Abstract
Considering the impending applications of room temperature ionic liquids (RTILs) in various areas involving high optical and radiation fields, it is pertinent to probe the structure–property correlation of these solvents exposed to such conditions. Herein, femtosecond Z-scan technique (at high pulse repetition rate, 80 MHz) was employed to investigate the non-linear optical response of imidazolium RTILs in 3 scenarios: (1) -OH functionalization, (2) C2 methylation, and (3) influence of high radiation fields. Large negative non-linear refractive values (n 2) were observed in all the RTIL samples and have been attributed predominantly due to the thermal effects. In order to isolate and determine the contribution of electronic Kerr effect, the Z-scan experiments were also carried out at low pulse repetition rate (i.e. 500 Hz) by means of a mechanical chopper. The closed aperture transmittance profile showed the valley-peak pattern, which signifies positive non-linearity. Nonetheless, the variation in the n2 values of the RTILs follows the same trend in low pulse repetition rate as was observed in case of high pulse repetition rate. The trend in the n 2 values clearly showed the decrease in the non-linearity in the first two cases and has been attributed to the weakening of the ion-pair formation, which adversely affects the charge transfer between the ionic moieties via C2 position. However, an increase in the n 2 values was observed in case of ILs irradiated to high radiation doses. This enhancement in the non-linearity has been assigned to the formation of double bond order radiolytic products. These results clearly indicate a strong correlation between the non-linearity and the strength of cation–anion interaction amongst them. Therefore, such information about these solvents may significantly contribute to the fundamental understanding of their structure–property relationships.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Room temperature ionic liquids (RTILs) are a rapidly expanding family of condensed-phase media and are being considered as a potential “green” alternative to traditional organic solvents with important applications in synthesis, extraction, catalysis, and electrochemistry [1–4]. Essentially, RTILs have been widely studied due to their unique physicochemical properties such as high polarity, negligible vapour pressure, high ionic conductivity, and thermal stability [1, 5, 6]. Furthermore, these liquids are also known as ‘designer solvents’, because alterations in the molecular structure of either the cation or the anion facilitates control over their physicochemical properties such as viscosity, solvation, catalytic activity, hydrophobicity, and melting point [1–4].
It is now an established fact that RTILs are microheterogeneous in nature with the co-existence of different types of interactions (such as coulombic, dipolar, van der Waals, and H-bonding), which make these fluids unique and complex in comparison to conventional solvents [7–9]. Essentially, RTILs comprise co-existing polar and non-polar domains i.e. the charged cationic head groups and the anions form a polar network, while the alkyl side chains aggregate to form non-polar tail domains. It is to be mentioned here that ILs may also exhibit liquid crystalline phase on increasing the amphiphilic character of the cation. Ionic liquid crystals contain anions and cations, and this ionic character differentiates them from that of conventional liquid crystals. Briefly, liquid crystals are also microheterogeneous in nature, and well-known as strong candidates in the field of non-linear optics, and possess optical non-linearities 108 times larger than CS2 [10]. Owing to such large non-linearity, various non-linear optical processes (i.e. optical bistability, switching, limiting) have been demonstrated [10, 11] in liquid crystals. Nevertheless, some preliminary studies exploring the non-linear optical response of RTILs have been carried out in the recent times. For instance, Souza et al. [12] investigated the non-linear optical properties of 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIM][BF4]) and 1-butyl-3-methylimidazolium hexafluorophosphate ([BMIM][PF6]) using the Z-scan technique for two excitation wavelengths, 514 and 810 nm. Large negative values of non-linear refractive index, n 2 (of the order of 10− 8 to 10− 9 cm2/W) and thermo-optical coefficients, dn/dT (10− 3 to 10− 5 K− 1) were obtained therein, while no non-linear absorption was observed. Trejo-Durán et al. [13] reported the anion effect on the non-linear optical properties of RTILs with same cation, [BMIM] and three different anions i.e. BF4, bis((trifluoromethyl)sulfonyl)imide (NTF2) and trifluoroacetate (CF3COO). The RTIL, [BMIM] [NTF2] was found to exhibit largest non-linearity amongst them. Santos et al. [14, 15] investigated the influence of the anionic and cationic parts on the non-local nature of optical non-linearity of RTILs using the Z-scan technique. The RTILs comprise BF4, PF6, and NTF2 as anions, while the cationic part was composed of five different derivatives of imidazolium (CnMIM, with n = 4, 6, 8, 10, and 12). Their results indicated that the RTILs with NTF2 (as the anion) exhibit lower non-linear response in comparison with other ILs having same cation. According to these authors, this trend was probably due to the ion-pairing effect, which follows the order as BF4 > PF6 > NTf2 [16, 17]. Also, a similar study regarding the non-local non-linear optical response of RTILs under violet excitation was carried out [15], where the authors observed an enhancement in the thermal non-linear refractive index (n 2) values by about two orders of magnitude higher than in the infrared region. The influence of anions and cations on the thermal lens strength was investigated by Nóvoa-López et al. [18]. These authors observed that both cation and anion significantly affect the thermal lens strength. Their results indicated stronger thermal lens signals for the phosphonium-based RTILs as compared to pyridinium-based RTILs. However, the authors did not observe any clear and systematic relation between the thermal refraction strength and the structural parameters of the RTILs investigated in their work. Apart from these, other non-linear spectroscopic techniques have also been applied to investigate the structure–property correlation in RTILs. For instance, Namboodiri et al. [19] employed femtosecond time-resolved coherent anti-Stokes Raman scattering (CARS) to monitor the vibrational dynamics of 1, 3-dialkylimidazolium RTILs containing [NTf2] as anion. Iwahashi et al. [20] investigated the interfaces of water/ RTIL by infrared–visible sum frequency generation (IV-SFG) vibrational spectroscopy and molecular dynamics (MD) simulation. Giraud et al. [21] probed the ultrafast solvent dynamics of RTILs by studying the effects of cation and anion substitution on the low frequency librational modes using optical heterodyne-detected Raman-induced Kerr effect spectroscopy (OHD-RIKES). The simultaneous determination of sound speed and thermal diffusivity in the RTIL and its mixture with organic solvents was carried out by Kozlov et al. [22] using laser-induced gratings (LIGs) technique. Recently, systematic studies combining Raman and hyper-Raman have been employed to probe the nature and multipolar symmetry of the local structure in RTILs [23].
From the aforementioned literature, it is clear that RTILs possess a huge potential to be exploited as a non-linear material (with a strong non-local character) for optical applications. However, non-linear studies on these media are still in the initial phase and to the best of our knowledge, the effect of functionalization or alkyl substitution of the cations on the non-linear optical response of imidazolium-based RTILs has not been investigated so far. Besides these, it is a well-established fact now that the RTILs, especially imidazolium based, are highly radiation stable [24–27]. Of late, we have also reported the high radiation stability of flouro alkyl phosphate (FAP) based imidazolium RTILs even at high radiation doses of 400–500 kGy [28]. The physicochemical properties of FAP imidazolium ILs were found to vary insignificantly on irradiation; however, the photophysical behaviour of the pre- and post-irradiated FAP ILs categorically pointed towards considerable changes in the orientations and the molecular rearrangements of the ions on irradiation [29]. Since, the optical non-linearity of materials has a strong correlation with their molecular structure; it would be quite intriguing to determine the impact of high radiation fields on their non-linear optical properties. Moreover, considering the impending applications of RTILs involving high radiation fields, it is essential to determine the non-linear behaviour of RTILs exposed to such conditions. Therefore, in the present work, the non-linear optical response of the imidazolium-based RTILs was investigated using femtosecond (fs) Z-scan technique, with three main objectives as follows. The influence of the hydroxyl (–OH) group functionalization (in the alkyl side chain of the imidazolium moiety) and C2 methylation of the imidazolium cation on the non-linear optical response of the RTILs was investigated. Furthermore, the effect of high radiation doses on the optical non-linearity of RTILs was also examined. The n 2 values were measured for all the RTILs, and the differences observed have been correlated with their molecular structure and the bonding interactions existing between the ionic moieties. We believe that such molecular level information about these solvents may contribute significantly in the fundamental understanding of their structure–property relationships.
2 Materials and methods
The RTILs investigated in the present work are 1-ethyl-3-methylimidazolium tris(pentafluoroethyl) trifluorophosphate ([EMIM][FAP]), 1-(2-hydroxyethyl)-3-methylimidazolium tris(pentafluoroethyl) trifluorophosphate ([EOHMIM][FAP]), 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([BMIM][NTF2]), and 1-butyl-2,3-dimethylimidazolium bis(trifluoromethylsulfonyl)imide ([BMMIM][NTF2]). [EMIM][FAP] and [EOHMIM][FAP] were obtained from Merck KGaA, Darmstadt, Germany, with a purity >99%.while [BMIM][NTF2] and [BMMIM][NTF2] were purchased from Io-Li-Tec, Germany, with the stated purity >99%. The stated water content and halide ion concentration in the RTILs was <100 ppm. It is also supported by the fact that the ILs were colourless and transparent. Water content in the RTILs was further rechecked by Karl Fischer titration with the aid of Metrohm 831 KF coulometer, and found to be within limits as specified by the manufacturer. Since these ILs are highly hydrophobic and the water uptake is extremely slight, they were used as such without further processing. The samples of RTILs were stored in vials furnished with an air tight septum and kept in vacuum tight desiccators. Extreme care has been taken to avoid any type of contamination while performing various measurements. For instance, the sample cells were properly cleaned and dried to avoid any sort of contamination from any other impurities. The structures of the RTILs studied are shown in Fig. 1.
The RTIL, [EMIM][FAP] was irradiated with a 7 MeV electron beam (FWHM ~ 2 µs) obtained from a linear accelerator (LINAC, in conjugation with Pulse radiolysis system) described elsewhere [30]. The irradiation conditions have been reported in our earlier work [28, 29]. Briefly, the absorbed dose was measured using an air-saturated solution containing 5 × 10− 2 mol dm− 3 KSCN assuming Gε for \(({\text{SCN}})_2^{ \bullet - }\) = 2.6 × 10− 4 m2 J− 1 at 475 nm. The absorbed dose per pulse was kept at 160 Gy and the samples were irradiated with repeated pulses @ 50 pulses per second.
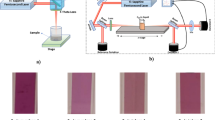
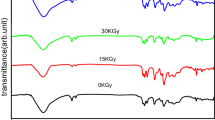
UV–Vis optical absorption studies were carried out on a JASCO V-650 spectrophotometer in a quartz cuvette having a path length of 1 mm. The UV–Vis absorption spectra of the RTILs (along with the RTIL, [EMIM][FAP] irradiated to a radiation dose of 100 and 200 kGy) are shown in Fig. 2. As can be seen, the unirradiated RTILs are transparent in the visible and near infrared regions while, the absorption spectra of irradiated RTILs showed a peak at ~300 nm accompanied by a shoulder at ~370 nm. On irradiation, the colourless and transparent RTIL ([EMIM][FAP]) become coloured and darkened with the increase in absorbed dose. The picture showing the colour evolution in the same IL on irradiation at various doses is provided in the inset of Fig. 2. The radiolytic products of the imidazolium cation (especially, oligomers and other species containing multiple bond order groups) were found to be primarily responsible for the colour evolution and emergence of peaks in the absorption spectra of the irradiated FAP ILs, as reported in our earlier work [28, 29].
The non-linear optical properties of the RTILs were investigated using the Z-scan technique [31]. The Z-scan setup is shown in Fig. 3 and the measurements were performed using a mode-locked laser beam of 20 fs (FWHM) delivered by a Ti:sapphire oscillator at λ = 800 nm with a pulse repetition rate of 80 MHz. The beam was focused onto the sample using a 15 cm focal length convergent lens with a beam waist radius (ω 0) of 31 ± 3 μm. The samples were taken in a quartz cuvette of 1 mm width. The cuvette was mounted on a motorized translation stage and moved around the focal point of the lens by a computer using Matlab-based programme. The transmitted intensity of the light was measured as a function of the sample position both in the closed aperture and open aperture configuration. The Z-scan setup was calibrated using CS2, and a negative non-linear refraction with n2 value of approximately 0.2 × 10− 18 m2/W was obtained, which matches closely with the values reported by Ganeev et al. [32] and Novoa-López et al. [33] at similar experimental parameters. The error in n2 value is estimated to be within 15–20%.
The analysis of the Z-scan data was performed using the model developed by Sheik-Bahae et al. [31] i.e. the normalized transmittance (T) of the sample (at the small aperture of the far-field detector) in the far-field condition is given by Eq. (1),
where \(X = z/z_{o}\), z0 is the Rayleigh range and \(\Delta{{\Phi }}_{0}\) is the on-axis phase change given by Eq. (2).
where I 0 is the on-axis irradiance at the focus, λ is the wavelength of the laser beam, and L eff is the effective length, given by \(L_{{\text{eff}}} = \frac{{1 - {e^{ - \alpha L}}}}{\alpha },\) where α is the linear absorption coefficient. It is well known that spatial variation of the refractive index of the material takes place when the intensity of the laser beam interacting with it is high enough to introduce non-linear effects. The variation in the refractive index is mainly attributed to either Kerr effect or thermal lens effect. The Kerr effect may take place due to different physical processes such as electronic, nuclear, or molecular orientation. All these processes involve different temporal responses ranging from few femtoseconds to picoseconds [34]. On the other hand, thermal effects involve slow and accumulative response. The efficiency of these effects strongly depends on the characteristics of the laser [32]. In the present work, considering the use of 20 fs pulses @ 80 MHz, the change in refractive index may be due to both electronic Kerr and thermal effects. However, the predominant effect among these two may be identified by varying the pulse repetition rate or their dependence on irradiance or fluence. Moreover, thermal effects usually prevail over the Kerr effect, when high pulse repetition rate lasers are used [18, 34]. Nonetheless, as already mentioned, the main objectives of this work were to determine the influence of functionalization, methylation, and high radiation fields on the non-linear optical response of the imidazolium-based RTILs. The analysis of the Z-scan traces was performed using Eq. (1). Also, an attempt was carried out to separate the electronic and the thermal originated non-linear optical response of the RTILs by reducing the pulse repetition rate to 500 Hz using a chopper.
It is to be mentioned here that the n 2 values of the RTILs have been determined using irradiance (I 0) values calculated from peak power in case of low pulse repetition rate laser. The I 0 values calculated using peak power (labelled as \(I_{\text{o}}^\prime\)) were determined using Eq. (3) [35].
where γ and Δt are the repetition rate (500 Hz) and duration (~50 fs, FWHM) of the laser pulses, respectively. The only dispersive elements in the experimental setup are a neutral density filter used to control the laser intensity and the lens used to focus the beam. The group delay dispersion due to these elements is calculated to be ~ 290 fs2. This changes the originally 50 fs pulse to a pulse of width ~ 52 fs (FWHM). We expect that this chirp is small enough not to make any major contribution to the observed results.
In case of high pulse repetition rate laser (80 MHz), the n2 values of the RTILs were determined using I0 values (labelled as \(I_{\text{o}}^{\prime\prime}\)) calculated from the average power. The \(I_{\text{o}}^{\prime\prime}\) values were determined using Eq. (4) [31].
The order of the n2 values for some of the RTILs (discussed later) calculated using \(I_{\text{o}}^{\prime\prime}\) match closely with those reported in earlier studies [12, 14] using similar experimental parameters (such as pulse duration and repetition rate).
3 Results and discussion
The closed aperture Z-scan traces of the [EMIM][FAP] (pre- and post-irradiated) and [EOHMIM][FAP] are shown in Fig. 4, while those of [BMIM][NTF2] and [BMMIM][NTF2] are shown in Fig. 5.
As can be seen from the transmittance curves, the peak is preceded by the valley, which signifies negative non-linearity or defocusing behaviour. The fitting of the curves was carried out with Eq. (1) to extract the on-axis phase shift, and the n 2 values were obtained using Eq. (2). The n 2 values (corresponding to \(I_{\text{o}}^{\prime\prime}\)) of the investigated RTILs are provided in Table 1. From the negative values of the n2 values, it is apparent that the thermal effects are predominant behind the as observed non-linear optical response. This is further substantiated from the fact that the peak-valley separation (ΔZ P−V ) calculated from the closed aperture Z-scan curves of was found to be ~2.2 Z0 (i.e. >1.7 Z 0).
Before, analysing these data, it is important to mention some of the structural aspects and bonding interactions prevailing in imidazolium-based RTILs. Although, cation–anion interactions in ILs are mainly dominated by coulombic (electrostatic) forces, additional interactions such as van der Waals, π–π stacking, and hydrogen bonding also play an important role. The extent of delocalization in the imidazolium cation is also of interest, which has been found to comprise 3-centre-4-electron configuration across the N1-C2-N3 moiety, a double bond between C4 and C5 at the opposite side of the ring, and a weak delocalization in the central region [36]. Besides, it has been shown by various researchers in the earlier reports that the proton at C2 position (C2H) has a large partially positive charge, and is the most acidic proton in the imidazolium cation [36–40]. Therefore, in case of imidazolium-based RTILs, the hydrogen bonding between the cation and the anion is mainly determined by the hydrogen at the C2 position [41–43]. Furthermore, the bonding strengths between cation and anion have been observed to be considerably influenced by the nature of the anions such as their size, charge delocalization, and basicity [41, 42]. For instance, voluminous and heavy anions only weakly interact, whereas small and light anions provide strong interaction potential due to their higher surface charge density, as reported by Fumino et al. [43]. Apart from this, charge transfer between the cation–anion ion-pair, and related partial atomic charges is an important descriptor for RTILs [44]. For instance, XPS investigations carried out by Cremer et al. [42] into cation–anion interactions have indicated a degree of charge transfer from anion to cation, which is correlated to the anion basicity. Such charge transfer is expected to be smallest for the large and weakly coordinating anions while, for the smaller, basic, and strongly coordinating anions, relatively higher localized charge is transferred from the anion to the cation, leading to a less positively charged ring. In fact, Hunt et al. [36] in their theoretical investigation on RTIL i.e. 1-butyl-3-methylimidazolium Chloride (in gas phase) observed a correlation between the amount of charge transferred and the relative stability of ion pairs such that the more stable a dimer pair the more charge is transferred. Also, from the natural bond orbitals (NBO) analysis, it was found that, on forming the ion-pair, the charge transfer from the chloride anion to the imidazolium cation increased the electron density in the π-system. It is to be noted here that the ion-pair formation in imidazolium-based ILs involves two types of scenarios, which can be categorized as either (1) typical ion–ion interaction, with the anion interacting from above or below the imidazolium plane or (2) hydrogen-bonding interaction, with the anion interacting with the hydrogen at the C2 position of the imidazolium cation [45–48]. However, both types of interactions were found to induce similar charge transfers, as reported by Izgorodina et al. [45].
All these aforementioned facts regarding the electronic structure and the bonding interactions prevailing in the imidazolium-based ILs have been used in the analysis of the as observed non-linear optical behaviour exhibited by the investigated RTILs.
From Table 1, it is shown that, on –OH functionalization, the non-linearity decreases, which is evident from the decrease in the n2 values (magnitude) of [EOHMIM][FAP] as compared to non-hydroxyl IL ([EMIM][FAP]). The probable explanation for this trend in the non-linearity can be provided as follows. It has been reported that the introduction of –OH group at the end of alkyl moiety of imidazolium-based IL reduces cation–anion electrostatic interactions because of the extended distances between the proton at C2 position (of imidazolium moiety) and an anion [49, 50]. This is due to the fact that for non-hydroxyl ILs, the anion is located in front of the imidazolium ring, close to the C2H unit. On the contrary, the anions in hydroxyl ILs are somewhat above the imidazolium ring, moving close to the –OH group [50]. Besides these, –OH group exhibits both, electron donating as well as withdrawing effect by resonance and inductive effect, respectively, depending on its attachment to the various sites of the aromatic ring [28]. Substitution at the ring site (hydrogen attached to the imidazolium ring) results in a direct electron donation from the lone pairs of the –OH group to the delocalized π-orbitals in the imidazolium ring, and thus the electron donating resonance effect dominates over the electron withdrawing inductive effect. When attached to the N-site (hydrogen on the methyl group attached to a nitrogen atom) or C-site (hydrogen on the alkyl group attached to the carbon atom), the opposite is true and the inductive effect dominates. Various sites of imidazolium ring are shown in Fig. 6.
Apparently, due to the combined effect of the reduction in the cation–anion electrostatic interactions (mentioned earlier) in –OH containing IL as well as the electron withdrawing nature of –OH, the electron density of the imidazolium cation is anticipated to be less. This probably results in the decline in the optical non-linearity, which is reflected by the decrease in the n 2 values (magnitude) of [EOHMIM][FAP] as compared to [EMIM][FAP].
As mentioned earlier, the effect of C2 methylation on the optical non-linearity of RTILs ([BMIM][NTF2] and [BMMIM][NTF2]) was investigated. The n 2 values (magnitude) of [BMIM][NTF2] were found to be higher than [BMMIM][NTF2], as shown in Table 1. This signifies the decrease in the non-linear optical behaviour of the imidazolium IL on C2 methylation. The most probable explanation for this observation can be provided as follows. It is quite obvious that the primary difference between [BMIM]+and [BMMIM]+ is the termination of the ability of the latter to form hydrogen bond through the proton on the C2 position of the imidazolium ring [51]. Furthermore, C2 methylation enables the anion to move above or below the cation instead of co-planar with the ring near the C2 position [51, 52]. Consequently, the interaction between the cation and anion decreases (in comparison to that of the non-methylated one) [51, 52], which possibly obstructs the charge transfer between the cation–anion ion-pair. Moreover, it has been confirmed from various studies (i.e. Raman, IR, and NMR spectra) that methylation at the C2 position of the imidazolium cation alters the electron density distribution and strength of the interionic interactions [53]. Essentially, methylation at C2 position (of imidazolium cations) blocks the most important site for C2–H···anion hydrogen bonding. However, despite eliminating this site for hydrogen bonding, the C2-methylated RTIL is more viscous than the non-methylated one. The reason for this observation is not yet fully understood, and different explanations have been proposed [54, 55]. Nonetheless, other non-linear spectroscopic techniques such as CARS [19], 2D-IR spectroscopy [55], and OHD-RIKES [21] have revealed that the interionic interactions affect not only the vibrational frequencies but also their dynamics. For instance, CARS investigations [19] showed that the directional, and strong hydrogen bonds in non-methylated (at C2 position) RTILs open a possibility for vibrational energy transfer between the counter ions, and also act as molecular dampers leading to a faster decay in the vibrational dynamics. However, such scenario was not observed in case of C2-methylated RTILs. Indeed, this observation corroborates the findings of the present work.
Essentially, charge transfer is one of the fundamental processes which can induce significant enhancements in the optical non-linearity [56–58]. Taking this into account, the observed non-linear optical behaviour (of investigated RTILs) appears to have a strong correlation with the strength of cation–anion interaction facilitating charge transfer between the two moieties. Similar proposition was also mentioned by Santos et al. [14], where these authors related the non-linear optical response of RTILs to the ion-pairing effect.
The influence of high radiation fields on the non-linear optical behaviour of RTILs was investigated considering their futuristic applications in areas involving high radiation levels. The n 2 values of [EMIM][FAP] irradiated to high radiation doses (100 and 200 kGy) are provided in Table 1. It can be observed that not only the n 2 values (absolute) of post-irradiated [EMIM][FAP] are higher than the unirradiated one, they also exhibit an increasing trend with the radiation dose levels. This clearly signifies the enhancement in the optical non-linearity of [EMIM][FAP] on irradiation. The most probable explanation for this trend can be provided as follows. It has been already mentioned that the radiation chemical studies (carried out earlier by our group) have clearly indicated high radiation stability of FAP based imidazolium RTILs even at high radiation doses of 400–500 kGy [28]. The amount of radiolytic products formed on irradiation was very less. However, we were able to identify some of the possible radiolytic products possessing double bond units (conjugated as well as non-conjugated) from their mass spectrometric studies [28, 29]. Furthermore, the photophysical behaviour of the pre- and post-irradiated ILs categorically pointed towards considerable changes in the orientations and the molecular rearrangements of the ions on irradiation [29]. Besides, the average fluorescence lifetime of the RTILs increased with the rise in the absorbed dose imparted, which could be due to the formation of new intermolecular hydrogen bonds and other non-covalent bonding interactions between the radiolytic products and the ionic moieties (indicated from the vibrational studies [29]). Considering these aforementioned facts, the enhancement in the optical non-linearity of irradiated IL i.e. [EMIM][FAP] could be attributed to the formation of radiolytic products possessing double bond units as well as to the stronger interionic interactions (as revealed from the photophysical studies reported earlier [29]).
In case of high pulse repetition rate lasers, the contribution of the thermal effects is predominant. However, in order to determine the contribution of electronic Kerr effect to the non-linear refractive index, the Z-scan experiments were also carried out at low pulse repetition rate (i.e. 500 Hz). The closed aperture transmittance profile showed the valley-peak pattern (shown in Fig. 7), which signifies positive non-linearity or self-focusing behaviour. This is contrary to the negative non-linearity or defocusing behaviour (peak-valley pattern) observed for the RTILs in case of high pulse repetition rate laser (@ 80 MHz). The n2 values (corresponding to \(I_{\text{o}}^\prime\) values) of the investigated RTILs have been provided in Table 2. As can be seen, the n 2 values of the RTILs are of the order 10− 16 cm2/W. Some common organic solvents (utilized in non-linear optics) such as CS2, toluene, DMF exhibit n2 values of the order 10− 14 to 10− 15 cm2/W [32, 33, 59] at similar experimental parameters. Therefore, it shows that the n2 values (obtained with low pulse repetition rate) of the RTILs are low as compare to these commonly used organic solvents.
Z-scan curves of RTILs (800 nm @ 500 Hz): a [EMIM][FAP], b [EOHMIM][FAP], c [EMIM][FAP]-100 kGy, d [EMIM][FAP]-200 kGy, e [BMIM][NTF2], f [BMMIM][NTF2]. The pulse duration, average power (P avg), and the pulse repetition rate in all the measurements were 50 fs, ~78 µW, and 500 Hz, respectively. Circles correspond to experimental data, while solid lines illustrate the theoretical fit
It is to be noted that the peak-valley separation (ΔZ P−V ) calculated from the closed aperture Z-scan curves of RTILs at low pulse repetition rate (@ 500 Hz) was found to be ~1.7 Z0. However, in case of high pulse repetition rate (@ 80 MHz), ΔZ P−V was found to be ~2.2 Z0. A peak-valley separation of more than 1.7 times the Rayleigh range (Z 0) is a clear indication of predominantly thermally originated non-linearity. Therefore, it can be said that the investigated RTILs exhibited non-linear optical response primarily due to the electronic effects at low pulse repetition rate, while thermally originated non-linearity predominates in case of high pulse repetition rate. Nonetheless, the variation in the n2 values of the RTILs (on –OH functionalization, C2 methylation, and at high radiation fields) follows the same trend (see Tables 1, 2) in low pulse repetition rate as was observed in case of high pulse repetition rate laser.
4 Conclusions
In summary, femtosecond Z-scan technique was employed to investigate the non-linear optical response of the imidazolium-based RTILs on functionalization, alkylation, and irradiation. However, from the negative values of the n 2 values (at high pulse repetition rate), it is apparent that thermal effects predominantly contribute towards the observed non-linear optical response. The trend in the n 2 values clearly showed the decrease in the optical non-linearity on –OH group functionalization and C2 methylation of the imidazolium cation. This decrease in the non-linearity has been most plausibly attributed to the weakening of the ion-pair formation, which adversely affect the charge transfer between the cationic and anionic moieties via C2 position. However, an increase in the n2 values was observed in case of RTILs irradiated to high radiation doses. This enhancement in the non-linearity has been attributed to the formation of radiolytic products possessing double bond units as well as to the stronger interionic interactions. The low pulse repetition rate experiments revealed positive non-linearity. This further substantiates that the investigated RTILs exhibit non-linear optical response primarily due to the electronic effects at low repetition rate, while thermal originated non-linearity predominates in case of high pulse repetition rate. Nonetheless, the variation in the n 2 values of the RTILs (on –OH functionalization, C2 methylation, and at high radiation fields) follows the same trend in low pulse repetition rate as was observed in case of high pulse repetition rate. We believe that such information may significantly contribute in further investigation (to improve their priori design) and exploitation of these solvents in various applications, especially related to optics and photonics.
References
T. Welton, Chem. Rev 99, 2071 (1999)
P. Wasserscheid, W. Keim, Angew. Chemie Int. Ed. 39, 3772 (2000)
R. Sheldon, Chem. Commun. 2399 (2001)
A.M. Funston, T.A. Fadeeva, J.F. Wishart, E.W. Castner, J. Phys. Chem. B 111, 4963 (2007)
V.I. Pârvulescu, C. Hardacre, Chem. Rev 107, 2615 (2007)
J.P. Hallett, T. Welton, Chem. Rev 111, 3508 (2011)
Y. Wang, G.A. Voth, J. Am. Chem. Soc. 127, 12192 (2005)
L.M.N.B.F. Santos, N. José, C. Lopes, J.A.P. Coutinho, J.M.S.S. Esperança, L.R. Gomes, I.M. Marrucho, L.P.N. Rebelo, J. Am. Chem. Soc. 129, 284 (2007)
Y. Wang, W.E.I. Jiang, T. Yan, G.A. Voth, Acc. Chem. Res 40, 1193 (2007)
P. P. Banerjee ed., Nonlinear Optics: Theory, Numerical Modeling, and Applications (CRC Press, London, 2003)
I.C. Khoo, Acta Phys. Pol. A 86, 1 (1994)
R.F. Souza, M.A.R.C. Alencar, M.R. Meneghetti, J. Dupont, J.M. Hickmann, J. Phys. Condens. Matter 20, 155102 (2008)
M. Trejo-Durán, E. Alvarado-Méndez, J.A. Andrade-Lucio, K.A. Barrera-Rivera, J.M. Estudillo-Ayala, I.A. Sukhoivanov in International Conference on Laser Fiber-Optical Networks Model (IEEE, Sevastopol, 2010), pp. 131–132
C.E.A. Santos, M.A.R.C. Alencar, P. Migowski, J. Dupont, J.M. Hickmann, Chem. Phys 403, 33 (2012)
C.E.A. Santos, M.A.R.C. Alencar, P. Migowski, J. Dupont, J.M. Hickmann, Adv. Mater. Sci. Eng. 104914, 2013 (2013)
R. Lungwitz, V. Strehmel, S. Spange, New J. Chem. 34, 1135 (2010)
F.C. Gozzo, L.S. Santos, R. Augusti, C.S. Consorti, J. Dupont, M.N. Eberlin, Chemistry 10, 6187 (2004)
J. A. Nóvoa-López, E. López Lago, M. Domínguez-Pérez, J. Troncoso, L. M. Varela, R. De La Fuente, O. Cabeza, H. Michinel, J. R. Rodríguez, Opt. Laser Technol. 61, 1 (2014)
M. Namboodiri, M.M. Kazemi, T.Z. Khan, A. Materny, J. Kiefer, J. Am. Chem. Soc. 136, 6136 (2014)
T. Iwahashi, Y. Sakai, D. Kim, T. Ishiyama, A. Morita, Y. Ouchi, Faraday Discuss. 154, 289 (2012)
G. Giraud, C.M. Gordon, I.R. Dunkin, K. Wynne, J. Chem. Phys. 119, 464 (2003)
D.N. Kozlov, J. Kiefer, T. Seeger, A.P. Fröba, A. Leipertz, J. Phys. Chem. B 115, 8528 (2011)
V. Rodriguez, J. Raman Spectrosc. 43, 627 (2012)
X. Sun, H. Luo, S. Dai. Chem. Rev. 112, 2100 (2012)
D. Allen, G. Baston, A. E. Bradley, T. Gorman, A. Haile, I. Hamblett, J.E. Hatter, M.J.F. Healey, B. Hodgson, R. Lewin, K.V. Lovell, B. Newton, W.R. Pitner, D.W. Rooney, D. Sanders, K.R. Seddon, H.E. Sims, R.C. Thied, Green Chem. 4, 152 (2002)
M. Qi, G. Wu, S. Chen, Y. Liu, Radiat. Res. 167, 508 (2007)
L. Berthon, S.I. Nikitenko, I. Bisel, C. Berthon, M. Faucon, B. Saucerotte, N. Zorz, P. Moisy, Dalton Trans. 2526 (2006)
A. Guleria, A.K. Singh, S. Adhikari, S.K. Sarkar, Dalton Trans. 43, 609 (2013)
A. Guleria, A.K. Singh, S. Adhikari. Phys. Chem. Chem. Phys. 17, 11053 (2015)
T. Mukherjee, in Atomic, Molecular and Cluster Physics, ed. S.A. Ahmad (Narosa Publishing House, New Delhi, 1997), pp. 299–316
M. Sheik-Bahae, A.A. Said, T.H. Wei, D.J. Hagan, E.W. Van Stryland, IEEE J. Quantum Electron. 26, 760 (1990)
R.A. Ganeev, A.I. Ryasnyansky, M. Baba, M. Suzuki, N. Ishizawa, M. Turu, S. Sakakibara, H. Kuroda, Appl. Phys. B Lasers Opt. 78, 433 (2004)
J.A. Novoa-López, P. Martín-Ramos, H. Michinel, I. J. Sola, P. Chamorro-Posada, Opt. Mater. Express 5, 503 (2015)
D.N. Christodoulides, I.C. Khoo, G.J. Salamo, G.I. Stegeman, E.W. Van Stryland, Adv. Opt. Photon 2, 60 (2010)
N. Wickremasinghe, X. Wang, H. Schmitzer, H.P. Wagner, Opt. Express 22, 23955 (2014)
P.A. Hunt, B. Kirchner, T. Welton, Chemistry 12, 6762 (2006)
M. Babucci, A. Akçay, V. Balci, A. Uzun, Langmuir 31, 9163 (2015)
S.B.C. Lehmann, M. Roatsch, M. Schöppke, B. Kirchner, Phys. Chem. Chem. Phys. 12, 7473 (2010)
H.S. Schrekker, D.O. Silva, M.A. Gelesky, M.P. Stracke, C.M.L. Schrekker, R.S. Gonçalves, J. Dupont, J. Braz, Chem. Soc 19, 426 (2008)
S.E. Norman, A.H. Turner, T.G.A. Youngs, RSC Adv. 5, 67220 (2015)
A.M. Fernandes, M.A.A. Rocha, M.G. Freire, I.M. Marrucho, J.A.P. Coutinho, L.M.N.B.F. Santos, J. Phys. Chem. B 115, 4033 (2011)
T. Cremer, C. Kolbeck, K.R.J. Lovelock, N. Paape, R. Wölfel, P.S. Schulz, P. Wasserscheid, H. Weber, J. Thar, B. Kirchner, F. Maier, H.P. Steinrück Chemistry 16, 9018 (2010)
K. Fumino, S. Reimann, R. Ludwig, Phys. Chem. Chem. Phys. 16, 21903 (2014)
R.P. Matthews, T. Welton, P.A. Hunt, Phys. Chem. Chem. Phys. 17, 14437 (2015)
E.I. Izgorodina, D.R. MacFarlane, J. Phys. Chem. B 115, 14659 (2011)
K. Bica, M. Deetlefs, C. Schröder, K.R. Seddon, Phys. Chem. Chem. Phys. 15, 2703 (2013)
H. Jin, B. O’Hare, J. Dong, S. Arzhantsev, G.A. Baker, J.F. Wishart, A.J. Benesi, M. Maroncelli, J. Phys. Chem. B 112, 81 (2008)
Z.J. Chen, J.M. Lee, J. Phys. Chem. B 118, 2712 (2014)
S. Zhang, X. Qi, X. Ma, L. Lu, Y. Deng, J. Phys. Chem. B 114, 3912 (2010)
S. Zhang, X. Qi, X. Ma, L. Lu, Q. Zhang, Y. Deng, J. Phys. Org. Chem 25, 248 (2012)
A. Aggarwal, N.L. Lancaster, A.R. Sethi, T. Welton, Green Chem. 4, 517 (2002)
M.H. Kowsari, M. Fakhraee, S. Alavi, B. Najafi, J. Chem. Eng. Data 59, 2834 (2014)
K. Noack, P.S. Schulz, N. Paape, J. Kiefer, P. Wasserscheid, A. Leipertz, Phys. Chem. Chem. Phys. 12, 14153 (2010)
V.H. Paschoal, L.F.O. Faria, M.C.C. Ribeiro, Chem. Rev. doi:10.1021/acs.chemrev.6b00461
Z. Ren, A.S. Ivanova, D. Couchot-Vore, S. Garrett-Roe. J. Phys. Chem. Lett 5, 1541 (2014)
Q. Chen, L. Kuang, E.H. Sargent, Z.Y. Wang, Appl. Phys. Lett. 83, 2115 (2003)
R. Boyd, Contemporary Nonlinear Optics (Academic Press, 2012)
J. Messier, P. Prasad, D. Ulrich, Nonlinear Optical Effects in Organic Polymers (Springer, New York, 2012)
K. Iliopoulos, D. Potamianos, E. Kakkava, P. Aloukos, I. Orfanos, S. Couris. Opt. Express 23, 24171 (2015)
Acknowledgements
The authors thank LINAC staff for their technical help and co-operation in carrying out experiments in the LINAC facility of RPCD, BARC. The authors thank Dr. D. K. Palit, Head, RPCD and Dr. B. N. Jagatap, Director, Chemistry Group, BARC for their support and encouragement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Namboodiri, V.V., Guleria, A. & Singh, A.K. Effect of –OH functionalization, C2 methylation, and high radiation fields on the non-linear optical response of imidazolium ionic liquids. Appl. Phys. B 123, 103 (2017). https://doi.org/10.1007/s00340-017-6695-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00340-017-6695-x