Abstract
The hyperfine structure of the 5d6s 2 2D3/2 → 5d6s6p 4F5/2 transition of the radioactive isotope 177Lu has been investigated by laser photoionization spectroscopy. Measured spectra permitted the determination of hyperfine magnetic dipole constants and electric quadrupole constants for ground and excited state as well as the isotope shift of the 177Lu isotope. The data obtained were used to confirm the selective photoionization of 177Lu from a neutron-irradiated sample that initially had a natural isotope composition. A concentration for 177Lu of 50 % was achieved, and the photoionization efficiency was estimated as suitable for technological application.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
An increased interest in the isotope 177Lu over the last decade has been associated with its growing use in cancer treatment owing to its special nuclear properties: a β − decay half-life of 6.7 days, a low energy of 0.13 MeV, a short absorption path for the beta emission (<2 mm), and γ-emissions suitable for imaging [1–4]. Various therapies require different specific activities of 177Lu. In particular, 177Lu with a concentration >20 % (specific activity >740 GBq/mg) is used in peptide receptor radionuclide therapy [5]. Given that natural lutetium contains only 175Lu (97.4 %) and 176Lu (2.6 %), 177Lu must be produced artificially. Current production methods use either highly enriched isotopes of 176Lu (direct route) or 176Yb (indirect route), which are irradiated in a nuclear reactor with further radiochemical processing. The enriched material use efficiency is very low, and even for the direct route the efficiency does not exceed 25 % [6]. Another possible production route, proposed in this work, is to use a neutron capture reaction 176Lu (n,γ) → 177Lu, where natural lutetium metal is irradiated in a nuclear reactor. After irradiation, the lutetium target consists of the natural isotopes 175Lu, 176Lu, and 177Lu together with the nuclear isomer 177mLu. The concentration of 177Lu, which depends on the neutron flux and the spectral characteristics, is typically <0.1 %. For medical applications, 177Lu needs to be extracted from the mixture and this step can be performed by atomic vapor laser isotope separation technology (AVLIS technology) [7–9]. It is considered that this approach involving isotope separation at the final stage of 177Lu radionuclide production is promising, particularly from the standpoints of efficiency and low-waste technology.
It should be noted that laser isotope separation has been achieved for indirect 177Lu production [10]. Using this laser technology, the authors succeeded in extracting 176Yb enriched up to 97 % from natural ytterbium.
A three-step selective photoionization scheme (most typical for AVLIS) for lutetium has recently been proposed and investigated for a natural isotope mixture [11] (Fig. 1). The scheme was used successfully for 176Lu extraction from a natural isotope mixture. Despite a small isotope shift (−388.8 MHz) [12], 176Lu could be separated from 175Lu. Some hyperfine structure (HFS) components of 176Lu and 175Lu were sufficiently separated from each other, thus permitting selective and efficient photoionization of 176Lu. In this way, a weight amount of 176Lu with a concentration of about 70 % was prepared.
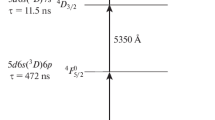
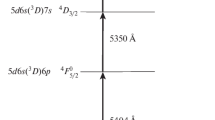
The present study is concerned with application of the 177Lu extraction scheme to neutron-irradiated natural lutetium. The possibility for this process is critically dependent on whether there are HFS 177Lu components that may be used for selective photoionization. The HFS spectroscopy of the optical transition at 451.9 nm for 177Lu was explored [13], but this transition was not applicable to conventional AVLIS with dye lasers pumped by copper vapor lasers (CVL). Therefore, the structure of the transition from the ground state 5d6s 2 2D3/2 to the excited state 5d6s6p 4F5/2 at 540.4 nm (also further wavelengths in the vacuum) was investigated. Despite data on the HFS of the 177Lu ground state being available [13–15], there is no information on isotope shifts and hyperfine interaction constants for the 5d6s6p 4F5/2 level, hence the raison d’être for this work.
2 Experimental
Research was performed at a purpose-built facility for spectroscopic measurements. The instrumentation featured narrow collimated atomic beams, and specialized equipment was used for determination of photoion isotopic composition following laser resonance selective photoionization. This technique and setup are quite similar to conventional resonance ionization spectroscopy setups described elsewhere [10, 16, 17]. The facility included a vacuum chamber with pumping system, a tantalum oven mounted inside the chamber, a quadrupole mass spectrometer, and photoion and fluorescence registration units. The CVL pumped dye laser system consisted of three single-mode master oscillators and two dye amplifiers. The master oscillators were constructed on the grazing incidence diffraction grating scheme [18]. The laser dye, pyrromethene-556 pumped with the 510.7-nm CVL component, was applied on the first and second steps (Fig. 1). Sulforhodamine-640 pumped by the 578.3-nm CVL component was used on the third step. Master oscillators were able to scan the radiation wavelength by changing the drive voltage on the piezoelectric supports of the cavity-dispersive elements. High-precision meters (LM-007, Laser 2000 GmbH) provided laser wavelength measurements with an absolute accuracy of 0.0005 Å and served also as stabilized etalons for active wavelength stabilization of master oscillators. Dye amplifiers [19] were used to increase power at the second and third steps. Three output laser beams were expanded to a diameter of 12 mm and then collimated. Semitransparent and dichroic mirrors were used to combine the radiation from the three dye lasers into one beam. The second- and third-step laser pulses were time delayed by 25 ns with respect to the first laser step pulse to avoid the effect of multiphoton processes on wavelength determination. Given that the lifetime of the 5d6s6p 4F5/2 level is 472 ns [20], the second and third laser radiation affects the atoms in the excited state after termination of the first laser step pulse. The performance parameters of the laser system are presented in Table 1.
The 12-mm-diameter, three-color laser beam was transmitted through a 2-mm diaphragm and directed into the mass spectrometer.
Five milligrams of natural lutetium metal was irradiated by a 1.5 × 1013 1/cm2 s thermal neutron flux for 1 day, and after a decay time of 1 week, the irradiated sample was loaded into the tantalum oven. The concentration of radioactive 177Lu atoms, as determined by gamma spectroscopic analysis, was 10−5. Vaporized lutetium atoms (several milligrams per hour) passed through the diaphragm system and formed an atomic beam (3° divergence angle) that was orthogonal to the laser beam. The estimated Doppler broadening was ~100 MHz. The laser beam intersected the atomic beam directly within the ionization (electron impact) chamber of the mass spectrometer. The intersection region had the shape of a cylinder with a diameter and length of 2 mm. Photoions were detected by a secondary electron multiplier, and fluorescent photons were measured by a photomultiplier located on an ion-optical spectrometer axis in a direction opposite to the photoion flow (Fig. 2). The fluorescence registration system had low sensitivity and could not detect 177Lu fluorescence. This system was used for measuring 175Lu HFS. Control of the laser system and data recording were accomplished on line, and this allowed the experiment to be completed in 1 h.
To measure the 177Lu photoion spectrum, the first wavelength was scanned, while the second- and third-step wavelengths were fixed and stabilized. The whole HFS structure of the transition cannot be measured in one scan as the wavelengths of the second and third step are different for each HFS component. Each scan included one or several HFS resonances, which are close to each other and can be measured when the second- and third-step laser wavelengths are fixed. For different scans, the wavelengths of the second and third step were roughly adjusted to maintain the ion signal value. The photoion signal versus wavelength of the first transition is shown in Fig. 3. Because of the imperfections in the second and third wavelengths tuning mentioned above, the amplitude of the components did not represent the real oscillator strength.
Hyperfine structure of the 5d6s2 2D3/2 → 5d6s6p 4F5/2 transition. The low number F is the ground-state total angular momentum of atom, while the upper number F′ is the momentum of the excited state. All transitions were identified except the 4 → 3 and the 2 → 3 transitions, which were not resolved. The arrow marks these resonances
3 Results and discussion
The experimental data permitted calculation of the ground- and excited-state hyperfine interaction constants and the center of gravity (CG) energy for the 5d6s6p 4F5/2 state based on the expression:
where E (cm−1) is the CG energy of the excited level (zero for ground state), Δ F and Δ F′ (cm−1) are the low- and upper-level hyperfine splitting, and λ FF′ (cm) is the transition wavelength. The center of each line was determined by Voigt line shape fitting performed using packet PAW of the CERN library [21]. The best fit found corresponded to 10 (+10; −5) MHz Lorentzian and 125 (±10) MHz Gaussian widths (confidence level of 95 %) and was the same for all peaks (Fig. 4).
The constants for the hyperfine magnetic dipole and electric quadrupole interactions of the excited A e, B e, and ground states A g, B g are included with a linear relationship in the equations for Δ F′ and Δ F [22]. The A- and B-factors as well as the CG energy found by the least square fit are listed in Table 2.
The 177Lu ground-state constants obtained in this study coincided with published values [13–15] within experimental error. It is possible to compare the measured 177Lu excited-state constants with the estimates using the appropriate 175Lu values [23]. Actually, apart from a small hyperfine anomaly, the ratio of the 177Lu and 175Lu constants was equal to the ratio of the known nuclear magnetic µ I /I factors for Ae (µ I —nuclear magnetic moment, I—nuclear spin) and the quadrupole moments for B e of these isotopes [13]. The measured values are in good agreement with estimates (Table 2, row 4 and 6). It was also possible to compare the 175Lu and 177Lu isotope shifts measured and estimated. In heavy atoms, the isotope shift is mainly caused by differences in nuclear charge density distribution (field shift). For the transition of interest, 5d6s 2 → 5d6s6p, the isotope shift is almost completely a field shift because the normal and the specific mass shifts are small and do not exceed ~20 MHz [24]. The isotope shift measured in our study can be compared with that using the mean square charge radii difference δ‹r2› data [13]. The difference δ‹r 2› for 175Lu and 177Lu exceeds that for 175Lu and 176Lu by about three times. This means that the isotope shift of 177Lu relative to 175Lu should be three times greater than that between 176Lu and 175Lu [25]. Taking as a reference the isotope shift between 176Lu and 175Lu as −388.8 MHz [12], we obtain in this case an isotope shift between 177Lu and 175Lu of −1147 MHz. Analogous estimates of constants and isotope shifts can be made for the 177mLu isomer (Table 2).
The relative positions of the studied 177Lu HFS components and those calculated for the natural isotopes are shown on the common frequency scale (Fig. 5). Calculations were made using the literature values for the constants [12, 13, 23, 26].
Figure 5 shows that transitions 2 → 1, 2 → 3, 3 → 3, 4 → 3, 4 → 5, and 5 → 6 are suitable for 177Lu selective photoionization because these components are well separated from the components of the other isotopes.
The result of 177Lu selective photoionization on the 5–6 first transition is shown in Fig. 6. The mass spectrum was recorded at the wavelength of the first transition tuned to resonance with the 5–6 component, while the second- and third-stage wavelengths were tuned to the maximum current of 177Lu. After wavelength adjustment, the mass spectrometer (resolving power at 10 % of peak height was 200) was scanned over the mass range of 170–180 a.m.u and the spectra were recorded. The first-, second-, and third-step laser average power densities were 10, 10 mW/cm2, and 3.1 W/cm2, respectively.
Laser-induced photoion mass spectrum. Vertical bar is photoion signal, mV. Radiation at the first stage was tuned to resonance with component 5–6. The initial 177Lu concentration was 10−5. The first-, second-, and third-step laser average power densities were 10, 10 mW/cm2, and 3.1 W/cm2, respectively
The selectivity of photoionization depended strongly on the first- and second-step laser intensities, the 177Lu concentration being reduced to ~6 % for the first and second laser average power densities of 20 and 300 mW/cm2, respectively. The selectivity factor generally used in separation techniques can be represented as the ratio of the photoionization probability for the target isotope W 177 and the non-target isotope W:
The number of target photoions is W 177 · C F177, where C F177 is the initial concentration of the target isotope.
The number of non-target photoions is W(1 − C F177). The photoion concentration of the target isotope measured by the mass spectrometer is therefore:
Selectivity may be expressed in terms of concentrations by the formula:
For a 177Lu initial sample concentration C F177 = 10−5 and a 177Lu photoion concentration C P177 = 0.5, the selectivity (4) is 105. The selectivity measured exceeded significantly the ratio of the first-step laser intensities applied to the 177Lu and 175Lu isotopes. For the 5–6 transition (Fig. 5), the shift between the 177Lu and 175Lu components was 1300 MHz. The ratio of the Voigt intensity (with Lorentzian width 10 MHz and Gaussian width 125 MHz) at the center of the line to the intensity at a frequency of 1300 MHz from the center was 8 × 103. This excess selectivity may be explained by the additional selectivity at the second step. This finding is in good agreement with the experimental result that selectivity strongly depends on the second laser wavelength and intensity. The effect of improved selectivity by multistep ionization has been demonstrated by several groups [10, 27, 28].
The upper limit of photoionization efficiency may be determined by measuring the dependence of the photoion signal on laser intensity. A typical response curve showed a linear increase in photoion signal at low laser intensity, but with a further increase in intensity, the photoion signal became saturated. The reason for saturation is owing to a depletion of ground-state atoms. Figure 7 shows the experimental dependence of photoion signal on the first (a)-, second (b)-, and third (c)-stage laser intensity. It can be seen that saturation was readily attained for 10 mW/cm2 average power density at the first stage and 10 mW/cm2 and 2 W/cm2 at the second and third stages, respectively. In the case of saturation, the photoionization efficiency was proportional to the population of the start level. The 177Lu ground-state population at the vaporization temperature of ~1700 K was 77 %. The ground state consists of four sublevels with F quantum numbers 2, 3, 4, and 5. The population of the sublevels is proportional to the statistical weight 2F + 1 of the sublevel, i.e., 15.6, 21.9, 28.1, and 34.3 % for the sublevel with F = 2, 3, 4, and 5, respectively. For example, if the F = 5 sublevel is used as the initial level, the 34.3·0.77 ~26 % of 177Lu atoms may be converted into photoions.
a Dependence of ion signal on first-step laser intensity. The second- and third-step laser average power densities were 0.47 and 3.1 W/cm2. b Dependence of ion signal on second-step laser intensity. The first- and third-step laser average power densities were 10 mW/cm2 and 3.1 W/cm2. c Dependence of ion signal on third-step laser intensity. The first- and second-step laser average power densities were 10 and 20 mW/cm2
In the case of the second and third stages of photoionization where there is a 25-ns delay, a smaller portion of atoms may be ionized. This portion is equal to the amount of atoms that remained in the first excited state after the first-step pulse. This amount, in the case of saturation, is equal to the ratio of the statistical weight of the upper state to the statistical weight of the lower state. For the 5–6 transition, this ratio is 0.54 and the portion of 177Lu atoms is reduced to ~14 %.
The remarkable feature of 177Lu extraction technology is that it does not require significant mass productivity, in contrast to, for example, AVLIS for the production of 100 kg of 150Nd [9]. The mass equivalent for a typical patient dose of 7 GBq is ~1.8 × 10−6 g of pure 177Lu. This makes it possible to consider a production facility with the Doppler broadening of atom flow similar to that used in spectroscopic experiments to maintain the selectivity of photoionization. The many problems associated with ion extraction and ion sputtering disappears because of the low photoion current and extraction voltage. One source of loss, however, does remain, and this is connected with the probability of atom irradiation by the pulsed laser. This type of loss may be significantly reduced by stretching the laser irradiation volume along the atom flow. Therefore, it is reasonable to expect that the total extraction of 177Lu in a production facility will be not less than 10 %.
Given that selectivity is invariant to variations in concentration, expression (4) may be used for the calculation of the 177Lu photoion concentration, CP177, at different initial concentrations CF177. The result of the calculation is presented in Fig. 8 as a function of the sample neutron irradiation time.
Concentration of 177Lu in irradiated sample multiplied by 104 (black line). 177Lu photoion concentration given by expression (4) with a selectivity of 105 (red line)
The black line represents the dynamics of the production of 177Lu concentration in an IR-8 reactor at a thermal neutron flux of 1.5 × 1013 1/cm2s. The red line is the calculation of the photoion 177Lu concentration, CP177, given by expression (4) with a selectivity of 105. The figure shows that even in the case of a relatively low neutron flux facility, it is possible to obtain a photoion 177Lu concentration above 80 %. A selectivity of 105 is achieved at laser saturation intensities that permit significant photoionization efficiency, suitable for the technological applications.
An important advantage of the indirect route for 177Lu production is the low content of 177mLu. The metastable isomer 177mLu with a decay half-life of 160 days complicates radioactive waste handling in medical practice. A typical value for the 177mLu/177Lu ratio for the direct route varies between 0.01 and 0.02 % [6]. The AVLIS extraction of 177Lu from a neutron-irradiated natural lutetium target has the potential for additional purification of 177mLu because the absorption lines of 177mLu (according to estimates in Table 2) are shifted from the 177Lu lines by 670 MHz for the 5–6 transition and by 1700 MHz for the 2–1 transition.
4 Conclusions
The measurement of hyperfine structure and isotope shift for the 5d6s 2 2D3/2 → 5d6s6p 4F5/2 transition revealed 177Lu HFS components with a significant gap of ~1 GHz from the other isotopes. These spectral lines can be employed for the extraction of 177Lu from an isotope mixture of irradiated natural lutetium by the AVLIS method. Experimentally, a selectivity of 177Lu photoionization of 105 was achieved. The photoion concentration of 177Lu of 50 % was obtained for irradiation of a natural lutetium sample for 1 day in a neutron flux of 1.5 × 1013 1/cm2s. The high 177Lu photoion concentration occurred at laser intensities corresponding to the photoion current saturation range, and this is a good basis for further refinement and development of the separation technology.
References
NuPECC Report “Nuclear Physics for Medicine”: 136 (2014)
D.J. Kwekkeboom, W.W. de Herder, B.L. Kam, C.H. van Eijck, M. van Essen, P.P. Kooij, R.A. Feelders, M.O. van Aken, E.P. Krenning, J. Clin. Oncol. 26, 2124 (2008)
S. Khan, E.P. Krenning, M. van Essen, B.L. Kam, J.J. Teunissen, D.J. Kwekkeboom, J. Nucl. Med. 52, 1361 (2011)
A. Dash, M.R.A. Pillai, F.F. Knapp Jr., Nucl. Med. Mol. Imaging 49, 85 (2015). doi:10.1007/s13139-014-0315-z
W.A. Breeman, R.A. de Zanger, H.S. Chan, E. Blois, Curr. Radiopharm. 8(2), 119–122 (2015)
R. Henkelmann, A. Hey, O. Buck, K. Zhernosekov, T. Nikula, Workshop “Physics for health in Europe”, Contribution ID: 82 (2010)
J.A. Paisner, Appl. Phys. B 46, 253 (1988)
P.T. Greenland, Contemp. Phys. 31, 405 (1990)
A.P. Babichev, I.S. Grigoriev, A.I. Grigoriev, A.P. Dorovskii, A.B. D’yachkov, S.K. Kovalevich, V.A. Kochetov, V.A. Kuznetsov, V.P. Labozin, A.V. Matrakhov, S.M. Mironov, S.A. Nikulin, A.V. Pesnya, N.I. Timofeev, V.A. Firsov, G.O. Tsvetkov, G.G. Shatalova, Quantum Electron. 35, 879 (2005). doi:10.1070/QE2005v035n10ABEH006601
H. Park, D.H. Kwon, Y.H. Cha, T.S. Kim, J. Han, K.H. Kon, D.Y. Jeong, C.J. Kim, J. Nucl. Sci. Techn. Supp. 6, 111 (2008)
A. D’yachkov, S. Kovalevich, A. Labozin, S. Mironov, V. Panchenko, V. Firsov, G. Tsvetkov, G. Shatalova, Quantum Electron. 42, 953 (2012). doi:10.1070/QE2012v042n10ABEH014929
W. Jin, M. Wakasugi, T. Inamura, T. Murayama, T. Wakui, H. Katsuragawa, T. Ariga, T. Ishizuka, M. Koizumi, I. Sugai, Phys. Rev. A 49, 762 (1994). doi:10.1103/PhysRevA.49.762
U. Georg, W. Borchers, M. Keim, A. Klein, P. Lievens, R. Neugart, M. Neuroth, P.M. Rao, Ch. Schulz, the ISOLDE Collaboration, Eur. Phys. J. A 3, 225 (1998)
F.R. Petersen, H.A. Shugart, Phys. Rev. 126, 252 (1962)
R. Müller, E.W. Weber, Z. Physik A 275, 305 (1975). doi:10.1007/BF01434016
S. Rothe, A.N. Andreyev, S. Antalic, A. Borschevsky, L. Capponi, T.E. Cocolios, H. De Witte, E. Eliav, D.V. Fedorov, V.N. Fedosseev, D.A. Fink, S. Fritzsche, L. Ghys, M. Huyse, N. Imai, U. Kaldor, Y. Kudryavtsev, U. Köster, J.F.W. Lane, J. Lassen, V. Liberati, K.M. Lynch, B.A. Marsh, K. Nishio, D. Pauwels, V. Pershina, L. Popescu, T.J. Procter, D. Radulov, S. Raeder, M.M. Rajabali, E. Rapisarda, R.E. Rossel, K. Sandhu, M.D. Seliverstov, A.M. Sjödin, P. Van den Bergh, P. Van Duppen, M. Venhart, Y. Wakabayashi, K.D.A. Wendt, Nat. Commun. 4, 1835 (2013). doi:10.1038/ncomms2819
B.L. Feary, D.C. Parent, R.A. Keller, C.M. Miller, J. Opt. Soc. Am. B 7, 3 (1990)
I. Grigoriev, A. Diachkov, V. Kuznetzov, V. Labosin, V. Firsov, in Proceedings of SPIE 5121 Laser Processing of Advanced Materials and Laser Microtechnologies 411 (2003)
I. Grigoriev, A. D’yachkov, V. Labozin, S. Mironov, S. Nikulin, V. Firsov, Quantum Electron. 34, 447 (2004)
J.A. Fedchak, E.A. Den Hartog, J.E. Lawler, P. Palmeri, P. Quinet, E. Biemont, Astrophys. J. 542, 1109 (2000)
I. Sobelman, Introduction to theory of atomic spectra (in Russian) (Nauka, Moscow, 1977)
A. Kuhnert, A. Nunnemann, D. Zimmermann, J. Phys. B 16, 4299 (1983). doi:10.1088/0022-3700/16/23/013
S. Witte, E. van Duijn, R. Zinkstok, W. Hogervorst, Eur. Phys. J. D 20, 159 (2002)
L. Landau, E. Lifshitz, Quantum Mechanics, vol. 3 (Pergamon Press, New York, 1965)
J. Verges, J.F. Wyart, Phys. Scripta 17, 495 (1978)
B.A. Bushaw, W. Nörtenhäuser, K. Wendt, Spectrochim. Acta B 54, 321 (1999)
P. Müller, B.A. Bushaw, K. Blaum, S. Diel, Ch. Geppert, A. Nähler, N. Trautmann, W. Nörtenhäuser, K. Wendt, Fresenius J. Anal. Chem. 370, 508 (2001)
Acknowledgments
The authors express their gratitude to D.Y. Chuvilin, Y.V. Vyazovetskiy, and V.A. Zagryadskiy as well as to the scientific group of the “Kurchatov Institute” IR-8 reactor for the preparation and analysis of the sample. The work was partially supported by a RFBR 12-08-00561 grant for laser technology and by the Ministry of Education and Science of the Russian Federation project RFMEF160714X0041 as part of radiochemical sample preparation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
D’yachkov, A.B., Firsov, V.A., Gorkunov, A.A. et al. Photoionization spectroscopy for laser extraction of the radioactive isotope 177Lu. Appl. Phys. B 121, 425–431 (2015). https://doi.org/10.1007/s00340-015-6248-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-015-6248-0