spectrometers. However, the increasing demand of applications of in situ techniques requires an increased efficiency of LIBS analytical performance when using non-intensified spectrometers, which are commonly used in portable LIBS systems due to their low cost and small size.
In this context, the objective of this study was to perform a comparative elemental analytical LIBS analysis using a system consisting of a spectrometer coupled to a non-intensified charge-coupled device (CCD) camera using two Nd:YAG lasers operating at two different wavelengths, i.e., 532 nm and 1064 nm, applied to soils, citrus leaves, and various synthetic solid matrices. In particular, the dependence of the optimal laser wavelength on the upper energy level of the emission lines of interest was investigated.
2 Experimental
2.1 Samples
Twelve soil samples were collected in duplicate at different depths under eight native pastures and four forests in the fields around the Southeast Livestock Research Center of Embrapa located near the city of São Carlos, São Paulo State, Brazil. Six different citrus leaf samples were collected at Fazenda da Toca located at Itirapina in São Paulo State.
Plant roots were removed from soil samples that successively were dried, ground, and sieved to obtain particles smaller than 0.15 mm, whereas citrus leaves were dried and milled manually to obtain homogenized powder. A 8-ton press was used for 30 s to obtain one pellet for each soil and leaf sample.
Pellets of different synthetic solid matrices, including calcium sulfite (CaSO3), chromium (III) oxide (Cr2O3), and manganese dioxide (MnO2), each containing 30% of boric acid (H3BO3), were also prepared and tested.
2.2 Instrumentation
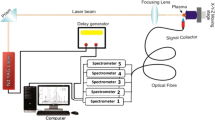
The LIBS spectra were acquired using two different laser systems, the first one was a Q-switched pulsed Nd:YAG laser (Quantel—Ultra 50) operating at the wavelength of 1064 nm (IR), and the second one a Nd:YAG laser (Quantel—Brilliant) operating at the wavelength of 532 nm (VIS). The two laser systems had a pulse width of about 6 ns, a repetition rate fixed at 1 Hz, and a similar power density on the focal spot.
The beam spot size before the focal lens for each laser was 2.8 mm (VIS laser) and 5.32 mm (IR laser); both were determined by knife edge method [28]. Using an ABCD method for Gaussian beam propagation, the beam waist estimated in the focal region (w0) was approximately 12.0 µm (for VIS laser) and 12.7 µm (for IR laser). The pulse energy close to the focal lens was approximately 40 mJ (for VIS laser) and 50 mJ (for IR laser), both measured using an energy meter (Coherent, FieldMax II), and the power density on the focal position was estimated for both. In this optical setup, the power densities of the two beams were approximately 1.7 ± 0.1 × 1012 Wcm−2 and they were used for the acquisition of LIBS spectra. The beams were focused and aligned by dichroic mirrors to hit the target sample in the overlapping mode. For an efficient collection of emitted plasma, a silica lens was placed between the sample and the optical fiber tip. The sample holder was placed in a micro-controlled XY stage for speed scanning of the laser beam impinging on it.
A spectrometer coupled to a non-intensified CCD ranging from 188 to 400 nm with an optical resolution of 0.4 nm (Stellar Net Inc, model EPP2000-HR) was also used for all measurements. The delay time between the laser shot and the spectrum acquisition was set at 2 μs with an integration time of 1 ms. The emission lines considered were rigorously checked by measuring each sample with an optical resolution of 0.1 nm (Ocean Optics, model LIBS2500). Fifteen measurements with a single shot of each laser system were performed on each pellet. In order to correct the background near the emission line considered, the selected spectral range was subtracted by the average of five pixels with no transition and close to the element emission line [29]. After the background correction, the signal/noise relation was improved by averaging the 15 spectra. The intensity of the emission lines in the LIBS spectra was determined and evaluated comparatively for the two laser systems used. All specific peak transitions were normalized by the highest value of the 30 spectra acquired (15 for each laser wavelength).
The images of the craters generated by the two laser systems on soil pellets were analyzed by scanning electron microscopy (SEM) (JSM-6510/JEOL, Thermo Scientific). The diameter and depth of the craters were measured on x–y–z axes from the image obtained by a confocal microscope (LSN 780, Zeiss) coupled with a micrometer. To estimate the volumes of the craters, they were approximated to cylinders. The ablated mass was estimated on the bases of the ablated volume and sample density [30].
2.3 Known features of laser-induced plasma
A phenomenon known as bremsstrahlung effect commonly occurs during light absorption and plasma ignition. It consists in the deceleration of electrons in the ionic Coulomb field with consequent emission of photons. However, in the presence of an external laser field (e.g., the LIBS laser pulse), electrons absorb photons continuously from this field. On average, absorption exceeds emission and the plasma is heated by electron–ion collisions, yielding the so-called inverse bremsstrahlung (IB) [31], which depends on the laser wavelength and is proportional to λ 3 [32, 33].
Another important effect that influences plasma heating is photon ionization (PI) or multiphoton ionization (MPI) [32], i.e., during the interaction of laser radiation with matter, the ions produced can absorb photons and be photoionized. This process increases with higher photon energy, i.e., it is inversely proportional to the laser wavelength.
The combination of these two processes determines the physical properties of plasma dynamics and, consequently, influences the atomic emission lines. Thus, the PI and MPI effects become greater at the VIS (532 nm) wavelength, whereas the IB was greater when using the IR (1064 nm) wavelength [1, 32, 33].
3 Results and discussion
Figure 1 shows the emission spectra acquired at the excitation wavelengths of 532 nm (gray) and 1064 nm (black) of a soil sample, in a spectral range of 188–400 nm. In particular, Fig. 1 shows the spectral window (inset) where it is possible to observe that the Fe I—368.75 nm (4.220 eV) emission line was more intense when using the 1064 nm than the 532 nm laser light. The spectra of soils and leaves showed clearly that each peak intensity depended on the laser excitation wavelength used. As an example, Fig. 2 shows the average intensity of the emission lines of C I (193.03 nm) (a), Fe I (368.60 nm) (b), Al II (198.99 nm) (c), and Ti II (336.12 nm) (d) obtained using a laser wavelength of either 532 nm or 1064 nm for the soil samples examined. Apparently, the wavelength of 532 nm was more efficient for the C I and Al II lines, whereas the wavelength of 1064 nm was more efficient for the Fe I and Ti II lines. As a result, by choosing the proper excitation wavelength the signal intensity can be increased up to 80% in analyzing soil and leaf samples.
In order to find the most appropriate wavelength for each transition, after correction of the electronic spectral background, the mean ratios between LIBS signal intensity measured at the wavelength of either 532 or 1064 nm were calculated by dividing point by point the two spectra, and plotted as a function of wavelength (Fig. 3). Ratios >0 indicated that the 532-nm laser provided a higher emission line intensity, whereas ratios <0 indicated that the 1064-nm laser performed better. Values of the ratio <5% were disregarded. This procedure thus allowed to find the optimal (most sensitive) laser wavelength for each emission line, i.e., element, of the spectrum.
A similar analysis was performed for the elements Al, Ca, Cr, Fe, Mg, Mn, S, Si, and Ti and the corresponding results are summarized in Table 1. All lines were rigorously checked by measuring each sample using a high-spatial resolution spectrometer (Δλ = 0.1 nm). The last column in Table 1 lists the most efficient laser excitation wavelength, either 532 or 1064 nm, for each element, based on the average intensity increase of the corresponding emission lines. A paired statistical t test was performed, which yielded a statistically significant difference between line intensities at either 532 or 1064 nm for all samples.
The average plasma temperature calculated by the Boltzmann plot for Fe lines was 8500 ± 2000 K for all samples examined, with no statistically significant difference among the samples. In this way, it is not possible to ascribe the differences in emission intensities to the temperature differences. This result could be expected due to the spectrometer temporal and spectral limitations, i.e., non-intensified CCD and low spectral resolution typical of most portable low-cost LIBS equipments for in situ measurements. Therefore, the sensitivity of the technique can be ascribed to the laser excitation wavelength used, and it is related to the upper energy level of the emission lines. In particular, the results of this study showed that LIBS signal is more sensitive for detecting emission lines of elements with higher upper energy level at VIS wavelength (532 nm) and with lower upper energy level at IR wavelength (1064 nm).
A tentative interpretation of the results described above can be based on two basic effects of laser ablation, i.e., the bremsstrahlung and MPI effects. For the detection of higher upper energy level emission lines, the MPI process [34] must be favored because of higher sensitivity at the smallest wavelength, i.e., 532 nm. Furthermore, the excitation wavelength at 532 nm has a more energetic photon plasma excitation than the wavelength at 1064 nm, in order to reach higher energy levels through resonance absorption. On the other hand, for the detection of lower energy level emission lines the bremsstrahlung effect would be dominant, which features higher line intensities at higher wavelengths, i.e., 1064 nm.
The SEM images of the soil surface after impinging with IR (a) and VIS (b) laser radiation are shown in Fig. 4. The ablated mass per laser shot calculated when using the IR laser (20 ± 4 μg) was greater than that obtained by the VIS laser (0.7 ± 0.1 μg). The IR laser thus produces an intense ablation in forming the crater and is able to excite lower energy level emission lines more efficiently. However, even if atomization is incomplete, the VIS laser appears efficient and selective for “higher upper energy level” emission lines.
4 Conclusion
The preliminary results obtained by the comparative elemental analysis of a number of soil, leaf, and synthetic samples performed using the Nd:YAG pulsed lasers operating at the excitation wavelength of either 532 or 1064 nm suggested that LIBS spectral line intensities depend on the laser wavelength used as a function of the upper energy level of the emission lines analyzed. In particular, elements of lower upper energy level showed a higher sensitivity to the IR laser, whereas elements of higher upper energy level were better detected using the VIS laser.
The obtained results also showed that the LIBS excitation wavelength could be optimized using a non-intensified CCD detection system not provided with an electronic shutter or an intensified detector for time control acquisition. Thus, although no time-resolved spectroscopy was used, which would have allowed to select the time window and obtain a more stable plasma, the present approach allowed to obtain an increased signal intensity (up to 80%) for the samples examined. In conclusion, the choice of the optimal wavelength for any specific element can help optimize the construction of portable LIBS systems valuable for environmental applications on site. Further work will be conducted using a tunable laser source (OPO) for more wavelength capability to better explain the wavelength-dependent LIBS signal, but for now the important aspect is that it has been observed for the first time.
References
S. Awasthi, P. Kumar, G.K. Rai, A.K. Rai, Opt. Las. Eng. 79, 29–38 (2016)
A.N. Kadachi, M.A. Al-Eshaikh, Spectrosc. Lett. 48, 403–410 (2015)
S. Siano, J. Agresti, Stud. Conserv. 60, sup1, S106–S119 (2015)
E.C. Ferreira, D.M.B.P. Milori, E.J. Ferreira, L.M. dos Santos, L. Martin-Neto, A.R. de Araújo Nogueira, Talanta 85, 435–440 (2012)
G. Nicolodelli et al., Appl. Optics 53, 2170–2176 (2014)
A. Segnini. et al, Am. J. Anal. Chem 5, 722–729 (2014)
E.C. Ferreira, E.J. Ferreira, P.R. Villas-Boas, G.S. Senesi, C.M. Carvalho, R.A. Romano, L. Martin-Neto, D.M.B.P. Milori, Spectrochim. Acta Part B 99, 76–81 (2014)
G.S. Senesi, M. Dell’Aglio, R. Gaudiuso, A. de Giacomo, C. Zaccone, O. DePascale, T.M. Miano, M. Capitelli, Environ. Res. 109, 413–420 (2009)
S. Pandhija, N.K. Rai, A.K. Pathak, A.K. Rai, A.K. Choudhary, Spectrosc. Lett. 47, 579–589 (2014)
M. Pouzar, T. Cernohorsk, M. Prusova, P. Prokopcakova, A. Krejcova, J. Anal. At. Spectrom. 24, 953–957 (2009)
M. Hanif, M. Salik, M.A. Baig Opt. Laser Eng. 49, 1456–1461 (2011).
D.J. Santos, L.C. Nunes, G.G.A. Carvalho, M.S. Gomes, P.F. Souza, F.O. Leme, L.G.C. Santos, F.J. Krug, Spectrochim. Acta Part B 71–72, 3–13 (2012)
C. Barnett, E. Cahoon, J.R. Almirall, Spectrochim. Acta Part B 63, 1016–1023 (2008)
E. Tognoni, V. Palleschi, M. Corsi, G. Cristoforetti, Spectrochim. Acta Part B 57, 1115–1130 (2002)
V. Zorba, X. Mao, R.E. Russo, Appl. Phys. Lett. 95, 041110 (2009)
P.K. Diwakar, S.S. Harilal, J.R. Freeman, A. Hassanein, Spectrochim. Acta Part B 87, 65–73 (2013)
J.K. Antony, N.J. Vasa, V.L.N.S. Raja, A.S. Laxmiprasad, Appl. Phys. A 112, 215–219 (2013)
C.W. Ng; W.F. Ho, N.H. Cheung, Appl. Spectrosc. 51, 976–983 (1997).
Z. Da-Cheng, M. Xin-Wen, W. Wei-Qiang, Z. Peng-Ju, Z. Xiao-Long, L. Bin, L. Hui-Ping, Chin. Phys. Lett. 27, 063202 (2010)
R.E. Russo, X. Mao, H. Liu, J. Gonzalez, S.S. Mao, Talanta 57, 425–451 (2002)
R. Ahmed, M.A. Baig, Opt. Laser Technol. 65, 113–118 (2015)
G.S. Senesi, I. Carrara, G. Nicolodelli, D.M.B.P. Milori, O. De Pascale, Microchem. J. 124, 296–305 (2016)
G. Nicolodelli, G.S. Senesi, R.A. Romano, I.L.O. Perazzoli, D.M.B.P. Milori, Spectrochim. Acta. Part B, 111, 23–29 (2015).
L. Fornarini, V. Spizzichino, F. Colao, R. Fantoni, V. Lazi, Anal. Bioanal. Chem. 385, 272–280 (2006)
W. Sdorra, J. Brust, K. Niemax, Mikrochim. Acta. 108, 1–10 (1992).
L.M. Berman, P. Wolf, J. Appl. Spectrosc. 52, 438–443 (1998)
N. Elsherbiny, O.A. Nassef, Sci. Justice 55, 254–263 (2015)
Y. Suzaki, A. Tachibana, Appl. Optics, 14, 2809–2810 (1975)
B.S. Marangoni, K.S.G. Silva, G. Nicolodelli, G.S. Senesi, J.S. Cabral, P.R. Villas-Boas, C.S. Silva, P.C. Teixeira, A.R.A. Nogueira, V.M. Benites, D.M.B.P. Milori, Anal. Methods 8, 78–82 (2016)
G. Nicolodelli, G.S. Senesi, I.L.O. Perazzoli, B.S. Marangoni, V.M. Benites, D.M.B.P. Milori, Sci. Total Environ. 565, 1116–1123 (2016)
M. Moll, M. Schlanges, Th. Bornath, V.P. Krainov New J. Phys. 14, 065010 (2012).
V.N. Raia, S.N. Thakurb, Physics of plasma in laser-induced breakdown spectroscopy, in Laser-Induced Breakdown Spectroscopy, ed. by J.P. Singh, S.N. Thakur (Elsevier press, Amsterdam, 2007)
R.E. Russo, X.L. Mao, J.H. Yoo, J.J. Gonzalez, in Laser Ablation, in Laser-Induced Breakdown Spectroscopy, ed. by J.P. Singh, S.N. Thakur (Elsevier press, Amsterdam, 2007)
D. Kleppner, in Interaction Laser-Plasma. Laser-Plasma Interaction, ed. by R. Bailan, J.C. Adam (North-Holland Publishing Company, Amsterdam, 1980)
Acknowledgements
The authors thank FAPESP (Process: 2012/24349-0 and 2013/07276-1), CNPq, and EMBRAPA for their financial support to this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nicolodelli, G., Senesi, G.S., Romano, R.A. et al. Laser-induced breakdown spectroscopy of environmental and synthetic samples using non-intensified CCD: optimization of the excitation wavelength. Appl. Phys. B 123, 127 (2017). https://doi.org/10.1007/s00340-017-6699-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00340-017-6699-6