Abstract
Fugitive gas emissions from agricultural or industrial plants and gas pipelines are an important environmental concern as they contribute to the global increase of greenhouse gas concentrations. Moreover, they are also a security and safety concern because of possible risk of fire/explosion or toxicity. This study presents standoff detection of CH4 and N2O leaks using a quantum cascade laser open-path system that retrieves path-averaged concentrations by collecting the backscattered light from a remote hard target. It is a true standoff system and differs from other open-path systems that are deployed as point samplers or long-path transmission systems that use retroreflectors. The measured absorption spectra are obtained using a thermal intra-pulse frequency chirped DFB quantum cascade laser at ~7.7 µm wavelength range with ~200 ns pulse width. Making fast time resolved observations, the system simultaneously realizes high spectral resolution and range to the target, resulting in path-averaged concentration retrieval. The system performs measurements at high speed ~15 Hz and sufficient range (up to 45 m, ~148 feet) achieving an uncertainty of 3.1 % and normalized sensitivity of 3.3 ppm m Hz−1/2 for N2O and 9.3 % and normalized sensitivity of 30 ppm m Hz−1/2 for CH4 with a 0.31 mW average power QCL. Given these characteristics, this system is promising for mobile or multidirectional search and remote detection of gas leaks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Fugitive gas emissions from agricultural or industrial plants or natural gas pipeline infrastructure are a concern due to their impact on the global warming [1] and their possible toxicity or fire and explosive risks. For these reasons, optical methods to detect fugitive emission of greenhouse or hazardous gases over large areas have been developed [2–5]. Among these methodologies, open-path Fourier transform infrared spectroscopy (OP-FTIR) has been commonly used for gas concentration measurement [6–9], and in the near-UV and near-ir, differential optical absorption spectroscopy (DOAS) has been applied [10–13]. Differential absorption lidar (DIAL) and Raman scattering lidar (RSL) are also mature techniques for active remote sensing of atmospheric trace gases and water vapor. More recently, a lidar system based on optical correlation spectroscopy using spectrally broadband laser coupled to pulse shaping techniques achieved range resolved gas concentration measurements in the atmosphere [14, 15]. In addition, technological improvements in tunable diode sources have been achieved, making tunable diode laser absorption spectroscopy an important method for gas concentration measurement or leak detection [16], using either direct transmission or backscattered light from a topographic target [17, 18].
Similarly, the quantum cascade laser (QCL) has become a reliable mid-infrared light source for various trace gas sensing applications [19–22] either in pulsed [23–25] or continuous mode [25, 26]. QCLs can operate at room temperature or with thermoelectric cooling (TEC), are relatively compact, and can address mid- to long-wave infrared spectral range applications where many trace gases exhibit significant absorption features. As a consequence, QCLs have successfully been used in many applications such as cigarette smoke analysis [27, 28], NO detection [29, 30], NO2 detection [31], engine exhaust analysis [32–34] and breath analysis [35, 36].
One trace gas detection technique takes advantage of the intra-pulse frequency chirp observed in output of a pulsed distributed feedback (DFB) QCL. More specifically, the wavelength of a DFB-QCL when used in pulsed mode is rapidly chirped during the course of the pulse resulting in a well-defined frequency sweep, allowing this type of laser to be used for intra-pulse spectroscopy [37]. In a previous contribution [38], we demonstrated ambient trace gas measurements in a 215-m multipass cell based on this technique using a pulsed DFB-QCL at 7.7 µm. We showed that the system is capable of fast retrieval (~5 ms) of simultaneous path-averaged methane and nitrous oxide ambient concentrations with <1 % ambient accuracy. We further discussed an open-path quantum cascade laser (OPQCL)-based system that deployed a retroreflector to fold the beam back to a telescope/detector receiver system and showed preliminary field measurements. More recently, the OPQCL was deployed in the field to make long term (>30 h) simultaneous path-averaged methane and nitrous oxide measurements for a roundtrip path length of 500 m. A manuscript on this work is under preparation. The system utilizes a retroreflector (similar to an OP-FTIR system) to define a measurement path (fence-line) and is a field-deployable fast response sensor for simultaneous detection of path-averaged methane, nitrous oxide ambient concentrations with <1 % ambient accuracy.
Most of the measurement techniques mentioned above are either point samplers that deploy some sort of multipass cell or cavity, or fence-line systems that deploy a retroreflector. The latter systems are not ideal for mobile or multidirectional measurements since the direction of the beam is defined by the system layout and has to be realigned when the system is moved, while point samplers cannot do standoff remote detection of fugitive gas emissions. Remote leak detection systems require fast measurement cycle (>10 Hz), portability and sufficient range to scan large areas in short time [17].
We present in this study a potential system for remote gas leak detection using the backscattered light off a hard target with a fast measurement cycle. The measurement cycle for retrieving concentration using this system is 68 ms, corresponding to the time to averaging 1024 laser shots at 20 kHz (50 ms) and the data processing time (18 ms). Actual topographic targets present a wide variety of backscattering coefficients and angular distributions [51]. Therefore, we carried out our measurements with a reference reflectance target, Infragold from Labsphere. The Infragold target is a diffuse scattering target for mid-IR light and is used as a well-characterized target with typical reflectance of >94 % and a near-Lambertian behavior. We performed gas concentration measurements using a modified QCLOPS system in a backscatter configuration with path length of up to 45 m (~148 ft.). Our novel approach allows for simultaneously high spectral and temporal resolution gas spectroscopy and precise measurements of the target range using time resolved observations. These measurements are obtained with short measurement cycle and at sufficient range that fulfill the requirement for fast, mobile, or multidirectional gas leak monitoring.
Measurements were performed on methane (CH4) and nitrous oxide (N2O), respectively, the second and third most potent anthropogenic greenhouse gases [1]. Both are the sources of major concern for environmental issues. Nitrous oxide has a global warming effect 300 times higher than CO2 mostly because of its lifetime in the atmosphere of 114 years and strong mid-IR absorption bands; however, its effect is limited by its relatively low ambient concentration of 0.3 ppm [1]. Methane has a global warming potential 25 times higher than carbon dioxide [1]. In addition, its ambient concentration has increased by a factor of 2.5 since preindustrial times, from 0.7 to 1.8 ppm [1]. Methane is also a highly explosive gas when its concentration is >5 % in the ambient air, making it urgent to remotely and quickly detect methane leaks [39, 40].
This paper is organized as follows. In Sect. 2, the QCLOPS is described, presenting the optical layout and its functioning principle. In Sect. 3, we present the gas concentration retrieval method based on the least squares analysis. Finally, in Sect. 4, the specifications of our system are given, a signal-to-noise (SNR) analysis for the system for CH4 concentration measurements is presented together with the experimental measurements and results for both CH4 and N2O. The paper ends with the conclusion and outlooks for the presented work.
2 QCLOPS methodology
This section presents the principle of the standoff QCLOPS together with the related system optical layout. The system is based on active differential optical absorption spectroscopy (DOAS) [13]. A quantum cascade laser emits a laser pulse, with intensity I0(t), expressed as a function of time t. This laser pulse is directed toward a scattering target. A receiver system based on a Newtonian telescope, coaxial with the laser, and a mid-ir detector are used to collect and detect the backscattered light from the target, noted here as I(t). During propagation from the laser to the target and then back to the detector, the laser light undergoes optical extinction due to the presence of gas molecules as displayed in Fig. 1.
A remarkable feature of this QCLOPS is that its laser source is not actively scanned, nor does it use a spectrally resolved detector. In order to measure an absorption spectrum, it uses the intra-pulse frequency chirp of a pulsed DFB-QCL. The performance and reliability of intra-pulse spectroscopy using chirped QCLs have been established and demonstrated for gas concentration measurement such as ozone [41], ammonia and ethylene [42], methane and nitrous oxide [38], carbon dioxide and water vapor [43], difluoroethylene [44], nitric oxide [29, 30, 45], and methane isotopic composition [46] to name a few. As the laser is emitting the pulse for a few 100 ns, the substrate temperature varies causing a change in its refractive index. As a consequence, within the pulse duration, the frequency of the DFB-QCL is shifted from high to low with a given chirp rate (1 cm−1 in ~200 ns in our case), in a stable and repeatable manner [37]. Therefore, as shown in Fig. 2, the time resolved intensity I(t) measurement can be converted to a spectrally resolved intensity I(υ) using the experimentally determined chirping rate of the QCL (details of this conversion are presented in Sect. 4.2). For this reason, the outgoing signal intensity I 0(t) (herein referred to as reference signal) and the return signal intensity I(t) can be mapped to a function of frequency υ:
The optical intensity I(υ) received by the detector from a scattering target at range L is described by:
where β is the backscatter coefficient of the hard target expressed in sr −1, and K is a coefficient accounting for the optical efficiency of the system. While both β and K are also frequency dependent, they can be considered as nearly constant over a frequency micro-window of ~1 cm−1. The absorbance of the target gas is denoted as a gas(υ) and is defined as:
where σ(υ) is the absorption cross section, C is the concentration to be determined and 2L is the round-trip distance between the transmitter and the scattering target. This treatment is presented for a single gas species; however, multiple gas analysis is carried to take into account contribution from gases other than the target species by considering the sum of the absorbance for each gas [38]. In leak detection applications, typically absorption from the target gas dominates.
From Eq. 1, the negative logarithm of the ratio between the measured signal I(υ) over the reference signal I 0(υ) is equal to the measured relative absorbance a meas(υ):
where a meas(υ) can be expressed as:
where A and B are the coefficients of a first-order polynomial that accounts for constant and linear terms and include \(- \ln \left( {\beta K/L^{2} } \right)\) term and other background losses, biases, etc.
The measured relative absorbance a meas(υ) is the result of the convolution of the actual absorbance and the instrument function denoted as Ψ(υ), which gives the spectral resolution limit. Based on the laser bandwidth, the chirp rate of the laser, and the bandwidth of the detector and acquisition system, the spectral resolution has been found to be equal to 0.03 cm−1 and the instrument function Ψ(υ) to be a Gaussian function having a FWHM equal to the spectral resolution.
3 Gas concentration retrieval method
This section briefly presents the methodology used to retrieve the gas concentration using the least squares analysis, background information can be found in the literature [38, 47–49].
From the reference and the measurement signal, respectively, I 0(υ) and I(υ), the gas concentration can be retrieved using the least squares fitting technique. As presented in Fig. 3, the least squares fit is operated between:
-
The measured relative absorbance a meas(υ)
-
The reference absorbance a ref(υ) obtained from the convolution of the instrument function of our system Ψ(υ) and the absorbance generated from the HITRAN database and GENSPECT [50] for the target gas and other interfering ambient gases and for reference concentrations and path length.
More precisely, Eq. 2 can be rewritten as:
where \(a_{\text{ref}}^{i} \left( \upsilon \right)\) is the reference absorbance, \(a_{\text{ref}}^{i} \left( \upsilon \right)\) is the absorption cross section, and \(C_{\text{ref}}^{i}\) is reference concentration (usually on the order of typical ambient concentrations) for gas species i, L ref is a reference length on the order of typical operating path length. From Eq. 2, the absorbance due to all gas species is then:
where C i is the concentration of gas species i. Using Eq. 6, Eq. 4 can be rewritten as:
where
Coefficients A, B, and D i are obtained using least squares fitting [47–49]. From Eq. 8, the studied gas concentrations and uncertainty associated with them can be calculated as:
In the case of leaks, typically the absorbance of the target species dominates. The least square data analysis presented in this paper below takes into account contributions from methane, nitrous oxide, and water vapor gas species (index i in treatment above); where either methane or nitrous oxide leaks are introduced and the two other gases are interfering species (at their respective ambient levels).
Laser intensity fluctuations and changes in hard-target backscattering coefficient, path length, broad background absorption features, and scaling factor related to measured reference signal alter the measured relative absorbance a meas(υ) as evident in Eq. 1. However, as long as these effects do not introduce spectral structures within the frequency micro-window used, change in a meas(υ) will consist of a constant bias, or at most a linear term, that is compensated by the coefficients A and B and do not affect the retrieved gas concentrations.
The sensor principle and retrieval method described above provide operational characteristics that are essential to carry out mobile and remote detection of leaks. In addition, as the technique presented in this work has high temporal resolution, the path length L is calculated for every measurement by resolving the delay between the transmission and reception of the laser pulse, and thus, variations of path length in potential mobile/multidirectional application of the system may be automatically taken into account.
4 Measurements of CH4 and N2O concentrations
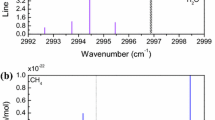
This section begins with QCLOPS system details and specifications; then, a numerical analysis is performed to evaluate the expected SNR and uncertainties of the retrieved concentration for methane. Finally, experimental results are presented and discussed for both CH4 and N2O. For this study, the N2O and CH4 vibrational–rotational absorption bands located between 1295 and 1296 cm−1 are considered. This spectral range has been chosen since both gases can be addressed within the tunable range of a single DFB-QCL. Figure 4 presents the calculated reference absorbance spectrum of ambient CH4 (1.8 ppm) and N2O (0.3 ppm) for an 80-m path using the HITRAN database [50].
4.1 Experimental setup
The infrared source used is an Alpes Laser pulsed DFB-QCL operating with a pulse length of 200 ns and at 20 kHz repetition rate. The average power is 0.31 mW. The laser beam is then expanded by a 4× beam expander to decrease the divergence. The stability of the DFB-QCL and its chirp rate has been established in a number of earlier works as noted in Sect. 2. Here, it was studied by measuring the fluctuations on the FWHM of gas absorption bands over a few days. Results show fluctuations of less than 6 % in the chirp rate, with no evidence of long-term drift. We note that these observations are also consistent with our continuous long-term (30 h) field measurements of ambient methane and nitrous oxide concentrations with a fence-line system utilizing the same laser (paper in preparation) [38].
The scattering target used is a 160 cm2 Infragold target by Labsphere. This target possesses high diffuse (near-Lambertian) reflectance in the near and middle infrared (larger than 94 %). The target is placed ~40 m away from the QCLOPS sensor. When using different topographic targets such as brick, wood, rock, plastic, or metal, and at different angles of incidence, the received signal can vary above and below the level of signals observed with the Infragold target, for example, we observed a higher backscatter signal from a painted metal door. We have investigated the diffuse reflection of topographic targets in an earlier work [51], and the Infragold target is used here as it is a reference diffuse reflectance standard in the mid-infrared.
The backscattered light is collected by a coaxial F/3.7 Newtonian telescope with an 8″ primary mirror diameter and focused on the PVI-3TE-10.6 infrared detector by Vigo Systems with a 1 mm2 effective optical area. The detector is coupled with a transimpedance amplifier (TIA) with gain ranging from 0.6 to 2.8 kV/A, and the resulting voltage responsivity ranges from 0.51 to 2.4 kV/W with a noise equivalent power (NEP) equivalent to 29–135 nV Hz−1/2 and a 100 MHz bandwidth. The detector output is digitized using the Gage Applied Technologies CS1622 16 bit ADC card with a 200 MSamples/s sampling rate and 125 MHz input analog bandwidth. Its onboard FPGA averages 1024 acquired waveforms at high repetition rate and then transfers this averaged waveform to the computer.
Gas puffs are obtained using a controlled leak of either pure CH4 or pure N2O. A valve is used to control when the gas is released at approximately one-meter range from the optical path. In the case of methane, the leak has a flux of approximately 50 mL/s (≈2 × 10−3 mol/s, or ≈30 mg/s). The valve was open for 5 s to release a total of 150 mg of CH4. For nitrous oxide, the leak flux was 2 mL/s (≈10−4 mol/s, or ≈4 mg/s), and the valve is only open for 0.5 s, releasing 2 mg of N2O. Gas dispersion in the air once released was not controlled.
As mentioned in Sect. 2, both signals I(t) and I 0(t) are recorded as functions of time and converted to a functions of frequency using the frequency chirp property of the QCL. Figure 5 shows an example of the reference signal I 0(t) and a measurement signal I(t) using our system for the N2O leak through the laser optical path. The reference signal I 0(t), measured using a set of flip mirrors that redirect the outgoing laser beam to the detector, is representative of the shape of the laser pulse in the absence of a leak; the measurement signal I(t) is displayed as a dashed line, showing the gas absorption effects on the laser pulse when a puff of N2O is released.
As a first step, the chirp rate of the laser is studied using an uncoated Germanium Fabry–Perot etalon interferometer. A signal is obtained by placing the etalon in front of the detector while recording the reference signal I 0(t). This signal shows an amplitude modulation, sometimes referred to as fringes [41] and is used for time to frequency conversion. Figure 6 presents the reference signal I 0(t) (dashed line) as well as the modulated signal (solid line). The latter is obtained through the interferometer which slightly attenuates the signal. The signals display overshoots at the beginning and end of the pulse, and these segments of the pulse are not considered in performing spectroscopic analysis. The signal modulation collected through the etalon is adequate for chirp-rate analysis. The low contrast is most likely due to the instrument function (0.03 cm−1) and that the light incident on the etalon placed in front of the detector is highly convergent.
The oscillations visible on the modulated signal have a period Δt corresponding to the known etalon-free spectral range Δυ = 0.048 cm−1 which allow the retrieval of the chirp rate α:
α decreases from 6.10−3 to 4.5.10−3 ± 2.10−4 cm−1/ns. The uncertainty in α also contributes to the relative uncertainty retrieved in the least squares fitting.
4.2 Signal-to-noise ratio (SNR) analysis
SNR simulation was made for methane measurements using the specification of the various components in the experimental setup described in Sect. 4.1. Based on the methodology described in Sect. 2 and Eq. 1, the expected signal intensity was evaluated. The total noise was evaluated based on the effective detectivity of the detector/amplifier package and its noise equivalent power (NEP), the noise associated with the acquisition system, and the shot noise from the signal and background. The noise budget is mainly due to the NEP of the detector/TIA, accounting for at least 98 % of the resulting noise, the measured noise on our signal (1024 pulse average) is 10 µV.
With 80 mW laser peak power, considering the loss on our various optical elements and a perfectly aligned system, the backscattered optical power at 45 m range with an Infragold diffuse scattering target (from Labsphere) is in the range of 0.4 µW peak power. Such optical power leads to a signal in the range of 400 µV, with a transimpedance gain set at 0.6 kV/A. Figure 7 presents the theoretical SNR for a perfectly aligned system as a function of range, SNR reaches 21 at 45 meters range.
In addition, returned signals were simulated for various SNR and methane path-averaged concentrations. Using the least square analysis, the methane mixing ratio and relative uncertainty have been retrieved from the simulated signals. Ensembles of retrievals for various realizations of random noise were analyzed to study statistical errors. Figure 8 shows the methane mixing ratio relative uncertainty as a function of SNR and methane mixing ratio over 45 m range.
Results obtained from the numerical simulation show that with relatively low SNR (~10), methane mixing ratio above 30 ppm can be measured with a relative uncertainty better than 20 %. Higher SNR is required for low concentration measurement, for example, an SNR of 80 leads to measurement of 5 ppm path-averaged concentration with a 10 % relative uncertainty.
4.3 N2O concentration measurements
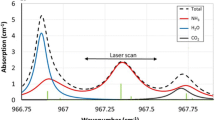
Measurements to detect N2O were performed with the scattering target placed at 40 meters away from the sensor system. In these conditions, the signal-to-noise ratio predicted by our numerical simulation for a perfectly aligned and aberration-free system is 27, and the experimental SNR was equal to 22 (likely due to unaccounted optical losses and uncertainty in component specifications). Fig. 9 presents an example of the measured relative absorbance corrected for constant (A) and linear (B.ν) terms from the least squares fit (Eq. 6) for clarity. The solid line corresponds to gas absorbance fit from least squares (Eq. 7), where NO2, CH4, and water vapor are considered. Nitrous oxide dominates the gas absorbance. The residuals of the fit are displayed on the lower graph. When studying the residual of various measurement cycles, no significant bias was found.
Figure 10 presents the path-averaged N2O mixing ratio, retrieved by the least squares analysis, with a 68-ms measurement cycle and range of 40 m. Before the valve is open (t < 0.58 min), the average N2O ambient mixing ratio is about 0.18 ppm with fluctuations (±0.32 ppm). Then, at t = 0.58 min, the valve is opened for 0.5 s, releasing approximately 2 mg of N2O. At this time, the strong extinction due to the high concentration of N2O leads to a saturation of the signal (absorption ~100 %). Shortly after, the retrieved mixing ratio reaches a maximum of 15.9 ppm. The minimum relative retrieval uncertainty is 3.1 %. As the gas dissipates, the concentration of N2O slowly decreases until it returns to the initial ambient values (evident with longer measurement cycle). The time-evolution of the retrieved mixing ratio illustrates the fast response and effectiveness of our system to perform gas leak detection. At ambient levels, the N2O mixing ratio uncertainty is ~0.32 ppm (for 15 Hz measurement cycle), leading to a detection limit of the path-averaged mixing ratio of 12.8 ppm m over the 40 m range for measurements at 15 Hz.
4.4 CH4 concentration measurements
The procedure and experiment described above using N2O was replicated to detect leaks of CH4; in this case, the scattering target was placed 45 meters away from the sensor system. The SNR predicted by our numerical simulation and based on component specifications is 21, and the experimental SNR was equal to 16 (likely due to unaccounted optical losses and uncertainty in component specifications). Figure 11 shows an example of the measured relative absorbance corrected for constant (A) and linear (B.ν) terms from the least squares fit (Eq. 6) for clarity. The solid line corresponds to gas absorbance fit from least squares (Eq. 7). Again, NO2, CH4, and water vapor are considered but methane absorbance dominates. The residuals show no evidence of any systematic bias on the measurement.
Figure 12 shows the detection of a methane leak with a measurement cycle of 68 ms, same as for the measurements involving N2O. Instants before the CH4 gas is released, the initial mixing ratio oscillates around the ambient mixing ratio (1.8 ppm) as expected, but cannot be measured precisely with the 15-Hz measurement cycle since the associated error can be as large as ±2.6 ppm. At t = 8 s, 150 mg of CH4 gas is released and the retrieved mixing ratio rapidly rises to a maximum of 146 ± 31 ppm (relative uncertainty of 21 %) while the gas puff occurs. Then, the gas dissipates until it reaches the background values once again (evident with longer measurement cycle). The uncertainty reaches a minimum of 2 ppm with a mixing ratio of 21.6 ppm (relative uncertainty of 9.3 %). The detection limit for the CH4 measurements can be determined in the same way as for N2O. For ambient level, the CH4 mixing ratio uncertainty is 2.6 ppm for 45 m (for 15 Hz measurement cycle), leading to a detection limit of 117 ppm m.
Using our numerical simulation described in Sect. 4.2, experimental CH4 mixing ratio relative uncertainties are compared to relative uncertainties predicted by the numerical simulation. Figure 13 presents these simulated relative uncertainties as function of the methane mixing ratio for a SNR of 16, as in our experiment, together with the retrieved relative uncertainty from our measurements. Results show good agreement between the retrieved relative uncertainties from the experiment and those from the numerical simulations.
5 Summary and conclusions
A standoff leak detection technique is presented based on a backscatter quantum cascade laser open-path system (QCLOPS) to measure path-averaged methane and nitrous oxide concentrations with fast measurement cycle and at moderate ranges, allowing us to remotely detect and evaluate the presence of gas leaks. The system takes advantage of the remarkable spectral stability of the intra-pulse frequency chirped DFB-QCL to carry out intra-pulse gas spectroscopy in a backscatter configuration. The methodology to retrieve the path-average gas mixing ratio using a least square analysis is presented as well as a numerical simulation to evaluate the expected SNR and the relative uncertainty for methane mixing ratio measurement. Experimental measures are presented for both methane and nitrous oxide. Small puffs of gas (~150 mg of methane and ~2 mg of nitrous oxide) were released nearby a 40 + meters path between the QCLOPS and a scattering target. We demonstrate retrieval of the path-averaged gas mixing ratio with minimum uncertainties of 9.3 % for CH4 and 3.1 % for N2O with a 68-ms measurement cycle. These results are in good agreement with the uncertainty predicted by the SNR analysis for methane. With the low pulse power QCL used (0.31 mW average power), the normalized sensitivity is 30 ppm m Hz−1/2 for methane and 3.3 ppm m Hz−1/2 for nitrous oxide.
It is important to note that the range to the scattering target is simultaneously retrieved from time resolved measurements for the relatively short pulses deployed and incorporated in the path-averaged concentration retrievals. A full spectrum is recorded for every pulse. Due to the relatively small frequency window used (~1 cm−1) and the spectral stability of the QCL, the gas concentration revivals are not sensitive to the laser intensity fluctuations or the frequency dependence/variation in the backscatter coefficient of various hard (topographic) targets.
The resulting detection limits from Sect. 4 for CH4 and N2O, respectively, 117 and 12.8 ppm m are for 15 Hz measurement cycle and limited by the laser power used in this work (0.31 mW average power with 20 kHz pulse repetition rate) and the short measurement cycle. These are high when compared to gas measurements systems referred to earlier (including work by our group) that use gas sampler based on laser transmission through a multipass cell, or open-path fence-line systems with retroreflectors [38, 52]. However, it is important to note that such systems are not standoff systems, and while sensitivities of laser transmission systems are higher than backscatter systems, applications are very different, as an example, backscatter systems are suitable for mobile or multidirectional standoff leak detection applications.
Our normalized sensitivity of 30 ppm m Hz−1/2 for CH4 and 3.3 ppm m Hz−1/2 for N2O may be compared with other standoff gas detection techniques in the literature. Macleod et al. [53] present a coherent detection technique based on a QCL for standoff measurements of chemicals using quantum cascade laser with a normalized sensitivity of 13.7 ppm m Hz−1/2 for N2O despite having almost 100 times more laser power (30 mW, 0.31 mW in our case). Frish et al. [17] claim a 5 ppm m sensitivity at 10 Hz (1.6 ppm m Hz−1/2) for methane up to 30 m range using a semiconductor diode laser in the near-infrared, with 32 times more average laser power. However, we note that according to our analysis presented earlier, SNR increases linearly with the laser power. Higher power lasers are now available and could notably improve our detection limits and range if deployed. Similarly, the QCL can operate at higher repetition rate, and we have observed that the SNR indeed decreases with the square root of the number of averaged pulses. In our case, going from 20 kHz repetition rate to 100 kHz would increase our SNR by a factor of 2.2, and gas detection sensitivities for the same measurement cycle (15 Hz) could be enhanced by about the same factor as the SNR for methane based on our simulations above. Therefore, taking into consideration our low average laser power, the system presented here has performance that is at least on-par or better than other standoff techniques.
Future work will be dedicated to the demonstration of the backscatter QCLOPS gas sensor for leak detection in filed applications using topographic targets to investigate the feasibility of the instrument to perform mobile or multidirectional search and remote detection of gas leaks. As an example, if set on a platform moving at 50 km/h, the instrument could perform one concentration measurement every meter. Thus, it makes possible the localization of gas leaks over large distances in a short time frame and with an excellent spatial resolution.
References
T.F. Stocker, D. Qin, G.K. Plattner, M. Tignor, S.K. Allen, J. Boschung, B.M. Midgley: IPCC, 2013: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (2013)
W. Demtröder, Laser Spectroscopy 2. Experimental Techniques (Springer, Berlin, 2008)
C. Weitkamp, Lidar, Range-Resolved Optical Remote Sensing of the Atmosphere (Springer, Berlin, 2005)
T. Fujii, T. Fukuchi, Laser Remote Sensing (CRC Press, Boca Raton, 2005)
J. Hodgkinson, R.P. Tatam, Meas. Sci. Technol. 24, 012004 (2013)
Z. Bacsik, J. Mink, G. Keresztury, Appl. Spec. Rev. 40, 327 (2005)
M.J. Wooster, P.H. Freeborn, S. Archibald, C. Oppenheimer, G.J. Roberts, T.E.L. Smith, N. Govender, M. Burton, I. Palumbo, Atmos. Chem. Phys. 11, 11591 (2011)
T.E.L. Smith, M.J. Wooster, M. Tattaris, D.W.T. Griffith, Atmos. Meas. Technol. 4, 97 (2011)
H.M. Heise, U. Müller, A.G. Gärtner, N. Hölscher, Field Anal. Chem. Technol. 5, 13 (2001)
J.M.C. Plane, A. Saiz-Lopez, in UV–Visible Differential Optical Absorption Spectroscopy (DOAS), Analytical Techniques for Atmospheric Measurement, ed. by D.E. Heard (Blackwell, Oxford, 2006), pp. 147–188
R. Volkamer, L.T. Molina, M.J. Molina, T. Shirley, W.H. Brune, Geophys. Res. Lett. 32, L08806 (2005)
A.J. McGonigle, J. Volcanol. Geotherm. Res. 162, 111 (2007)
U. Platt, J. Stutz, Differential Optical Absorption Spectroscopy (Springer, Berlin, 2008)
B. Thomas, A. Miffre, G. David, J.P. Cariou, P. Rairoux, Appl. Phys. B 108, 689 (2012)
B. Thomas, G. David, C. Anselmo, J.P. Cariou, A. Miffre, P. Rairoux, Appl. Phys. B 113, 265 (2013)
J. Li, B. Yu, W. Zhao, W. Chen, Appl. Spec. Rev. 49, 666 (2014)
M.B. Frish, R.T. Wainner, M.C. Laderer, B.D. Green, M.G. Allen, IEEE Sens. J. 10, 639 (2010)
R.T. Wainner, B.D. Green, M.G. Allen, M.A. White, J. Stafford-Evans, R. Naper, Handheld, Appl. Phys. B 75, 249 (2002)
A. Kosterev, G. Wysocki, Y. Bakhirkin, S. So, R. Lewicki, M. Fraser, F. Tittel, R.F. Curl, Appl. Phys. B 90, 165 (2008)
C. Gmachl, F. Capasso, D.L. Sivco, A.Y. Cho, Rep. Prog. Phys. 64, 1533 (2001)
R.F. Curl, F. Capasso, C. Gmachl, A.A. Kosterev, B. McManus, R. Lewicki, M. Pusharsky, G. Wysocki, F.K. Tittel, Chem. Phys. Lett. 487, 1 (2010)
L. Zhang, G. Tian, J. Li, B. Yu, Appl. Spec. 68, 1095 (2014)
G.W. Santoni, B.C. Daube, E.A. Kort, R. Jiménez, S. Park, J.V. Pittman, E. Gottlieb, B. Xiang, M.S. Zahniser, D.D. Nelson, J.B. McManus, J. Peischl, T.B. Ryerson, J.S. Holloway, A.E. Andrews, C. Sweeney, B. Hall, E.J. Hintsa, F.L. Moore, J.W. Elkins, D.F. Hurst, B.B. Stephens, J. Bent, S.C. Wofsy, Atmos. Meas. Technol. 7, 1509 (2014)
J. Manne, O. Sukhorukov, W. Jager, J. Tulip, Appl. Opt. 45, 9230 (2006)
J.C. Cao, Rev. Special Focus Sci. China Inf. Sci. 55, 16 (2012)
D.J. Miller, K. Sun, L. Tao, M.A. Khan, M.A. Zondlo, Atmos. Meas. Technol. 7, 81 (2014)
D.R. Crawford, M.E. Parrish, D.L. Gee, C.N. Harward, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 67, 4 (2007)
Q. Shi, D.D. Nelson, J.B. McManus, M.S. Zahniser, M.E. Parrish, R.E. Baren, K.H. Shafer, C.N. Harward, Anal. Chem. 75, 5180 (2003)
P. Kluczynski, S. Lundqvist, J. Westberg, O. Axner, Appl. Phys. B 103, 451 (2011)
L. Menzel, A.A. Kosterev, F.K. Tittel, C. Gmachl, F. Capasso, D.L. Sivco, J.N. Baillargeon, A.L. Hutchinson, A.Y. Cho, W. Urban, Appl. Phys. B 72, 859 (2001)
A. Karpf, G.N. Rao, Appl. Opt. 48, 408 (2009)
G. Duxbury, K.G. Hay, N. Langford, M.P. Johnson, J.D. Black, Mol. Phys. 109, 2131 (2011)
W.H. Weber, J.T. Remillard, R.E. Chase, J.F. Richert, F. Capasso, C. Gmachl, A.L. Hutchinson, D.L. Sivco, J.N. Baillargeon, A.Y. Cho, Appl. Spec. 56, 706 (2002)
D. Weidmann, A.A. Kosterev, C. Roller, R.F. Curl, M.P. Fraser, F.K. Tittel, Appl. Opt. 43, 3329 (2004)
T.H. Risby, F.K. Tittel, Opt. Eng. 49, 111123 (2010)
M.R. McCurdy, Y. Bakhirkin, G. Wysocki, R. Lewicki, F.K. Tittel, J. Breath Res. 1, 014001 (2007)
G. Duxbury, N. Langford, M.T. McCulloch, S. Wright, Chem. Soc. Rev. 34, 921 (2005)
P.C. Castillo, I. Sydoryk, B. Gross, F. Moshary, in Proceedings of the SPIE 8718, Advanced Environmental, Chemical, and Biological Sensing Technologies X, 87180J (2013)
C.L. Yaws, Chemical Properties Handbook (McGraw-Hill, New York, 1999)
M.G. Zabetakis, Flammability characteristics of gases and vapors. Bureau Mines Bull. 627, 24 (1965)
M. Taslakov, V. Simeonov, M. Froidevaux, H. van den Bergh, Appl. Phys. B 82, 501 (2006)
J. Manne, W. Jäger, J. Tulip, Appl. Phys. B 94, 337 (2009)
M.T. McCulloch, E.L. Normand, N. Langford, G. Duxbury, D.A. Newnham, JOSA B 20, 1761 (2003)
E. Normand, M. McCulloch, G. Duxbury, N. Langford, Opt. Lett. 28, 16 (2003)
A.A. Kosterev, F.K. Tittel, IEEE J. Quantum Electron. 38, 582 (2002)
A.A. Kosterev, R.F. Curl, F.K. Tittel, C. Gmachl, F. Capasso, D.L. Sivco, J.N. Baillargeon, A.L. Hutchinson, A.Y. Cho, Opt. Lett. 24, 1762 (1999)
P.R. Bevington, D.K. Robinson, Data Reduction and Error Analysis (McGraw-Hill, New York, 2003)
S. Chapra, R. Canale, Numerical Methods for Engineers (McGraw-Hill, Boston, 2010)
W.H. Press, S.A. Teukolsky, W.T. Vetterling, B.P. Flannery, Numerical Recipes 3rd edition: The Art of Scientific Computing (Cambridge University Press, Cambridge, 2007)
L.S. Rothman, I.E. Gordon, A. Barbe, D. ChrisBenner, P.F. Bernath, M. Birk, V. Boudon, L.R. Brown, A. Campargue, J.-P. Champion, K. Chance, L.H. Coudert, V. Dana, V.M. Devi, S. Fally, J.-M. Flaud, J. Quant. Spectrosc. Radiat. Transf. 110, 533 (2009)
M. Lwin, P. Corrigan, B. Gross, F. Moshary, S. Ahmed, in Proc. SPIE, Infrared Technology and Applications XXXVI, 7660, 766043 (2010)
N.S. Daghestani, R. Brownsword, D. Weidmann, Opt. Exp. 22, 1731 (2014)
N.A. Macleod, R. Rose, D. Weidmann, Opt. Lett. 38, 3708 (2013)
Acknowledgments
This work was supported by the National Science Foundation, the Mid Infrared Technologies for Health and the Environment (MIRTHE) Engineering Research Center Award Number 0540832.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Diaz, A., Thomas, B., Castillo, P. et al. Active standoff detection of CH4 and N2O leaks using hard-target backscattered light using an open-path quantum cascade laser sensor. Appl. Phys. B 122, 121 (2016). https://doi.org/10.1007/s00340-016-6396-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00340-016-6396-x