Abstract
Dynamic laser speckle (DLS) technique or biospeckle is a noninvasive approach that can sensitively monitor the dynamics of a biological material such as the metabolism of cells. In turn, stimulation of cell activity is widely used in microbiology: it can be done by means of many agents, such as lipopolysaccharide (LPS), which is the most common component within the cell wall of Gram-negative bacteria, and it has been extensively used in in vitro models studying inflammation. The main challenge is to monitor the change of behavior inside the cells during their stimulation. This work tested the viability of the BSL to monitor the change in the metabolism of RAW cells when LPS is applied. The results show different answers of cells to distinct concentrations of LPS, and the inflammatory process promoted by the LPS could be best distinguished from the RAW cells alone in a concentration of 10 ng/mL. This proved the ability of the DLS to monitor the agent stimulus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The human body is constantly under attack by a wide range of pathogens, such as bacteria, fungi, viruses and other organisms. To fight these constant insults, evolution has provided the body with a set of defenses that are able to efficiently eliminate the pathogens, restore the damaged tissue and retain a memory of the pathogen for further exposures. Once the pathogen enters the body, inflammation, an important host mechanism of defense, is the first to take place, being rapidly activated to fight the pathogen and halt its progression, which favors an unspecific response against pathogens, leading to their elimination and to restoring the tissue function and structure [1].
This fast and unspecific response fights the pathogen initially, gaining time for the specific immune system to take action, where specific T cells and antibodies against the microorganism are produced, completely eliminating the insult and retaining a memory of it [2].
During this process, molecules called cytokines are produced to regulate the immune system’s response and to communicate between all the different immune cells so as to work together against the pathogen. Macrophages, along with other phagocytes, such as neutrophils or dendritic cells, are key players of the immune system since they are the main cytokine producers and constitute the first cellular mediators of the innate immune defense [3].
An inflammatory response has a very complex network of regulation with multiple points of control, progressing from the production of pro-inflammatory cytokines to finally being resolved by the production of anti-inflammatory cytokines that will inhibit inflammation, eliminating completely the pathogen and inducing wound healing and tissue remodeling [4]. Such strict control is needed, since a prolonged or exacerbated inflammatory response may be responsible for or contribute to different serious autoimmune diseases associated with inflammation, such as the autoinflammatory diseases type 2 diabetes mellitus, Crohn’s disease or atherosclerosis [5]. The possibility of using human samples for monitoring inflammation has been hindered by ethical issues and the limited amount of samples.
The inflammatory response can be analyzed by using in vivo models or alternatively, in a simpler way, using ‘in vitro’ models with established cell lines. One of the most widely used cell lines in inflammatory studies is that of mouse macrophages such as RAW 264.7 cells, which is a mouse leukemic monocyte–macrophage cell line established from an ascites of a tumor induced in a male mouse by intraperitoneal injection of Abelson leukemia virus. These cells are commonly used for metabolic, apoptosis and inflammation studies, where incubation with lipopolysaccharide (LPS) is the most common pro-inflammatory stimulus [6–9].
LPS is the most common component within the cell wall of Gram-negative bacteria, and it has been extensively used in in vitro models studying inflammation [6–8]. After the first in vitro exposure to LPS (which does not affect cell viability), macrophages become activated and rapid intracellular signaling pathways are turned on; a downstream signaling is carried out through serial phosphorylations, resulting in transcription factor activation and translocation to the nucleus [9–11]. These transcription factors are in charge of activating the transcription of genes related to innate immunity and the inflammation responses to specific stimuli. One of the first effects of LPS ligation is the secretion of inflammatory cytokines like IL-1B, activated by the transcription nuclear factor kappa B NF-κB, which in turn will control the secretion of other cytokines over time making up the final response to eliminate the pathogen [9–11].
These processes are tightly regulated by an extremely complex network with a wide variety of spatiotemporal points of control, which results in an array of responses even within the same intracellular pathways. These spatiotemporal regulations allow the expression of different responses using different concentrations of the same stimuli.
The measure of the biological activity of RAW cells stimulated with LPS is a challenge, and the main hypothesis of this work is that the dynamic laser speckle technique can be a reliable tool to monitor the evolution in time of the activity produced by the interaction of RAW cells and LPS. The dynamic laser speckle is based on the monitoring of the Doppler beating phenomenon caused by the multiple scattering of the coherent light of a laser returning from or passing by the illuminated sample. The signal observed is thus analyzed by means of many techniques, such as those associated with statistical analysis. Therefore, the ability to monitor the activity of the illuminated sample without any contact and the use of a low power light makes the dynamic laser speckle a potential tool in a broad range of applications in biological material and has also been referred to as ‘biospeckle laser’ [12].
More applications of dynamic laser speckle in biological fluid samples have been reported in the literature, for example, to the motility of bovine frozen semen [13], as well as to blood flow [14, 15] and bacterial chemotaxis [16]. Biospeckle has been recently applied for optical monitoring activities related to various physicochemical phenomena in biological tissues [17, 18] and associated with the efficacy of drugs on parasites [19, 20], where biospeckle laser parameters may, possibly, assess the biological activity resulting from the action of endogenous enzymes by a fast, nondestructive and low-cost method. Furthermore, dynamic laser speckle has also been applied for diagnosing cancer [21] and has successfully monitored the reaction of cancer cells of line MEL-RC08 (derived from a pericraneal metastasis of a cutaneous melanoma) to the application of the drug colcemid, showing a dose-dependent decrease in biological activity [22]. Hence, ME-RC08 cells were used in this manuscript as a comparative control for RAW cells biological activity.
Therefore, this work evaluated the potential of this technique for measuring the biological activity in RAW cells stimulated with different concentrations of lipopolysaccharide along time.
2 Materials and methods
2.1 Culture of RAW 264.7 cells
RAW 264.7 (ATCC® TIB71™) [23] is a macrophage-like cell line derived from tumors induced in male BALB/c mice by the Abelson murine leukemia virus. RAW cells were cultured in DMEM (Dulbecco’s modified Eagle’s medium) supplemented with 10% heat-inactivated fetal bovine serum, 1% nonessential amino acids, 4 mM l-glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin and maintained in monolayer cultures at 37 °C and atmosphere containing 5% CO2.
For the dynamic laser speckle analysis, 2 × 106 RAW cells per well were plated in four wells of multi-well cell culture plates with clear-bottom polystyrene treated with CellBIND® (Corning Life Sciences, Hazebrouck, France) and were left to adhere for at least 2 h at 37 °C and 5% CO2. Room temperature was approximately 30 °C and 56% relative humidity, measured before and after the imaging. Before starting the acquisition of images, the medium was removed from the RAW cultures and fresh medium containing 10 or 100 ng/mL of lipopolysaccharide (LPS; Sigma-Aldrich) was added. Images were taken right after the addition of the LPS during a time period of 8 h, enough time to induce a specific response.
2.2 Dynamic laser speckle setup
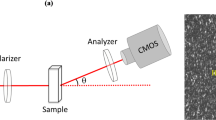
Figure 1 presents the experimental setup to acquire the speckle patterns generated in time by the illumination of the samples using a linear polarized He–Ne laser beam (632.8 nm, 30 mW). The beam size was expanded using a microscope’s objective with a 40× magnification in order have a round illuminated area with 110 mm of diameter, presenting a monitored area of a square edge with 38 mm. The well with a diameter of 16 mm was situated in the center of the illuminated area, which presented a uniform illumination provided by a diffuser [19], that also provided a high contrast of the speckle grains. The distance between the diffuser and the well was 7 mm. The well contained a sample with a thickness of 7.5 mm with a volume of 1.5 mL.
2.3 Methodology to speckle patterns acquisition
The images were acquired by a compact macro lens (SIGMA) with a focal length of 50 mm, numerical aperture of f/16, connected to an Allied Vision Technologies CCD Camera (AVT Marlin F-145B, pixel size of 4.65 μm).
The image quality was tested in order to avoid speckle grains with degraded information about the phenomena. Therefore, the setup was biased in order to avoid speckle grains smaller than the pixel size, to avoid large speckle grains with blurred appearance, as well as to avoid the saturation of the images. The blurred grains and saturation were avoided using contrast and saturation tests [24]. The setup was maintained during all the analysis, and thus, the speckle grain sizes remained in the same range. The speckle size in the image plane was 12.35 μm.
At the beginning of each hour, for 8 h, a collection of 120 images (8 bits, 640 × 480 pixels and shutter speed 1/125 s) was acquired at a rate of one frame per second.
2.4 Algorithms to process dynamic speckle image
2.4.1 Absolute values of differences (AVD)
The biospeckle activity was quantified by means of descriptors based on temporal analysis and descriptors based on frequency analysis. The behavior over time of the region of interest (ROI) was analyzed by means of the absolute values of the differences (AVD) method [25], after the creation of a time history speckle pattern (THSP) matrix [26]. The AVD method can be expressed by Eq. 1:
where the COM is the co-occurrence matrix related to the THSP and i and j represent the line i and the column j of each entry of the COM matrix [27, 28].
The data were also analyzed using by filtering the signals in different frequency bands. The wavelet transform was used based on the mother function Morlet to perform the filtering and the reconstruction of the signal in different bands in order to be analyzed again by the AVD [25, 28].
2.4.2 Test of a new protocol
The challenge presented in cells incubated with colcemid [22] was the analysis using the traditional methods to quantify numerically the biospeckle phenomena. Traditionally, this was done by means of a THSP matrix created using just one row in the middle of the ROI positioned in a square limited by the circle of the well. The drawback, despite the success of this earlier kind of analysis, was the variation of the results regarding the non-uniform disposition of the cells within the well [17].
In Fig. 2a, it is possible to see the new proposal where the THSP was created, but instead of using just one row, as presented for purposes of illustration, using a random number of pixels spread in the well. The comparison of the use of one row with using random points was conducted by dividing the ROI into 9 pieces as presented in Fig. 2b. A row was used to create a THSP in each of the nine squares, and the dispersion of their AVDs could be compared to that of the new proposed method. The proposed random assembling was carried out in the first image and with the random points fixed along the time history of the speckle pattern in order to construct the new THSP. Thus, in the whole ROI (a square in the well (244 × 250), as in Fig. 2b), the random points were selected at first and monitored in time. A check of this procedure was made by means of ten replications, i.e., ten collections of random points were tested by the AVD.
The size of the row inside each of the 9 pieces was 80 pixels and created a THSP of 80 × 120, where 120 represent the 120 images collected in time. The THSP constructed using the random values was 244 pixels by 120 images. The random points once selected were fixed along time.
3 Results and discussion
3.1 Comparative of biological activity of MEL-RC08 cellular line and RAW cells
Figure 3 shows the comparison of biological activity measured by AVD over time with biospeckle between the tumoral cell line MEL-RC08 and the mouse macrophage cell line RAW 264.7. The time rate acquisition was 15 frames per second for MEL-RC08 and one frame per second for RAW cells. If RAW cells were monitored using 15 frames per second, they did not present activity recorded by the BSL, confirming the lower activity of these cells compared with MEL-RC08 cells.
As expected, MEL-RC08, a novel human malignant melanoma cell line established from a pericraneal metastasis of a malignant melanoma of the skin, shows a higher biological activity than RAW cells. These data are in concordance with recent studies demonstrating the capability of biospeckle laser for monitoring physicochemical phenomena in biological tissues, where processes associated with cytoplasmic streaming, organelle movement, transport of substances in the cell, transport of water and solutes in vascular bundles, membrane ion channel functions, cell growth and division may account for the biological activity detected by BSL. They concluded that those involving the actin microfilaments affected the biospeckle activity the most, discarding cell division as a major cause of variation [19, 20, 29, 30].
The tumoral condition of MEL-RC08 cells implies the presence of different chromosomal aberrations common in melanoma [31], making these cells more biologically active than the monocyte–macrophage cell line RAW, as shown in Fig. 3. Due to the DNA alterations of these tumoral cells, different intracellular pathways are active, which leads to the activation of different biological processes, such as gene expression, and protein synthesis and secretion, making them active during the 8 h of the experiment, which allows their detection by the biospeckle. In contrast, RAW cells is a cell line derived from a mouse monocyte–macrophage, which until stimulated with LPS only exhibits basic cell physiological activities (cell adhesion, chemotaxis and proliferation), which in agreement with previous reports do not significantly affect biospeckle activity, which is the reason why the biological activity recorded by the biospeckle remains basal compared to MEL-RC08. When LPS is added to the RAW cells, these cell physiological activities slow down and the cells become activated, initiating the inflammatory genetic program [9–11].
3.2 Test of the new protocol using random points
The results of the traditional method using the row to create the THSP can be expressed by the variation of the AVD at each one of the nine pieces inside the ROI. The dispersion curve of the cells along time can be compared to the same dispersion of the ten replications of the random values inside the whole ROI, and in Fig. 4, it is possible to see the dispersion of the AVD values at 9 sub-ROIs along time and linked to two collections of RAW cells: one with 10 ng of LPS and the other without LPS.
The standard deviations of the traditional method to generate the THSP presented in the sub-ROIs (Fig. 4) can therefore be compared to the standard deviations presented by the random assembling of points within the ROI to generate the THSP (Fig. 5). It is clear that the best protocol to analyze the activity of cells within a well, by means of biospeckle laser, is the random assembling proposed here. Thus, it was used in the further biospeckle analysis to test the effect of LPS on RAW cells.
3.3 Temporal analysis of biological activity of RAW cells with and without LPS
Two different LPS concentrations were used for testing the biological activity over time in RAW cells imaged with BSL and using the new protocol developed. It has been shown that LPS arrests the proliferation of RAW cells without affecting cell viability and induces their activation through a pro-inflammatory response [9–11].
After activation of RAW macrophages with LPS, a genetic pro-inflammatory program is rapidly turned on and upstream pathway components are activated within minutes, which may explain the difference in AVD signal seen between RAW and RAW + LPS cultures at time 0 h (Figs. 5, 6). For example, NF-κB, an important transcription factor that regulates a pro-inflammatory genetic program in response to LPS, is quickly translocated to the nucleus after LPS ligation in the membrane of RAW cells. NF-κB translocation to the nucleus is a very rapid process, peaking around 30–60 min after LPS exposure [9]. Cytokine gene and protein expression have different time points, and depending on the concentration used, the peaking of the in vitro gene expression can vary from 2 to 8 h and that of the cytokine secretion from 4 to 8 h [10, 11].
These patterns of gene and protein expression are more complex if we study longer time points and increasing LPS concentrations. The intracellular physicochemical phenomena that are activated after LPS stimulation (change in morphology, activation of intracellular kinase-dependent pathways, and protein expression and synthesis) have been previously proven to be detected by variations in biospeckle patterns [20, 29, 32]. These authors experimentally inhibited specific cellular processes (actin cytoskeleton, microtubule reorganization and protein synthesis) and showed that cellular processes involving actin as a part of the cytoskeleton, ion channel and proton pumps events, and intra- and intercellular transport of proteins were what made the most significant contribution to the total biological activity [29, 30].
When analyzing biological activity of RAW cells with and without LPS during 8 h, different biospeckle patterns are obtained. During that time, the speckle pattern shows some fluctuations that can be due to the adjustment of the colony regarding the same factors related to basic cell activities, since the profiles of the variations are the same for all the cases and replications. These typical cell activities related to cell adhesion, chemotaxis and proliferation of the RAW cells can explain the same behavior at 3 and 4 h and 6 and 7 h for both the RAW cells with and those without LPS, as well as in all replicates.
When LPS is added at 10 ng/mL, the proliferation slows down and the RAW cells become activated, initiating the inflammatory response and increasing their biological activity, which also implies the reorganization of the cytoskeleton (changes in morphology), activation of intracellular kinases with increased ion flow, and intra- and intercellular mobilization of proteins and molecules (cytokines, transcription factors, etc.), events that are probably responsible for the difference in biological activity as shown in Fig. 5. Thus, we can assume that the difference observed between RAW cells and RAW + LPS (10 ng/mL) is due to the inflammatory effect induced by LPS that involves cellular processes that increase the signal detected by biospeckle. The offset observed between the curves (Fig. 5) can be explained by the action of LPS enhancing the inflammatory activation.
When RAW cells were also incubated with 100 ng/mL of LPS (Fig. 6), then, just as happened with 10 ng/mL, the biological activity is also increased at 0 h due to the fast activation of specific intracellular pathways in response to the LPS, something which happens within minutes. But, interestingly, the biological activity with 100 ng/mL of LPS does not show as great a difference from the RAW cells as happened with 10 ng/mL. This could be explained as being due to the different output obtained with these two different concentrations of LPS, yet they use the same intracellular pathways. The immune response has an extremely complex regulation network, where different concentrations of LPS can tune different pro-inflammatory responses inducing different patterns of cytokine expression.
This is of great importance, because an impaired or aberrant pro-inflammatory response can be harmful for the host. In this respect, the response that increases in 10 ng/mL of LPS is not the same as the response in the presence of bacteria. A lower concentration of LPS can be eliminated by a fast and strong inflammatory response, but a higher LPS concentration will require a more complex and long-term response in order to completely eliminate the pathogen. Although we used the same stimulus and activated the same intracellular pathways, the complex regulation of these responses led to different specific responses.
3.4 Comparison by frequency range of the temporal biological activity of RAW cells with and without LPS
In Fig. 7, we have analyzed the ratio of biological activity of RAW cells and RAW cells with different LPS concentrations at different frequency ranges.
The medium and high frequencies showed higher ratios at early times (0 and 1 h), presenting a decreasing tendency along time compared to the low-frequency signals. It can be seen that we obtain the same behavior at high and middle frequencies, allowing us to conclude that the LPS action can be seen in a transient phase (with high activity recorded by the biospeckle) at middle and high frequencies at early times and reducing along the next 3 h in the comparison of RAW + LPS and RAW cells alone. Complementary to Figs. 5 and 6, Fig. 7 clearly shows differences in biospeckle pattern that could be interpreted as a measure of the pro-inflammatory response to LPS and is sensitive enough to identify the tendency expected.
Complementary to previous results showing the ability to monitor cell behavior when drugs act on melanoma cells [ [21], [22] ], biospeckle can function as a very sensitive prognostic tool since it may be able to differentiate between very subtle biological processes in response to an inflammatory stimulus, hence showing its potential use in testing the efficacy of anti-inflammatory agents, protective agents inducing responses to kill a pathogen, treatments to empower the immune system against infection, or immunosuppressive agents for treating autoimmune diseases.
4 Conclusions
-
The change in the traditional processing of BSL using instead random points to create the secondary matrix THSP proposed here is the best protocol to analyze the activity of cells within a well.
-
RAW cells show lower biological activity measured by AVD (higher basal activity) compared to MEL-RC08.
-
RAW cells stimulated with 10 and 100 ng/mL of LPS show increased biological activity compared to RAW cell with medium due to the inflammatory process activated after LPS exposure.
-
RAW cells stimulated with 10 ng/mL show different biological activity than RAW cells with 100 ng/mL.
-
LPS-dependent activation of RAW cells shows different biological activity at medium- and high-frequency ranges.
-
Biospeckle is a reliable tool to monitor in vitro cell activities.
Abbreviations
- DLS:
-
Dynamic laser speckle
- LPS:
-
Lipopolysaccharide
- CCD:
-
Charge-coupled device
- AVD:
-
Absolute values of the differences
- THSP:
-
Time history speckle pattern
- COM:
-
Co-occurrence matrix
- ROI:
-
Region of interest
References
T. Lawrence, D.A. Willoughby, D.W. Gilroy, Nat. Rev. Immunol. 2, 787 (2002). doi:10.1038/nri915
A. Iwasaki, R. Medzhitov, Nat. Immunol. 16, 343 (2015). doi:10.1038/ni.3123
J.M. Cavaillon, Biomed. Pharmacother. 48, 445 (1994)
T. Hanada, A. Yoshimura, Cytokine Growth Factor Rev. 13, 413 (2002). doi:10.1016/S1359-6101(02)00026-6
S.L. Masters, A. Simon, I. Aksentijevich, D.L. Kastner, Annu. Rev. Immunol. 27, 621 (2009). doi:10.1146/annurev.immunol.25.022106.141627
E. Szliszka, D. Skaba, Z.P. Czuba, W. Krol, Molecules 16, 3701 (2011). doi:10.3390/molecules16053701
E. Bognar, Z. Sarszegi, A. Szabo, B. Debreceni, N. Kalman, Z. Tucsek, B. Sumegi, F.G. Jr, Plos One 8, e65355 (2013). doi:10.1371/journal.pone.0065355
V.W. Makene, E.J. Pool, Water Environ. J. 29, 353 (2015). doi:10.1111/wej.12127
C.-X. Xi, F. Xiong, Z. Zhou, L. Mei, W.-C. Xiong, J. Leukoc. Biol. 87, 415 (2010). doi:10.1189/jlb.0309125
E. Jones, I.M. Adcock, B.Y. Ahmed, N.A. Punchard, J. Inflamm. 4, 23 (2007). doi:10.1186/1476-9255-4-23
L.W. Soromou, Z. Zhang, R. Li, N. Chen, W. Guo, M. Huo, S. Guan, J. Lu, X. Deng, Molecules 17, 3574 (2012). doi:10.3390/molecules17033574
H.J. Rabal, R.A.B. Jr, Dynamic laser speckle and applications (CRC Press, New York, 2008)
P.H.A. Carvalho, J.B. Barreto, R.A. Braga Jr., G.F. Rabelo, Biosyst. Eng. 102, 31 (2009). doi:10.1016/j.biosystemseng.2008.09.025
Y.A. Ganilova, S.S. Ulyanov, Biophysics 51, 299 (2006). doi:10.1134/S0006350906020230
A.K. Dunn, Ann. Biomed. Eng. 40, 367 (2012). doi:10.1007/s10439-011-0469-0
S.E. Murialdo, G.H. Sendra, L.I. Passoni, R. Arizaga, J.F. Gonzalez, H. Rabal, M. Trivi, J. Biomed. Opt. 14, 64015 (2009). doi:10.1117/1.3262608
R.A. Braga, L. Dupuy, M. Pasqual, R.R. Cardoso, Eur. Biophys. J. EBJ 38, 679 (2009). doi:10.1007/s00249-009-0426-0
R.A. Braga Jr., G.F. Rabelo, L.R. Granato, E.F. Santos, J.C. Machado, R. Arizaga, H.J. Rabal, M. Trivi, Biosyst. Eng. 91, 465 (2005). doi:10.1016/j.biosystemseng.2005.05.006
J.A. Pomarico, H.O.D. Rocco, L. Alvarez, C. Lanusse, L. Mottier, C. Saumell, R. Arizaga, H. Rabal, M. Trivi, Eur. Biophys. J. 33, 694 (2004). doi:10.1007/s00249-004-0413-4
I.C. Amaral, R.A. Braga Jr., E.M. Ramos, A.L.S. Ramos, E.A.R. Roxael, J. Food Eng. 119, 135 (2013). doi:10.1016/j.jfoodeng.2013.05.015
S.S. Ul’yanov, V.N. Laskavyi, A.B. Glova, T.I. Polyanina, O.V. Ul’yanova, V.A. Fedorova, A.S. Ul’yanov, Quantum Electron. 42, 399 (2012). doi:10.1070/QE2012v042n05ABEH014885
R.J. González-Peña, J. Braga Roberto, A.R.M. Cibrián, R. Salvador-Palmer, R. Gil-Benso, T.S. Miguel, J. Biomed. Opt. 19, 57008 (2014). doi:10.1117/1.JBO.19.5.057008
ATCC: The Global Bioresource Center. http://www.lgcstandards-atcc.org/. Accessed 3 June 2016
J. Moreira, R.R. Cardoso, R.A. Braga, Opt. Lasers Eng. 61, 8 (2014). doi:10.1016/j.optlaseng.2014.04.005
R.A. Braga, C.M.B. Nobre, A.G. Costa, T. Sáfadi, F.M. da Costa, Opt. Commun. 284, 646 (2011). doi:10.1016/j.optcom.2010.09.064
A. Oulamara, G. Tribillon, J. Duvernoy, J. Mod. Opt. 36, 165 (1989). doi:10.1080/09500348914550221
R. Arizaga, M. Trivi, H. Rabal, Opt. Laser Technol. 31, 163 (1999). doi:10.1016/S0030-3992(99)00033-X
C. Torrence, G.P. Compo, Bull. Am. Meteorol. Soc. 79, 61 (1998). doi:10.1175/1520-0477(1998)079<0061:APGTWA>2.0.CO;2
A. Kurenda, P.M. Pieczywek, A. Adamiak, A. Zdunek, Food Biophys. 8, 290 (2013). doi:10.1007/s11483-013-9302-7
K.M. Ribeiro, B. Barreto, M. Pasqual, P.J. White, R.A. Braga, L.X. Dupuy, Ann. Bot. 113, 555 (2014). doi:10.1093/aob/mct271
R. Gil-Benso, C. Monteagudo, M. Cerdá-Nicolás, R.C. Callaghan, S. Pinto, A. Martínez-Romero, A. Pellín-Carcelén, T. San-Miguel, J.C. Cigudosa, C. López-Ginés, Hum. Cell 25, 61 (2012). doi:10.1007/s13577-011-0027-y
A. Zdunek, J. Cybulska, Sensors 11, 6317 (2011). doi:10.3390/s110606317
Acknowledgements
Thanks to the Department of Biochemistry of the Faculty of Medicine and Odontology of the University of Valencia and thanks to the Federal University of Lavras.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
González-Peña, R.J., Pérez-Montoyo, H., Braga, R.A. et al. Assessment of biological activity in RAW 264.7 cell line stimulated with lipopolysaccharide using dynamic laser speckle. Appl. Phys. B 122, 275 (2016). https://doi.org/10.1007/s00340-016-6549-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00340-016-6549-y