Abstract
An efficient application of fast remote diagnostics for carbon material (CM) bulk particles was demonstrated. Porous layers of CM particles with different oxidation levels were characterized by self-action of picosecond laser pulses at 1064 nm. Nitrogen adsorption, Boehm titration, and thermal analysis of the oxidized CMs revealed diverse specific surface area \(S_\mathrm {BET}\), reasonable surface acidity, and high concentration of surface oxygen-containing groups. Dense CM porous layers showed a monotonous reduction of the absorptive nonlinear optical (NLO) response efficiency versus the oxidation level with characteristic magnitude Im(\(\chi _\mathrm{C}^{(3)})\sim 10^{-10}\) esu for the carbon particles fraction. The obtained Im(\(\chi _\mathrm{C}^{(3)})/S_\mathrm {BET}\) ratio remains approximately constant, which indicates the certain proportion between the absorptive NLO response efficiency and the specific surface area. We suggest to use Im(\(\chi _\mathrm{C}^{(3)})\) as a figure of merit for carbons subjected to the oxidation—the route to enhance the CM surface reactivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nowadays, the micro- and nanosize carbon materials (CMs), e.g., nanotubes, graphenes, fullerenes, and their derivatives, deserve their applications in electronics, sensors, and energy-related systems [1–4]. The CMs functionalization is widely used to obtain advanced nanoobjects from the surface precursors [5, 6]. Particular attention should be paid to the oxidation reactions and oxidized CMs [7, 8]. For example, graphene oxide (GO) has a large amount of oxygen-containing functional groups of different acidity [9]. These groups change surface interface and effect on the CMs ability to be dispersed in polar and nonpolar liquids. They also represent the surface sites for covalent functionalization and can be removed partially by reduction that restores \(\pi\) bonding of the conjugated matrix [10]. All the mentioned properties of the oxidized CMs attract much attention via the promising possibility of novel materials designed for miscellaneous applications with diverse modification of their functionalities [11, 12]. Thus the elaboration of new express methods for the resulted oxidized CMs diagnostics is important [12]. Recently the nonlinear optical (NLO) properties of carbon dots, including organophilic and hydrophilic [13], boron-doped [14], and natural carbon [15] nanoparticles (NPs), were reported. The variations of the photoinduced absorption efficiency for different carbons NPs allotropes were shown [13, 16]. It is promising to use this kind of response as a figure of merit of carbon media. According to this, we suggest the absorptive NLO response readout as a diagnostic technique providing the comparative assessment of the porous CMs oxidized surfaces. The presented studies are based on the high sensitivity of the NLO methods to the intrinsic defects in solid state materials [17]. In this paper, the surface characteristics of the CMs subjected to the oxidation treatment were monitored with pulsed laser radiation self-action effects at 1064 nm as nondestructive remote testing. The obtained results were compared with the reference data for the nanosized carbons.
2 Experiments
A lignocellulosic biomass was used as a source material to prepare CM according to the route reported in [18]. This kind of new CMs from natural renewable resources is characterized by the developed texture and the tunable surface chemistry that provides the surface reactivity control.
The prepared initial carbon material CM0 (2 g) was refluxed with 60 ml of 5, 10, and 30 mass% nitric acid (\(\hbox {HNO}_3\)) for 2 h. Decanted oxidized CM was washed with distilled water to pH = 6.5 and air-dried at 120 °C. The samples were assigned as CM0, CM5, CM10, and CM30, where the number corresponds to mass% of the used \(\hbox {HNO}_3\) (\(\omega\)(\(\hbox {HNO}_3\))). The nitrogen adsorption–desorption data for these samples were collected on a Quantachrome NovaWin2 porosimeter at −196 °C. Brunauer–Emmett–Teller specific surface area \(S_\mathrm {BET}\) was determined from the adsorption isotherms with the relative error less than 10%. The pore size distribution (PSD) versus the pore width was calculated within the carbon—N2 2D–NLDFT model by the means of Micromeritics SAIEUS program (www.nldft.com). The total open pore volume \(V_\mathrm {S}\) was measured by the isopiestic method via water vapor sorption at 25 \(^\circ\)C [19]. The bulk density \(\gamma\) was found by the weighting of the presets compact volume. The adsorption and pycnometric data were used to estimate the gravimetric porosity p. The concentration of the surface functional groups C fg was found from the data of Boehm titration at different pKas [20, 21]. The concentrations of carbon C(CO2) and C(CO) oxides released by the thermal destruction of the surface functional groups [22, 23] were measured by the simultaneous thermal analysis (TA) technique—a combination of the thermogravimetric (TG) and thermal programmed desorption (TPD) methods [24, 25]. The outlet gas composition was screened quantitatively by the infrared (IR) spectroscopy. The heat capacity of CM0 was measured by differential scanning calorimetry (DSC) with a model Q2000 instrument.
The absorptive CM NLO response was measured via the self-action of the picosecond range laser pulses (42 ps FWHM) at 1064 nm with the repetition rate of 15 Hz. The experimental technique is described in [26]. We studied photoinduced transmittance variation of the porous CMs powder layers that contained the CM grains within 50–100 \(\mu\)m size range. The powder was put into the bed with ϕ \(\sim 8\) mm in the Teflon film placed between two glass microscope slides (Marienfeld, \(76\times 26\times 1\) mm\(^3\)). The film thickness \(d = 340\) μm was chosen to provide an optical homogeneity of the CM layer with sufficient for the registration transmittance level. The sample was positioned directly at 1 cm aperture of the photodetector to avoid scattering extinction impact by the almost total collection of the transmitted light.
The photoinduced variation of the total transmittance T versus the peak laser intensity I for the CM5 porous layer due to the self-action of the picosecond laser pulses at 1064 nm. Each point corresponds to the data averaged over 25 laser shots. The photographs of the CM5 layer, before and after ablation, are presented in the upper and the lower insets, correspondingly
At the preliminary stage, the samples were positioned at 16 cm after the focusing lens, with a focal length of 11 cm. The laser beam spot at the sample was about 0.5 mm in order to perform representative optical response spatial averaging over the inhomogeneous CM layer cross-section. The geometry of the experiment provided the photoinduced transmittance variation T(I) acquisition in the wide peak laser intensity I range 10–900 MW/cm\(^2\).
The typical experimental T(I) dependence for the CM5 layer is presented in Fig. 1. We have observed two regimes of the response: (A) reversible photodarkening \(\Delta T < 0\) for \(I < 600\) MW/cm\(^2\) and (B) irreversible bleaching \(\Delta T > 0\) for the higher intensities. For the (A) regime, the T(I) response was reversible for the consequent peak intensity rise/reduction. Obviously, this response originates from the photoinduced absorption in the CM porous layer. The sharp irreversible rise of the laser beam transmittance \(\Delta T(I) > 0\) was caused by the carbon layer ablation [27, 28]. The damaged after ablation of the CM5 layer (marked with the dashed circle) is clearly seen in the lower inset of Fig. 1.
In the (B) regime, we have observed the white flashes that transformed into a uniform “sparkling” emission from the beam spot. It corresponds to a blackbody radiation at \(\sim\)4500 °C [29] that is consistent with the microplasma formation in the carbon particles. To our point of view, the sharp rise of the total transmittance above the ablation threshold can be explained with two competitive mechanisms: (1) much higher quantum yield of the silicon photodetector to the visible emission of the carbon grains in comparison with the same at 1064 nm; (2) voids formation due to the ablation impact (see the insets in Fig. 1) that leads to the data acquisition at a system saturation.
In order to study the reversible absorptive NLO response below the ablation threshold, the rest of the study of the T(I) was performed with the CM porous layers position at 18 cm after the focusing lens. The corresponding beam spot at the sample was \(\sim\)0.8 mm. It provides the laser peak intensity range from 2 to 600 MW/cm\(^2\). Each experimental T(I) dependence (Fig. 2a) comprises ca. 5000 laser shots dataset. The maximum energy per pulse did not exceed 0.2 mJ. The low 15 Hz repetition rate of the single laser shots and high porosity of the CM layers prevents the sample from overheating during the experiment. For the CM0 sample with the highest optical absorption, we have simulated the magnitude of the temperature variation due to the laser shot impact with peak intensity \(I = 600\) MW/cm\(^2\) by the formula \(\Delta T_{\mathrm{peak}} = I\cdot \tau \cdot (1-\mathrm{exp}(-\alpha _\mathrm {_{eff}}d))/(C_\mathrm{p}\cdot d\cdot \gamma )\), where \(\Delta T_{\mathrm{peak}}\) is the peak temperature rise, \(\tau\) is the pulse duration (42 ps), \(C_\mathrm{p}\) is the specific heat capacity at 25 \(^\circ\)C from the DSC experiments, \(C_\mathrm{p}\) = 0.84 J/(g K). The estimated \(\Delta T_{\mathrm{peak}}\) does not exceed 2 \(^\circ\)C. Furthermore, the calculated heat dissipation time \(\tau _{_\mathrm{T}} =d^2 /( 4 \pi ^2 D_{_\mathrm{T}})\), where \(D_{_\mathrm{T}}\) is about 27 ms, that is 3 times less than the laser pulse repetition period; T is the thermal diffusion coefficient. The estimations were verified by remote IR thermometry measurements. The CMs average temperature increase was \(\le\)3 °C in the reversible NLO response range (A).
The photoinduced variations of the total transmittance due to the self-action of the picosecond laser pulses at 1064 nm: a the absolute magnitude of the total transmittance versus the peak laser intensity T(I); b the normalized \(T(I)/T_0\) smoothed data, where \(T_0\) is the transmittance in a linear regime (see Table 2), for the initial CM0 and the oxidized CMs
Analysis of the experimental data (Fig. 2a) has shown the CM layers transmittance T(I) variation in the studied excitation range to be slow. At the initial \(I \le 2\) MW/cm\(^2\) range, the total transmittance was approx. constant (\(T_0\)) that corresponded to the linear regime of the optical transmittance.
The response of the studied CMs high-porosity layers (\(p \ge 72\)%) has been modeled within the Looyenga effective media approximation [30]. The approximation has been approved to describe the response of the air-particles mixtures [31].
The dielectric susceptibility of the porous layer \(\varepsilon _\mathrm {eff}\) can be decomposed into the contributions of the bulk carbon fraction \(\varepsilon _{_\mathrm{C}}\) and of the voids \(\varepsilon _\mathrm {v} \equiv 1\):
According to the mentioned approach, the refractive index \(n_\mathrm {eff}\) of the CM porous layers has been estimated within the account of \(n_\mathrm {_\mathrm{C}}\) = 2.0 [32] for the bulk initial CMs:
Based on \(n_\mathrm {eff}\) magnitudes, we derived the layer transmittance \(T_\mathrm {eff}\) and the effective absorption coefficient of the CM porous layer \(\alpha _\mathrm {_{eff}}\):
by scaling the experimental values of \(T_0\) on the reflection losses at the couples of interfaces of air-glass (\(R_1\)) and glass-CM layer (\(R_2\)):
We estimated the effective cubic NLO susceptibility Im(\(\chi _\mathrm {eff}^{(3)}\)) of the CM porous layer that characterized an efficiency of the photoinduced absorption coefficient as \(\Delta \alpha _\mathrm {eff}= \alpha _\mathrm {eff}(I) - \alpha _\mathrm {eff} = \beta _\mathrm {eff} I \sim \mathrm {Im}(\chi _\mathrm {eff}^{(3)})I\). The transmittance variation T(I) can be described by the following expression with an account of the spatial and the temporal averaging over the Gaussian spatial and temporal profile of the laser radiation [26]:
where \(L_\mathrm {eff} = (1+\exp (-\alpha _\mathrm {eff}d))/\alpha _\mathrm {eff}\) is the effective self-action length [33]. A fit of the experimental T(I) data within Eq. 5 provides magnitudes of \(\beta _\mathrm {eff}\) and Im(\(\chi _\mathrm {eff}^{(3)}\)).
The resulting optical absorption of the bulk carbon fraction \(\alpha _\mathrm {C}(I)\) can be estimated with an account of the linear \(\alpha _\mathrm {C}\) and the photoinduced \(\beta _\mathrm {C}I\) absorptions contribution:
In the Looyenga effective media approximation:
the coefficients \(\alpha _\mathrm {C}\) and \(\beta _\mathrm {C}\) are proportional to the effective corresponding ones of the porous layer.
The imaginary part of the cubic NLO susceptibility Im(\(\chi ^{(3)}\)) (or effective two-photon absorption coefficient \(\beta _{\mathrm {eff}}\)) characterizes the efficiency of the absorptive NLO response in the condensed media [33]. For the carbon materials, particularly for GO, NLO response is related to the \(\hbox {sp}^2\) and \(\hbox {sp}^3\) hybridized regions [34]. The corresponding processes were described in details in [34]. The variation of the concentration of the \(\hbox {sp}^2\) and \(\hbox {sp}^3\) domains within the chemical reduction could lead to significant changes in the absorptive NLO response of the GO. Thus, in the paper, we used Im(\(\chi ^{(3)}\)) for the characterization of the CMs functionalization (\(\hbox {sp}^3\) hybridization contribution) due to the oxidization with different \(\hbox {HNO}_{3}\) concentration.
3 Results and discussion
Typically, carbons react with hot concentrated \(\hbox {HNO}_3\) solutions. The reaction causes the formation of acidic oxygen-containing surface groups and accompanies the partial destruction/dissolution of the carbon matrix. It reduces the \(S_\mathrm {BET}\) and \(V_\mathrm {S}\) magnitudes due to the removal of the carbon bulk fraction via gasification or washing out with the acid.
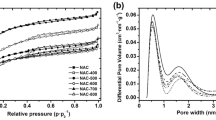
Table 1 presents the effect of the oxidation on the surface acidity and the texture of the microporous CM0 (for isotherms see Fig. S1a in the Supplementary Material). The highest one was observed for the CM30 sample prepared with 30 mass% \(\hbox {HNO}_3\): the \(S_\mathrm {BET}\) reduces \(\sim 3\) times on the background of \(\sim 17\)% \(V_\mathrm {S}\) decrease. In the CM5 and CM10 samples, the weaker texture changes were obtained: the reduction was \(\le\)40 and \(\sim\)8% for the \(S_\mathrm {BET}\) and \(V_\mathrm {S}\) correspondingly. The PSD performed by DFT analysis of the nitrogen adsorption isotherms showed that the oxidation suppresses the micropores contribution into the total porosity (see Fig. S1b). Nevertheless, the heterogeneous micropore size distribution is maintained with two different peaks at 5.5–6 and 13–15 Å. For the CM5 and CM10, sharp reduction of a number of micropores in 4–10 Å range is accompanied by an increase of that in 11–24 Å range. For the CM30, the significant micropores reduction was observed for the both ranges. The difference among the CM0 and the oxidized CMs PSDs observed over the mesopore range is not crucial due to the preferred microporosity of the CMs.
According to Table 1, the surface of CM0 involves \(\sim\)1 mmol/g of oxygen-contacting functionalities. The surface oxidation with nitric acid produces carboxylic (Cb), anhydride (A) and lactonic (L), and phenolic (Ph) groups that manifest weak acidic properties. The use of more concentrated solutions of \(\hbox {HNO}_3\) resulted in higher yield of groups, from 2.5 up to 9.0 mmol/g totally. This yield was determined from a significant mass loss on TG/DTG curves and C(CO2)/C(CO) concentrations monitored by the TPD-IR technique (for the details see Fig. S2a and b in the Supplementary Material). The TPD data provide the groups percentage distribution as 16–20% of Cb, 35–47% of A–L and 33–45% of Ph ones. Surprisingly, the ratio of the groups depends weakly on the oxidant concentration. Indeed, A–L and Ph groups dominate at the surface of the oxidized CMs. It corresponds to about 2.5–5.5 times increase of the acidic oxygen-containing groups concentration that is typical for the slight matrix destruction. A further rise of the mentioned groups’ concentration causes partial destruction of the microporous structure of the matrix for the CM30. Its C fg values determined from Boehm titration data display up to 5–20 times increase versus the initial CM0 ones at the each range of pKa. As expected, the provided moderate oxidation prevents selective functionalization in contrast with the barothermal oxidation [35]. Summarizing our findings, we can conclude that the oxidation of the CMs results in (1) the microporosity reduction and (2) reactivity level rise by the surface tailoring with diverse oxygen functionalities.
The effect of the CM oxidation on the total transmittance T(I) variation was examined versus the peak laser intensity. Figure 2a shows that the oxidation of the CM results in an increase of the total transmittance, which is much more pronounced when a reduction of p, see Table 2 for numeric data. We have attributed the transmittance \(T_0\), at the initial intensity range \(I < 2\) MW/cm\(^2\), to a linear regime that decreases with an increase of the magnitude of I. In order to substrate a manifestation of the photoinduced effects from the linear response of a background, we normalized T(I) on a linear transmittance, \(T_0\) (Table 2). The smoothed \(T(I)/T_0\) dependencies are shown in Fig. 2b, the relative error of the curves is about ±0.2% (for the details see Supplementary material, SM3).
Analysis of the presented data reveals three characteristic regimes of the carbons photoinduced absorption response (Fig. 2b). Each regime can be specified by the corresponding tangent (short-dash) lines that corresponded to the average slowly-varied slopes of the experimentally obtained dependency \(T(I)/T_0\) for the CM0 sample. A switch from one regime to another occurs at peak intensities \(I_\mathrm {s1}\) and \(I_\mathrm {s2}\) determined by the lines interception points. The first regime (1) is observed at \(I< I_\mathrm {s1} \sim 90\) MW/cm\(^2\) with efficient photodarkening manifestation \(\Delta T/T_0 \sim\)6–10% and Im\((\chi ^{(3)}_\mathrm {eff}) \sim 10^{-11}\) esu (see Table 3). The second one (2) in \(I_\mathrm {s1}< I < I_\mathrm {s2} \sim\)400–500 MW/cm\(^2\) range corresponds to the photoinduced absorption saturation of the initial NLO response mechanism. It means almost permanent transmittance within the experimental errors range for the CM0 and about the order of magnitude reduction of the photoinduced absorption efficiency in the oxidized CMs. The third (3) regime \(I > I_\mathrm {s2}\) corresponds to the enhancement of the NLO absorptive response. We kept peak laser intensity \(I < 600\) MW/cm\(^2\) in order to avoid CMs layers ablation impact (see Fig. 1) and to maintain reversibility of the NLO response.
The calculated values of \(n_\mathrm {_{eff}}\) of the initial and oxidized CMs are presented in Table 2. Their magnitudes and the linear transmittances \(T_0\) of the samples are increasing with the porosity reduction that agreed with the results reported in [32, 36]. We have estimated the linear transmittance of the porous layer \(T_\mathrm {eff}\) (Eq. 3), the optical absorption coefficients of the CM porous layers \(\alpha _\mathrm {_{eff}}\) (Eq. 3, the relative error \(\sim 1.5\)%), their bulk carbon fraction \(\alpha _\mathrm {_\mathrm{C}}\) (Eq. 7, relative error \(\sim\)4%), and the ratio of the values of \(\alpha _\mathrm {_\mathrm{C}}\) to \(S_\mathrm {BET}\) (see Fig. 3). The detailed data treatment for the sample CM5 is presented in SM3. One can see that the optical absorption of the carbon particles is \(\alpha _\mathrm {_\mathrm{C}}/\alpha _\mathrm {_{eff}}\sim 4.5\) times higher than that for their porous layer. The oxidation treatment decreases the porosity on \(\sim\)5% and reduces the optical absorption \(\alpha _\mathrm {_\mathrm{C}}\) at about 1.8 times.
For the studied CMs samples, the photoinduced absorption occurs efficiently only at so-called efficient self-action length \(L_\mathrm {eff} < d\) that is shorter than the porous layer thickness. Thus, we have observed about 40% reduction of the relative photoinduced transmittance variation \(\Delta T/T_0\) against the three-time rise of the linear transmittance \(T_0\) in the CM30 versus the initial CM0 sample.
We have estimated the NLO absorption coefficient \(\beta _\mathrm {_{eff}}\) and Im(\(\chi _\mathrm {_{eff}}^{(3)}\)) in the range (1) according to the approach described in [26] with the relative errors \(\sim\)8.0% and 9.3%, respectively (see SM3 for details). It was shown that the obtained reduction of \(\beta _\mathrm {_{eff}}/\mathrm {Im}(\chi _\mathrm {_{eff}}^{(3)})\) does not exceed the factor of 3.0/2.5 for the CM30 sample versus the CM0 porous layer. The aforementioned reduction of \(\beta _\mathrm {_{C}}/\mathrm {Im}(\chi _\mathrm {_{C}}^{(3)})\) is more pronounced \(\sim\)3.5/4.0 for the NLO response of the corresponding carbon particles fraction. The analysis of the decrease rate of the photoinduced absorption to linear response regime one shows the drastic decrease of the \(\beta _\mathrm {_\mathrm{C}} /\alpha _\mathrm {_\mathrm{C}}\) ratio for the samples of CM10 and CM30, that are of the higher degree of oxidation.
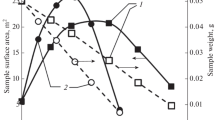
In order to estimate the impact of the specific surface area \(S_\mathrm {BET}\) variation on the linear and photoinduced light absorption, the ratios of \(\alpha _\mathrm {C}/S_\mathrm {BET}\) and Im\((\chi ^{(3)})/S_\mathrm {BET}\) were analyzed (see Fig. 3). The relative errors of the ratios are \(\sim\)11 and \(\sim\)22%, respectively.
If one compares the effect of the oxidation on the value of \(S_\mathrm {BET}\) and the optical absorption at 1064 nm, it can be clearly seen that the ratio of \(\alpha _\mathrm {C}/S_\mathrm {BET} \sim 4.4\times 10^{-5}\) g/cm\(^3\) is similar for the initial CM and CM5 that was oxidized with dilute \(\hbox {HNO}_3\). The difference in the oxidizing strength of diluted and concentrated nitric acid can be seen, on the example of the more oxidized CM10 and CM30 samples. Their \(S_\mathrm {BET}\) values decrease faster than optical absorption. This fact can be considered as strong evidence of the bulk optical absorption response versus the interface contribution.
The dependencies of the linear and the photoinduced absorption responses \(\alpha _\mathrm {C}/S_\mathrm {BET}\) and Im\((\chi ^{(3)})/S_\mathrm {BET}\) on variations in the surface area \(S_\mathrm {BET}\) of the CMs produced by the treatment with different \(\hbox {HNO}_3\) concentration \(\omega\)(\(\hbox {HNO}_3\))
We have obtained the most promising result for the Im\((\chi _\mathrm {_{C}}^{(3)})/S_\mathrm {BET}\) ratio variation of CMs studied. It remains close to the constant value of 0.25 within the experimental error range—the deviation that does not exceed 20% for the CMs of different degree of oxidation (see Fig. 3). This is indicated by a certain proportion between the absorptive NLO response and the \(S_\mathrm {BET}\) value. This fact supports the suggestion that the photoinduced variations of the absorption coefficient at wavelength 1064 nm (energy quanta 1.17 eV) are related to the CM surface states.
For the given excitation wavelength, we have compared the efficiency of the NLO response of the studied CMs samples with experimental data for the colloids of the different carbon-based nanoparticles (NPs) [13, 16, 37–39] due to the self-action of the pico- and nanosecond laser pulses. In order to estimate the contribution of the carbon fraction in the diluted colloids into the macroscopic NLO response, we applied an effective media approximation. For the two-component system, within the approximation of the nonlinear splitting [40] and negligible contribution of the solvent, \(\chi _\mathrm {_{sol}}^{(3)}\) in its transparency range, the NLO response of the composite can be given as:
where \(F=\partial \varepsilon _\mathrm {_{eff}}/\partial \varepsilon _\mathrm {_\mathrm{C}}\) and \(f_\mathrm {_{C}}\) is the volume fraction of carbon NPs. One can find the expression for F within the Maxwell–Garnett approximation for dilute solutions in [40].
According to the described approach, we have estimated the Im\((\chi _\mathrm {_{C}}^{(3)})\) magnitudes for the different carbon NPs (see Table 4). One can see that the obtained magnitudes for the C\(_{60}\) fullerene colloids in toluene, organophilic C-dots in chloroform and nanodiamonds in N,N-dimethylformamide (DMF) are in the range of \(\sim (1.2-7.5)\times 10^{-10}\) esu. It enhances for graphene-DNA in butanol \(\sim 1.1\times 10^{-9}\) esu, a single layer graphene oxide (GO) in water \((3.4-4.8)\times 10^{-9}\) esu and \(\sim 8.4\times 10^{-9}\) esu for few layered GO in DMF within nanosecond laser pulses excitation. So, we have experimentally proved that the photoinduced absorption efficiency of the bulk carbon particles Im\((\chi _\mathrm {_{C}}^{(3)} ) \sim (1-4)\times 10^{-10}\)esu (see Table 3) coincides with the NLO efficiency of the aforementioned carbon NPs response. The fact can reflect the same origin of the nonlinear response mechanism for different carbon materials, including that of NPs.
In order to explain the obtained versatility of the different carbon material NLO response efficiency we refer to the known GO tendencies. The GO 2D-network consisting of \(\hbox {sp}^2\) and \(\hbox {sp}^3\) hybridized regions, resulting in a heterogeneous electronic structure that features are both \(\pi\)-states from the \(\hbox {sp}^2\) carbon sites and a large energy gap between the \(\sigma\)-states of the \(\hbox {sp}^3\) bonded carbons [34]. The ultrafast relaxation dynamics and NLO study show that such electronic structure causes the manifestation of complex photoinduced absorption processes within a wide intensity range. In near IR excitation range the saturable absorption of the charge carriers from the \(\hbox {sp}^2\) hybridized clusters dominate in NLO response at low pump intensities, while the impact of the two-photon absorption in the \(\hbox {sp}^3\) hybridized domains—on the photoinduced transient absorption within excitation level rise [40]. This concept gives the possibility for the engineering of the linear and NLO properties of the carbon-based materials by manipulating the size, shape, and relative fraction of the different absorption domains [41].
The Raman spectroscopy is a versatile technique used to determine the number of the layers, nanosheet sizes, and the quality of edges in the GO [42–44]. It is due to the extremely efficient sensitivity of the phonons to the size effect in the CMs, while the NLO response of the delocalized electrons is similar in a wide scale range. The size effect manifestation is dealing with plasmonic oscillations in the far IR domain confinement at about tenth nm size nanosheets that produce an efficient nonlocal NLO response [45]. The last one is extremely sensitive to the nanosheet shape and its termination [46]. It was shown that a rise in the GO layers number efficiently impacts the electronic structure of the system that results in the absorptive NLO response enhancement [16]. Typically, in visible and near IR ranges, the absorptive NLO response of the CMs is determined by \(\hbox {sp}^2\) and \(\hbox {sp}^3\) hybridized domains fraction contribution [34]. It was shown that the reduction of the GO was used to tune the NLO properties of atomically thick graphene sheets [47].
Comparison of the data presented in Table 4 has shown that the NLO absorption efficiency Im\((\chi _\mathrm {_\mathrm{C}}^{(3)})\) in the studied oxidized CM powder porous layers is about 10 times less than the corresponding one of the GO-based materials at 1064 nm. It can be explained by at least two reasons: (1) inverse \(\hbox {sp}^2\) to \(\hbox {sp}^3\) hybridized domains fraction in the oxidized CM powder and GO NPs; (2) the specific surface to the bulk ratio is much smaller in the studied CMs grains in comparison with that in the GO NPs. To our point of view, the proposed control via oxidation/reduction routes gained in the efficient response of the few layered GO (see Table 4).
4 Conclusion
Summarizing the findings, we can conclude that for the first time the remote fast nonlinear optical diagnostics is used for characterization of the CMs bulk particles. The optically dense CMs porous layers were examined by a technique that is based on the self-action of the picosecond range laser pulses at 1064 nm. We have presented the effect of carbons oxidation on their photoinduced total transmission and related optomechanical characteristics. The linear optical absorption coefficient C and the photoinduced absorption efficiency Im\((\chi _\mathrm {_\mathrm{C}}^{(3)})\) are sensitive to the oxidation intensity of the carbons. The Im\((\chi _\mathrm {_\mathrm{C}}^{(3)})\) can be considered as a figure of merit of oxidized carbon media.
The oxidation decreases the porosity on \(\sim\)5% and reduces the values of \(\alpha _\mathrm {_\mathrm{C}}\) at about 1.8 times on the drastic reduction of the BET surface area from 1350 to 470 m\(^2\)/g. This fact can be considered as a strong evidence of the linear bulk optical absorption response versus the interface contribution. Accurate evaluation of the optical linear and photoinduced absorption coefficients shows that the higher CM oxidation level resulted in an essential decrease of the \(\beta _\mathrm {_\mathrm{C}}/\alpha _\mathrm {_\mathrm{C}}\) ratio. The most interesting result we have obtained is that the ratio Im\((\chi _\mathrm {_\mathrm{C}}^{(3)})/S_\mathrm {BET}\) remains close to the constant value. This observation indicates the certain proportion between the absorptive NLO response and the CM specific surface.
The photoinduced absorption efficiency of the bulk carbon particles Im\((\chi _\mathrm {_\mathrm{C}}^{(3)})\) is found to be of the order of ~10−10 esu. We have experimentally proved that the magnitude coincides well with the NLO efficiency of the nanosized carbons response reference data. The fact can reflect the same origin of the nonlinear response mechanism for different carbon materials, including NPs.
References
M. Notarianni, J. Liu, K. Vernon, N. Motta, Beilstein J. Nanotechnol. 7, 149 (2016)
S. Pilehvar, K. De Wael, Biosensors 5, 712 (2015)
A. Fraleoni-Morgera, Small 7, 321 (2011)
X. Wang, Y. Shi, in Nanofabrication and its Application in Renewable Energy, ed. by G. Zhang (RSC, London, 2014), pp. 1–30
B. Charleux, C. Copret, E. Lacte, Chemistry of Organo-Hybrids: Synthesis and Characterization of Functional Nano-Objects (Wiley, Hoboken, 2014)
C.-H. Andersson, H. Grennberg, Eur. J. Org. Chem. 26, 4421 (2009)
V.K. Thakur, M.K. Thakur, Chemical Functionalization of Carbon Nanomaterials: Chemistry and Applications (CRC, Boca Raton, 2016)
L. Meng, C. Fu, Q. Lu, Prog. Nat. Sci. 19, 801 (2009)
J. Chen, Y. Zhang, M. Zhang, B. Yao, Y. Li, L. Huang, C. Li, G. Shi, Chem. Sci. 7, 1874 (2016). doi:10.1039/C5SC03828F
A. Ganguly, S. Sharma, P. Papakonstantinou, J. Hamilton, J. Phys. Chem. C 115, 17009 (2011)
Y. Dai, Z. Li, J. Yang, ChemPhysChem 16, 2783 (2015)
H. Grennberg, Carbon nanotubes and graphene, in Organic Synthesis and Molecular Engineering, ed. by M.B. Nielsen (Wiley, Hoboken, 2013)
P. Aloukos, I. Papagiannouli, A.B. Bourlinos, R. Zboril, S. Couris, Opt. Express 22, 12013 (2014)
A.B. Bourlinos, G. Trivizas, M.A. Karakassides, M. Baikousi, A. Kouloumpis, D. Gournis, A. Bakandritsos, K. Hola, O. Kozak, R. Zboril, Carbon 83, 173 (2015)
I.M. Belousova, D.A. Videnichev, I.M. Kislyakov, T.K. Krisko, N.N. Rozhkova, S.S. Rozhkov, Opt. Mater. Express 5, 169 (2015)
S. Couris, N. Liaros, in Proceedings of 16th International Conference on Transparent Optical Networks (ICTON), ed. by M. Jaworski, M. Marciniak (IEEE & National Institute of Telecommunications, Warsaw, 2014), pp. 1–4. doi:10.1109/ICTON.2014.6876558
M.G. Papadopoulos, A.J. Sadlej, J. Leszczynski, Non-Linear Optical Properties of Matter: From molecules to Condensed Phases (Springer, Dordrecht, 2006)
V.E. Diyuk, A.N. Zaderko, K.I. Veselovska, V.V. Lisnyak, J. Therm. Anal. Calorim. 120, 1665 (2015)
S. Barnartt, J.B. Ferguson, Can. J. Res. 27b, 87 (1947)
S.L. Goertzen, K.D. Thriault, A.M. Oickle, A.C. Tarasuk, H.A. Andreas, Carbon 48, 1252 (2010)
A.M. Oickle, S.L. Goertzen, K.R. Hopper, Y.O. Abdalla, H.A. Andreas, Carbon 48, 3313 (2010)
J.L. Figueiredo, M.F.R. Pereira, M.M.A. Freitas, J.J.M. Orfao, Carbon 37, 1379 (1999)
W. Shen, Z. Li, Y. Liu, Rec. Pat. Chem. Eng. 1, 27 (2008)
V.E. Diyuk, A.N. Zaderko, L.M. Grishchenko, A.V. Yatsymyrskiy, V.V. Lisnyak, Catal. Commun. 27, 33 (2012)
K.I. Veselovs’ka, V.L. Veselovs’kyi, O.M. Zaderko, V.E. Diyuk, O.V. Ishchenko, J. Superhard Mater. 37, 39 (2015)
V.Ya. Gayvoronsky, A.S. Popov, M.S. Brodyn, A.V. Uklein, V.V. Multian, O.O. Shul’zhenko, in Nanocomposites, Nanophotonics, Nanobiotechnology, and Applications, ed. by O. Fesenko, L. Yatsenko (Springer, Heidelberg, 2015), pp. 147–164
L.P. Vera, J.A. Prez, H. Riascos, J. Phys. Conf. Ser. 511, 012063 (2014)
R. Zhang, Y. Achiba, K.J. Fisher, G.E. Gadd, F.G. Hopwood, T. Ishigaki, D.R. Smith, S. Suzuki, G.D. Willett, J. Phys. Chem. B 103, 9450 (1999)
L.W. Tutt, T.F. Boggess, Prog. Quantum Electron. 17, 299 (1993)
H. Looyenga, Physica 31, 401 (1965)
S.O. Nelson, D.P. Lindroth, R.L. Blake, Geophysics 54, 1344 (1989)
J.G. Speight, The Chemistry and Technology of Coal, 3rd edn. (CRC, Boca Raton, 2012)
W.L. Smith, in CRC Handbook of Laser Science and Technology, ed. by M.J. Weber (CRC, Boca Raton, 1988), pp. 229–258
Z.B. Liu, X.L. Zhang, X.Q. Yan et al., Chin. Sci. Bull. 57, 2971 (2012)
V.E. Diyuk, R.T. Mariychuk, V.V. Lisnyak, J. Therm. Anal. Calorim. 124, 1119 (2016)
B.J. Stagg, T.T. Charalampopoulos, Combust. Flame 94, 381 (1993)
N. Liaros, P. Aloukos, A. Kolokithas-Ntoukas, A. Bakandritsos, T. Szabo, R. Zboril, S. Couris, J. Phys. Chem. 117, 6842 (2013)
S. Husaini, A. Lesko, E.M. Heckman, R.G. Bedford, Opt. Mater. Exp. 5, 102 (2014)
I. Papagiannouli, A.B. Bourlinos, A. Bakandritsos, S. Couris, RSC Adv. 4, 40152 (2014)
N.L. Dmitruk, A.V. Goncharenko, E.F. Venger, Optics of Small Particles and Composite Media (Naukova Dumka, Kyiv, 2009)
Z. Liu, X. Zhang, X. Yan, Y. Chen, J. Tian, Chin. Sci. Bull. 57, 2971 (2012)
K.P. Loh, Q. Bao, G. Eda, M. Chhowalla, Nat. Chem. 2, 1015 (2010)
C. Neumann, S. Reichardt, P. Venezuela, M. Drögeler, L. Banszerus, M. Schmitz, K. Watanabe, T. Taniguchi, F. Mauri, B. Beschoten, S.V. Rotkin, C. Stampfer, Nat. Commun. 6, 8429 (2015)
I. Childres, L.A. Jauregui, W. Park, H. Cao, Y.P. Chen, in New developments in photon and materials research, ed. J.I. Jang, (Nova Science Publishers, Hauppauge, NY, 2013), pp. 403–419
F. Schedin, E. Lidorikis, A. Lombardo, V.G. Kravets, A.K. Geim, A.N. Grigorenko, K.S. Novoselov, A.C. Ferrari, ACS Nano 4, 5617 (2010)
B. Wang, X. Zhang, in Graphene Science Handbook: Electrical and Optical Properties, ed. M. Aliofkhazraei, N. Ali, W.I. Milne, C. S. Ozkan, S. Mitura, J. L. Gervasoni (CRC Press, Boca Raton, 2016), pp. 457–468
H. Shi, C. Wang, Zh. Sun, Y. Zhou, K. Jin, S.A.T. Redfern, G. Yang, Opt. Exp. 22, 19375 (2014)
Acknowledgements
The authors V.Ya.G., A.V.U., V.V.M. acknowledge a partial support from the NAS of Ukraine grant V-166. One of the authors, V.V.L. acknowledges supports from the National Scholarship Program of Slovak Republic for the Mobility of Students, Ph.D. Students, University Teachers, Researchers and Artists, SAIA Grant, n.o. in 2015, and the Agency of Ministry of Education, Science, Research and Sport of the Slovak Republic, the project ITMS: 26110230119.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Uklein, A.V., Diyuk, V.E., Grishchenko, L.M. et al. Characterization of oxidized carbon materials with photoinduced absorption response. Appl. Phys. B 122, 287 (2016). https://doi.org/10.1007/s00340-016-6561-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00340-016-6561-2