Abstract
We investigated the third-order nonlinear optical properties of 3,4-pyridinediamine solution. The nonlinear measurements were taken by using single-beam Z-scan technique with cw laser at 473 nm. The effect of varying glucose concentration in a sample solution has been studied. The experimental results show that the nonlinear refractive index, n 2, and nonlinear absorption coefficient, β, are strongly dependent on the glucose concentration in a sample solution. The optical limiting properties are measured by a transmission technique. We find that the limiting threshold can be improved by a proper choice of glucose concentration in sample solution. A 3,4-pyridinediamine with 80 mmol glucose concentration showed a good switching property. This phenomenon was demonstrated by waveguiding a transistor–transistor logic modulated cw 473 nm laser beam as an excitation beam modulated at 10 Hz frequency collinearly with a continuous-wave SDL-635-100T laser beam of wavelength 653 nm through a quartz cuvette of thickness 1 mm. The results of pump–probe experiments show that the time of switch-on and switch-off of the 3,4-pyridinediamine was in μs for the pump intensity. The energy-dependent transmission studies also reveal better limiting property of the sample compound at nanosecond regime. Also, thermo-optic coefficients have been determined by thermal lens (TL) technique (−9.54 × 10−5 K−1) and it was found to be temperature dependent. This value was compared with result obtained by Z-scan calculations (−7.46 × 10−5 K−1). Thus, the nonlinear response of the material suggests that it has a potential application for high-sensitive photonic devices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The most promising application of organic materials is the fabrication of an optical limiting solid-state device, which can be used to protect optical sensors and human eyes from the damages caused by high-energy laser light [1]. An ideal limiter exhibits a linear transmission below threshold and a constant transmission above threshold [2]. The threshold value of an optical limiting is dependent on the parameters of the system configuration such as aperture size [3], position of the sample behind the focal point of the lens [4], concentration of the solution [5–8], nature of the solvent [9], pH of sample [10], salt concentration of the solution [11, 12] and the wavelength of the laser used [13, 14]. All these parameters have been studied elsewhere. For our knowledge, still now no one studied the influence of glucose concentration on the optical limiting properties. Therefore, in this work we have studied the influence of this parameter on threshold value of an optical limiting.
Organic and metal–organic materials which possess optical power limiting (OPL) property have attracted great interests owing to their potential applications in sensor and eye protection against intense light [15–17]. All-optical switching (AOS)-related devices have gained interest in optical communication and form building blocks of future information processing systems. All-optical devices have been proposed for large variety of materials based on nonlinear absorption using pump–probe technique [18–20], and their theoretical and experimental investigations are based on resonant nonlinearity using fiber Bragg grating technique [21–24]. Based on the above consideration and in search for new materials for nonlinear optical (NLO) device applications, we have synthesized and measured the third-order NLO, OPL and AOS properties of a 3,4-pyridinediamine solution.
The nonlinear absorption mechanism in the NLO organic materials can be due to reverse saturable absorption (RSA), which leads to the increase in absorption in the material within crease in laser intensity, and saturable absorption (SA) which leads to the decrease in absorption in the material with the increase in laser intensity. RSA is observed when excited-state absorption is greater than the ground-state absorption, which results in a decrease in the transmission through the material within crease in the input intensity, while SA is observed when excited-state absorption is less than the ground-state absorption, which results in an increase in the transmission through the material with the increase in the input intensity.
In this paper, we report on the optical nonlinearity in organic material, namely 3,4-pyridinediamine in dimethyl sulfoxide (DSMO) solvent at low cw laser power levels, and demonstrate the optical limiting of a cw laser beam. The nonlinear refractive index and nonlinear absorption coefficient of 3,4-pyridinediamine solution under cw laser illumination are determined by the well-known Z-scan setup formulated by Sheik-Bahae [25]. Also we investigated the effect of glucose concentration in 3,4-pyridinediamine solution on the nonlinear optical and optical limiting properties. The optical power limiting and all-optical switching ability of the 3,4-pyridinediamine solution were also experimentally revealed by performing energy-dependent transmission and pump–probe experiments. Also, thermal lens spectrometry is applied to investigate the thermo-optical properties of 3,4-pyridinediamine with 80 mmol glucose concentration as a function of the temperature.
2 Experimental details
In the present study, we use two types of materials: First one is 3,4-pyridinediamine, and the second one is DMSO solvent. The samples were prepared as follows: 21.8 mg of the 3,4-pyridinediamine powder was dissolved in 20 ml of DMSO; the solution was stirred at room temperature for 50 min, and then the solution was filtered through a 0.2-mm syringe filter. The collected sample was transferred into five cleaned dry test tubes. Every tube contains 5 ml with concentration about 10 mmol; then, 900 mg of the glucose powder was added to the first tube, and the second, third and fourth tubes were left without the addition of glucose powder. The solution was mixed well and incubated at 37 °C for 15 min. The solution turned to pinkish red color. The prepared sample was further used for glucose measurements: seven drops from the first tube were added to the second tube (sample 1), five drops were added to the third tube (sample 2), and three drops were added to the fourth tube (sample 3). These samples have been used in this work to study the influence of glucose concentration on the nonlinear optical and optical limiting properties. Finally, the fifth tube has been used without the addition of any sample. So we can consider it as a sample with zero glucose concentration.
To determine the glucose concentration in samples 1, 2 and 3, a solid disk (4-aminophenazone glucose oxidase peroxidase) from Randox Co. was dissolved in buffer (phosphate-buffered phenol), and then, the collected mixture was transferred into cleaned dry test tube as sample 4. The 20-μl solution of the sample 1 was mixed with 2000 μl solution of the sample 4 at room temperature (25 °C). The final mixture was left for a few second ~25 s in a closed volume to get a limited solvent evaporation rate. This sample was taken in a quartz cuvette of thickness 1 mm for the measurement purposes. The other glucose sample mixtures were also prepared in a similar manner. The codes of mixtures samples 1, 2, 3 with 4 separately is A, B and C, respectively. The glucose concentration in samples A, B and C was calculated by the equation:
where E sample and E standard represent the optical absorption at 500 nm of sample and standard glucose, respectively.
The optical response was characterized by measuring the intensity-dependent refractive index n 2 of the medium using the Z-scan technique. The sample showed negative and large nonlinear refractive index values of the order of 10−8 cm2/W and reverse saturable absorption with high values of the nonlinear absorption coefficient of the order of 10−4 cm/W. The nonlinear refractive index was found to vary with the glucose concentration. These results indicate that the 3,4-pyridinediamine dye is a promising candidate for applications in the nonlinear optic field.
All-optical switching property of the sample was also investigated using standard pump–probe technique [26]. The sample is illuminated by two TEM00 Gaussian laser beams, one of which is used for exciting the sample to produce a local temperature increase, using a transistor–transistor logic (TTL) modulated laser beam (473 nm at 12 mW) as an excitation beam modulated at 10 Hz frequency and focused by +50-mm focal length lens, and the other to probe the thermal effect. The probe beam was a low-power (2 mW) continuous-wave laser beam of wavelength 653 nm. The probe beam is focused by +20-cm focal length lens and is aligned at an angle smaller than 1.4° with respect to the excitation beam. The sample was laced at \(\sqrt 3\) Z c from the probe beam waist. The signal was analyzed using a digital storage oscilloscope (lodestar-MOS-620CH) which was triggered by the transistor–transistor logic modulated laser.
3 Results and discussion
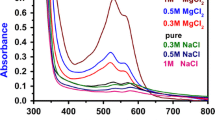
The ultraviolet–visible (UV–Vis) absorption spectra of the glucose samples with the wavelength region 400–800 nm at room temperature were recorded using an UV–Vis spectrophotometer (Cecil ReflectaScan Reflectance Spectrophotometer CE-3055.). The optical absorption of the samples A, B and C and standard concentration (a kit supplied by Randox Co.) is shown in Fig. 1. The values of optical absorption of the samples A, B and C and standard glucose at 500 nm are obtained from UV–Vis absorption spectrum (Fig. 1). Using Eq. 1, the values for glucose concentration of samples A, B and C are 27, 50 and 80 mmol, respectively, which also represent the glucose concentration in samples 1, 2 and 3, respectively.
Figure 2 shows the spectral distribution of absorbance for 3,4-pyridinediamine in DSMO solvent (sample with zero glucose concentration) and for 3,4-pyridinediamine in DSMO solvent with different glucose concentrations (samples 1, 2 and 3). The optical absorption for the samples shows an absorption peak at 520 nm, as shown in Fig. 2. Figure 2 shows that the absorbance of the sample increases with increasing the concentration of glucose in the sample solution.
The absorption coefficient (α) [27] is
where A is the absorbance and d is the sample thickness. The values of absorption coefficient (α) at wavelength 473 nm for 3,4-pyridinediamine in DSMO solvent with different glucose concentrations have been calculated using Eq. (2), and they are given in Table 1.
3.1 Nonlinear optical properties
The single-beam Z-scan is a well-known technique for measuring an absolute magnitude of the nonlinear refraction coefficient of the investigated substance [28, 29]. It allows the simultaneous measurement of both the nonlinear refractive index and nonlinear absorption coefficient. The basic idea behind the Z-scan technique is the self-focusing or self-defocusing. The study of nonlinear refraction by the Z-scan method is based on the intensity dependence of the investigated thin sample along a focused Gaussian laser beam. The sample causes additional focusing due to the positive nonlinear refraction or the defocusing due to a negative refraction. A Gaussian beam is focused by a spherical lens onto the sample, and the variation in the beam profile is observed at the far field as the sample is taken through the focus of the lens. The beam propagation direction is taken as the Z–direction, and the sample is moved along that direction, and hence, this technique is known as the Z-scan technique.
The Z-scan experiments were performed using a cw waveguide laser with 473 nm wavelength and power of 100 mW, which was focused by a 5-cm focal length lens. The laser beam waist ω 0 at the focus was measured to be 22.19 μm, and the Rayleigh length was evaluated by equation [30, 31]:
where Z R = 3.27 mm. It is greater than the thickness of the samples, which is an important requirement for Z-scan technique. The experimental setup used is shown in Fig. 3. A 1-mm-wide optical cell containing the sample solution was translated across the focal region along the axial direction that is the direction of the propagation of the laser beam. The transmission of the beam was measured using a photodetector fed to the digital power meter (Field Max II-To+OP-2 Vis Sensor). For an open-aperture Z-scan, a lens to collect the entire laser beam transmitted through the sample replaced the aperture.
To determine the nonlinear absorption, we performed Z-scan experiments without an aperture for sample solution with 0, 27, 50 and 80 mmol glucose concentration at incident intensity I o = 1.551 kW/cm2. The experimental results are shown in Fig. 4. Figure 4 shows that the normalized transmittance decreases when sample moves nearer to the focal point. The open-aperture curve exhibits the presence of reverse saturable absorption in the solution form. This is an indication of nonlinear absorption [32].
The measurable quantity ΔT P−V can be defined as the difference between the normalized peak and valley transmittances, T P − T V . The variation of this quantity as a function of Δϕ o is given by [33, 34].
The ratio of the light passing through the aperture on the light in front of the aperture was defined as S.
Or S is the linear transmittance of aperture, which can be calculated as [35]: \(S = 1 - \exp \left( { - r_{\text{a}}^{2} /\omega_{\text{a}}^{2} } \right)\) (S = 0.61), with r a denoting the aperture radius and ω a denoting the beam radius at the aperture in the linear regime. Δϕ o is the on-axis phase shift at the focus related to the third-order nonlinear refractive index by [36]:
where k = 2π/λ, L eff = (1 − exp(−αL))/α is the effective thickness of the sample, α the linear absorption coefficient, L the thickness of the sample, I o the on-axis irradiance at focus, and n 2 the third-order nonlinear refractive index.
The nonlinear absorption coefficient β can be estimated from the open-aperture Z-scan data [37]where ΔT is one-valley transmission.
where ∆T is one-valley transmittances at the open-aperture Z-scan curve and I o = 2P/πω 2 o is the intensity of the laser beam at focus. Also, Δn can be related to the total refractive index of the medium, n, and the background refractive index, n 0, as [38]:
Figure 5 shows the closed-aperture Z-scan data for sample solution with different glucose concentrations at incident intensity I 0 = 1.551 kW/cm2. The scan of all the samples has peak-valley configuration, corresponding to a negative nonlinear refraction index, i.e., self-defocusing occurs. In the previous work, we showed that in the case of sample solution and CW laser used in Z-scan technique, the defocusing effect is attributed to a thermal nonlinearity resulting from absorption of radiation. Localized absorption of a tightly focused beam propagating through an absorbing medium produces a spatial distribution of temperature in the sample solution and consequently a spatial variation of the refractive index that acts as a thermal lens resulting in phase distortion of the propagating beam. Practically, in the case of a closed-aperture scan, nonlinear refraction is accompanied by nonlinear absorption [39]. However, in order to differentiate nonlinear refraction from nonlinear absorption, one can follow division method described in Ref [40].
Separate Z-scan experiment was performed on pure DMSO solvent and found to have no contribution to nonlinearity at the incident intensity used. Hence, any contribution from the solvent to the nonlinearity of the samples is negligible. The nonlinear absorption coefficient β (cm/W) and nonlinear refractive index n 2 (cm2/W) for samples are calculated from the open- and closed-aperture normalized transmittance in Figs. 4 and 6, respectively, and they are given in Table 1. Also we noticed that values of n 2 and β increase with increasing glucose concentration. This may be due to the absorption coefficient (α) of the sample solution with zero glucose concentration being the smallest among the four concentrations, so that the n 2 and β values are the smallest for the sample solution with zero glucose concentration. The absorption coefficient (α) of the 80 mmol glucose concentration is the largest; hence, the n 2 and β values are the largest. The situation of the 29 and 50 mmol glucose concentrations is the intermediate case.
Finally, the thermally induced on-axis nonlinear refractive index of the materials can be extracted from the n 2 value obtained from the Z-scan trace using the expression [32]:
where dn/dT (K−1) is the change in the refractive index to the temperature or the thermo-optic coefficient of the material and k th is the thermal conductivity of the solvent.
Lastly, using Eq. (8) with the thermal conductivity, k th (=0.199 W m−1 K−1), the thermo-optic coefficient dn/dT of the 3,4-pyridinediamine solution with 0, 27, 50 and 80 mmol glucose concentrations has been found to be −11.47 × 10−5, −6.77 × 10−5,−6.98 × 10−5 and −7.46 × 10−5 K−1, respectively.
Figure 7 shows the linear dependence of the transmittance change ΔT p−v and ΔT (one-valley transmission) on the glucose concentration for the sample. It has also been found that the change in transmittance (i.e., peak to valley) and one-valley transmission increases with an increase in glucose concentration.
3.2 Optical limiting
The optical limiting behavior of sample solution was studied under cw laser illumination. The experimental setup is very similar to the Z-scan geometry. The optical power limiting property of the sample solution was performed by keeping the sample after the focal point of the laser beam, and the transmittances of the samples were recorded as a function of the input laser irradiance. The optical limiting behaviors were obtained by varying the input power. Before we study the optical limiting properties of sample, we can confirm that pure DMSO has no optical limiting properties. We then study the optical limiting properties of samples. Figure 8 shows the optical limiting behaviors of sample solution with different glucose concentrations, along with the corresponding transmittances. It is shown that the output–input power curves for sample solution with different glucose concentrations were nonlinear. The output power increased linearly when input power was low, while it was stable when the input power continued to increase. Accordingly, the transmittance was rapidly decreased under low incidence light intensity and decrease slowly with high incident light intensity. We also found that the limiting threshold of resultant sample is affected by glucose concentration (Table 1). As given in Table 1, the sample with 80 mmol glucose concentration showed a better optical limiting property than the samples with other concentrations, which is consistent with the result of the nonlinear optical property.
3.3 Mechanism for optical limiting
There are several different mechanisms that could explain the optical limiting behavior, such as reverse saturable absorption (RSA), two-photon absorption, nonlinear refraction and optically induced scattering [41, 42]. In our case, the optical limiting behavior appears to arise from the nonlinear refraction. Since the samples were pumped with CW laser beam, the arising nonlinearities are predominantly thermal in nature [43]. When we increased the laser power, at a certain point, the defocusing effect was pronounced, resulting in a greater part of the beam cross section being cut off by the aperture [44]. Thus, the transmittance recorded by the photodetector remains reasonably constant showing a plateau region and is saturated at a point defined as the limiting amplitude, i.e., the maximum output intensity, showing obvious limiting property. The saturated output value at which limiting occurs for the sample solution is shown in Fig. 9 for different concentrations. Figure 9 shows that the saturated output value decreases with an increasing concentration.
During the process of measuring, when the input power increases, the transmitted beam is photographed from a distance far from the sample. Figure 10 shows such photographs for 80 mmol concentration of sample solution when the input powers are 0.67, 1.04, 3.38, 5.66, 6.35, 7.12 and 7.85 mW, respectively. The photographs a, b and c show that the spot size increases with an increasing input power, which reflects the increasing output power. This explains the rapid increases in the output power for 0.67–3.38 mW input power in the limiting curve. Also, photographs d and e in Fig. 10 show that there is a small increase in the spot size. Hence, there is a little increase in the output power for 5.66–6.35 mW input power in the limiting curve. The photographs g and f show the similarity in spot size which means that the output power becomes constant.
Induced self-defocusing patterns are observed. Figure 11 shows laser spots taken for the following glucose concentrations of 0, 27, 50 and 80 mmol at 8 mW input power. As shown, the laser spot size of self-defocusing increases with increasing glucose concentration in the sample solution. For instance, the diameter of laser spots size is 4, 5.5, 7 and 8.5 mm for 0, 27, 50 and 80 mmol glucose concentrations, respectively. As the diameter of aperture photodetector is 2.5 mm, the sample with 80 mmol has a greater part of beam cross section which is cut off by the aperture; hence, they have low threshold power value, i.e., better optical limiting effect. This is because a sample with a high glucose concentration has more molecules per unit volume than that of the other samples [45]. This contributes to the laser heating which induced nonlinear effect (i.e., self-defocusing).
3.4 All-optical switching studies
The transistor–transistor logic (TTL) modulated cw 473 nm laser beam as an excitation beam modulated at 10 Hz frequency laser recorded in the pump–probe experiments on switching action of our sample, 3,4-pyridinediamine with 80 mmol glucose concentration, is shown in Fig. 12. When the pump signal laser passes through the sample, the output intensity of the probe beam from the wavelength 635 nm at 5 mW He–Ne laser gets reduced drastically (off-state). When the pump is turned off, the output probe beam regains its full to the increase in the population of triplet state [46]. The switching times are seen to be in the range of few microseconds. The relaxation of the triplet state to the ground state is forbidden, resulting in slow switching time of the molecules [47].
Figure 12 shows an oscilloscope trace of transient optical switching (below) together with the input TTL modulated pump signal (above) for the all-optical switching effect of the sample at room temperature with beam power at 12 mW. It is clear that when the beam is turned on, the intensity of the signal beam detected increases with a rising time about several milliseconds. When the beam is off, the transmitted intensity of signal beam decreases with a falling time about 10 ms. It is well known that without the control beam, 3,4-pyridinediamine with 80 mmol glucose concentration molecules is randomly oriented. The sample is optically isotropic and cannot exhibit any birefringence.
When the probe beam passes through the created lens, its optical path length undergoes a temporal change that can be observed by measuring the beam center intensity in the far field. According to this method, a lens focuses the laser beam into the sample resulting in a temperature gradient, which in turn produces a spatial gradient in the refractive index [48–51]. The transient signals of a dye compound, 3,4-pyridinediamine with 80 mmol glucose concentration, are shown in Fig. 13 for a pumping power of 12 mW at room temperature.
The heat generated in the region of absorption increases the local temperature, thereby modifying the refractive index and inducing an optical lens, which could be diverging or converging depending on the sign of ∂n/∂T, i.e., the temperature coefficient of the refractive index in the medium. The TL develops over a period of time governed by the rise time of the exciting beam and also characteristic of the thermal time constant of the medium. The transient signals of 3,4-pyridinediamine with 80 mmol glucose concentration are shown in Fig. 13 for a pumping power of 2 mW at room temperature. The red solid line corresponds to the data fitting of Eq. 4 [32] to the thermal blooming data. The fraction of absorbed energy, ϕ, is converted into heat per photon. In the case of non-luminescent samples, such as the samples studied in this work, all the absorbed energy is converted into heat, so that ϕ = 1. The values for dn/dT and the thermal diffusivity (D = K/ρC, ρ is the sample density, and C is the specific heat of the sample) for 2 mW power are 9.54 × 10−5 K−1 and 8.461 × 10−5 cm2/s, respectively. The standard error of the estimated optical properties was ±4.8999%.
4 Conclusion
The third-order nonlinear optical properties of 3,4-pyridinediamine was studied by using Z-scan technique. The effect of the glucose concentration in sample solution on nonlinear refraction index and nonlinear absorption coefficient was studied. The negative value of third-order nonlinear refraction index indicates that the sample solution shows self-defocusing effect. The mechanism of optical nonlinearity in the low-power regime is found to be predominantly of thermal origin. We also investigated the optical limiting properties of 3,4-pyridinediamine in DSMO solvent. The glucose concentration-dependent limiting threshold was observed in this sample solution. This material is expected to be a suitable candidate for sensor protection in the cw low-power regime. Further, our experiments indicate that the nonlinear absorption is due to RSA and the 3,4-pyridinediamine samples exhibit good optical power limiting behavior. We present the experimental investigation of all-optical switching exhibited by 3,4-pyridinediamine with 80 mmol glucose concentration. Hence, the metal–organic material investigated here emerges as a promising material for new photonic devices. Thermal lens spectrometry of 3,4-pyridinediamine with 80 mmol glucose concentration have been studied using TL technique. In this technique, a pump and a probe beam were aligned collinearly. A localized change in the refractive index of the sample due to the thermal heating produced a thermal lens, which was then detected by studying the focusing and defocusing of the pump and probe beam. Thermo-optic coefficients have been determined by thermal lens (TL) technique and Z-scan and it was found to be temperature dependent. The thermo-optic coefficients value of 3,4-pyridinediamine with 80 mmol glucose concentration obtained by this technique is in agreement with the value obtained by Z-technique.
References
Z. Hong Bing, C. Wen Zhe, W. Min Quan, Mater. Lett. 59, 1395 (2005)
P. Prem Kiran, D.R. Reddy, B.G. Maiya, D.N. Rao, Opt. Mater. 21, 565 (2002)
K. Sendhil, C. Vijayan, M.P. Kothiyal, Opt. Laser Technol. 38, 512 (2006)
X.Q. Yang, S.W. Qi, K. Chen, C.P. Zhang, J.G. Tian, Q. Wu, Opt. Mater. 27, 1358 (2005)
K. Sathiyamoorthy, C. Vijayan, M.P. Kothiyal, Opt. Mater. 31, 79 (2008)
M.D. Zidan, A.W. Allaf, Z. Ajji, A. Allahham, Opt. Laser Technol. 42, 600 (2010)
M.D. Zidan, A.W. Allaf, Z. Ajji, A. Allahham, Opt. Laser Technol. 42, 531 (2010)
R. Rathnagiri Krishnamurthy, R. Alkondan, Opt. Appl. 40, 187 (2010)
A.Y. Nooraldeen, M. Palanichant, P.K. Palanisamy, J. Nonlinear Sci. 7, 290 (2009)
H. Ali Badran, A. Imran, Q.M.A. Hassan, Optik 127, 5301 (2016)
H.A. Badran, Q.M.A. Hassan, A. Imran, Mater. Sci. Mater. Electron 26, 5958 (2015)
G. Wang, W. Sun, Phys. Chem. B 110, 20901 (2006)
Y.Z. Gu, Z.J. Liang, F.X. Gan, Opt. Mater. 17, 471 (2001)
H.A. Badran, Q.M. Hassan, A.Y. Al-Ahmad, C.A. Emshary, Can. J. Phys. 89, 1219 (2011)
G.J. Zhou, W.Y. Wong, Chem. Soc. Rev. 40, 2541–2566 (2011)
Q. Zheng, S.K. Gupta, G.S. He, L.S. Tan, P.N. Prasad, Adv. Funct. Mater. 18, 2770 (2008)
Q. Zheng, G.S. He, P.N. Prasad, Chem. Phys. Lett. 475, 250 (2009)
H. Abdeldayem, D.O. Frazier, M.S. Paley, Appl. Phys. Lett. 82, 1120 (2003)
C.P. Singh, S. Roy, Opt. Commun. 218, 55 (2003)
S. Roy, K. Kulshrestha, M. Prasad, I.E.E.E. Trans, Nanobioscience 8, 83 (2009)
Z.G. Zang, W.X. Yang, J. Appl. Phys. 109, 103106 (2011)
Z.G. Zang, Opt. Commun. 285, 521 (2012)
K.B. Manjunatha, R. Dileep, G. Umesh, B. Ramachandra Bhat, Opt. Mater. 35, 1366 (2013)
Z.G. Zang, Y.J. Zhang, J. Mod. Opt. 59, 161 (2012)
M. Sheik Bahae, A.A. Said, T. Wei, D.J. Hagan, E.W. Van Stryland, IEEE J. Quant. Electron. QE-26, 760 (1990)
H.A. Badran, Results Phys. 4, 69 (2014)
A.A. El-Fadl, G.A. Mohamad, A.B. Abd El-Moiz, M. Rashad, Phys. B 366, 44 (2005)
M. Sheik-Bahae, D.C. Hutchings, D.J. Hagan, E.W. Van Stryland, IEEE J. Quantum Electron. 27, 1296 (1991)
R. DeSalvo, A.A. Said, D.J. Hagan, E.W. Van Stryland, M. Sheik-Bahae, IEEE J. Quantum Electron. 32, 1324 (1996)
H.A. Badran, A.Y. Taha, A.F. Abdulkader, C.A. Emshary, J. Ovonic Res. 8, 161 (2012)
P.C. Haripadmam, M.K. Kavitha, H. John, B. Krishnan, P. Gopinath, Appl. Phys. Lett. 101, 0711031 (2012)
H.A. Badran, Appl. Phys. B 119, 319 (2015)
A.N. Dhinaa, A.Y. Nooraldeen, K. Murali, P.K. Palanisamy, Laser Phys. 18, 1212 (2008)
Q.M. Ali, A.Y. Al-Ahmad, M.F. Al-Mudhaffer, H.A. Badran, Rom. J. Phys. 58, 962 (2013)
D. Dorranian, Y. Golian, A. Hojabri, J. Theor. Appl. Phys. 6, 1 (2012)
H.A. Badran, A.Y. Al-Ahmad, Q.M. Ali, C.A. Emshary, Pramana j. Phys. 86, 135 (2016)
H.A. Badran, H.A. Sultan, Q.M. Ali Hassan, J. Mater. Sci. Mater. Electron 27, 6735 (2016)
F.A. Al-Saymari, H.A. Badran, A.Y. Al-Ahmad, C.A. Emshary, Indian J. Phys. 87, 1153 (2013)
H.A. Badran, A.Y. AL-Ahmad, M.F. AL-Mudhaffer, C.A. Emshary, Opt. Quant. Electron. 47, 1859 (2015)
H.A. Badran, H.F. Hussain, K.I. Ajeel, Optik 127, 5301 (2016)
G.S. He, B.A. Reinhardt, J.C. Bhatt, A.G. Dillard, G.C. Xu, P.N. Prasad, Opt. Lett. 20, 435 (1995)
F. Li, Q. Guang Zheng, G. Yang, N. Li Dai, P. Xiang Lu, Mater. Lett. 62, 3059 (2008)
A.Y. Al-Ahmad, Q.M.A. Hassan, H.A. Badran, K.A. Hussain, Opt. Laser Technol. 44, 1450 (2012)
H.A. Badran, K.I. Ajeel, H. Gazy, Lazim. Mater. Res. Bull. 76, 422 (2016)
A.Y. AL-Ahmad, M.F. AL-Mudhaffer, H.A. Badran, C.A. Emshary, Opt. Laser Technol. 54, 72 (2013)
C.P. Singh, K.S. Bindra, B. Jain, S.M. Oak, Opt. Commun. 245, 407 (2005)
F.Z. Henari, J. Opt. A: Pure Appl. Opt. 3, 188 (2001)
Q.M. Ali, H.A. Badran, A.Y. Al-Ahmed, Chin. Phys. B 22, 1140291 (2013)
J. Shen, A.J. Soroka, R.D. Snook, J. Appl. Phys. 78, 700 (1995)
H.A. Badran, K. Abd-Aladil, H. Gazy Lazim, A.Y. Al-Ahmed, Mater. Sci. Mater. Electron 27, 2212 (2016)
A.O. Marcano, C. Loper, N. Melikechi, Appl. Phys. Lett. 78, 3415 (2001)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Badran, H.A., Abul-Hail, R.C., Shaker, H.S. et al. An all-optical switch and third-order optical nonlinearity of 3,4-pyridinediamine. Appl. Phys. B 123, 31 (2017). https://doi.org/10.1007/s00340-016-6607-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00340-016-6607-5