Abstract
We report on the optical performances of laccaic acid dye in solution at different concentrations and dye–poly(methyl methacrylate) composite thin films. The linear spectral characteristics including optical constants, i.e. refractive index (n) and extinction coefficient (k), were carried out in a comprehensive way through absorbance, fluorescence and ellipsometric studies. The nonlinear optical parameters such as nonlinear absorption coefficient β eff (or β 2), the imaginary third-order susceptibility (Im[χ (3)]) and the imaginary part of second-order hyperpolarizability (γ) of the samples were evaluated using the open-aperture Z-scan technique with a laser pulse duration of 10 ns at 532 nm wavelength. The corresponding numerical values of these parameters were of 10−10, 10−11 and 10−32 order, respectively. Two-photon absorption was revealed to be the main driving physical mechanism in the nonlinear response. This suggests that laccaic acid dye can be a potential candidate for NLO materials application.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
With the extensive use of sensitive spectrophotometers and high-power lasers in many different applications, much interest is being directed towards the search for materials with linear and nonlinear optical (NLO) applications. Organic materials [1], including natural dyes [1–3] with large NLO responses, are of major interest for their potential applications in optical signal-processing devices, upconversion lasers [4], optical limiting for photo-sensors and human eyes protection from intense laser irradiation [5–8]. Additionally, materials with NLO properties such as broadband spectral response and fast response time are of great importance for realizing optical data storage [9, 10], two-photon microscopy [11] and three-dimensional optical storage micro-fabrication [12]. The design of devices of such typical applications requires physical mechanisms such as multiphoton absorption, nonlinear refraction and reverses saturable absorption, in which strong absorption at the excited state plays a key role. Thus, optical techniques such as absorption, fluorescence and ellipsometric spectroscopy are important tools for the optical properties characterization, while the Z-scan technique [13, 14] is employed for nonlinear absorption (NLA) and nonlinear refraction (NR) properties investigation. Through these techniques, a large number of organic compounds with a conjugated π-electron system have been investigated [16–17]. This includes natural dyes such as anthocyanin, Bixa orellana, chlorophyll, carotenoids, betanines [18–21], Hibiscus sabdarifa [22]. Recently, Pramodini et al. [23] investigated the NLO and optical properties of anthraquinone dyes and found reverse saturable absorption (RSA) response type. In conjugated organic materials, RSA associated with two-photon absorption (2PA) is the most likely NLO physical mechanism related to the creation of excited states of molecules whose absorption cross sections are larger than that of the ground state. In this paper, we report the linear and nonlinear absorption properties of laccaic acid dye belonging to the family of chromophores with anthraquinone structure based [24, 25].
2 Experimental details
The laccaic acid (Mw 537) was purchased from Kremer Pigmente Company (Kremer GmbH) in Germany. The molecular structure of laccaic acid dye shown in Fig. 1 presents an anthraquinone based. Four grades of 0.007, 0.014, 0.02 and 0.03 g were weighted and dissolved in 25 ml of acetone in containers. The solutions were thereafter filtered from residue. The initial concentrations were of 5.11 × 10−4, 1.02 × 10−3, 1.44 × 10−3 and 2.02 × 10−3 mol/l. For spin-coating purpose, 0.2 g of PMMA (Mw ≈ 550,000) powder weighted was mixed with 12 ml of each initial concentration and stirred for 1 h at 45 °C. The final concentration of the mixture was about 0.016 g/ml. Four thin films were therefore prepared at different rpm. The UV–Vis absorption spectra of both solution and thin film samples were recorded in the spectral range of 190–2500 nm using a LAMBDA 950 UV–VIS–NIR spectrophotometer. Fourier transform infrared (FTIR) spectrum of laccaic acid powder was recorded through a Bruker Vertex 70 FTIR unit with a spectral range of 200–4000 cm−1 for qualitative analysis. Fluorescence emission peaks were also recorded under an excitation wavelength of 523 nm using a QuantaMaster™ system. The optical constants refractive index n and extinction coefficient k of the thin films were estimated through the Tauc–Lorentz model using a spectroscopic ellipsometer Jobin–Yvon (UVSEL-NIR) in air at room temperature. The nonlinear optical response was examined through the fluence-dependent transmittance measurements using the standard open-aperture (OA) Z-scan technique employing a CW Nd: YAG laser with 10-ns pulses at 532 nm wavelength. In this particular experiment of Z-scan, the samples were mounted at the focal point during the fluence-dependent transmittance measurement while moving along the propagating direction of the laser beam.
3 Results and discussion
3.1 Absorption and vibrational spectroscopy
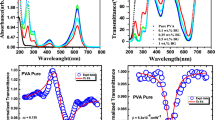
The electronic absorption spectra in both solution and spin-coated thin film samples are presented in Fig. 2a, b, respectively. The absorbance peaks in the liquid samples showed a convolution of peaks at three components at 467, 496 and 530 nm, attributed to the electronic \(\pi \to \pi^{*}\) transitions [25]. On the other hand, the thin films present sharp peaks at 485 nm. As shown in Fig. 1, laccaic acid dye contains electron-donating substituents (hydroxyl groups) and carbonyl groups as the acceptors. It is such typical donor–acceptor structure which leads to the electron density transfer through the delocalization mechanism. As a result, the spectral changes are due to the relaxation processes following \(\pi \to \pi^{*}\) interband absorption associated with the mobility of the electrons and holes and their highly delocalization. Moreover, the weak absorption signature observed between 325 and 380 nm could be attributed to the \(n \to \pi^{*}\) electronic transition in the C=O functional group. The FTIR spectrum of the laccaic acid dye is depicted in Fig. 3. The peak observed at 3117 cm−1 was assigned to the N–H stretching [25]. The absorption signature observed between 3650 and 3000 cm−1 can be assigned to the phenolic OH and OH of the carboxylic acid groups. The vibration modes observed between 1728 and 1000 cm−1 correspond to the anthraquinone-ring-based stretching with the presence of C=O, OH and NHC=O functional groups. The peaks at 1274 and 1200 cm−1 were assigned to the –C–O stretching of primary alcoholic group (–CH2–OH) and COOH group [25–27], respectively. The series of weak bands observed between 2000 and 1700 cm−1 can be attributed to simple aromatic group. The C–H bending vibration observed between 850 and 670 cm−1 can be used to sustain the presence of aromatic compound. The intramolecular H bonds resulting from the functional groups may induce a shift of each normal vibration band, making a difficult and complete interpretation of the vibrational modes of the laccaic acid molecule.
3.2 Fluorescence
Room-temperature fluorescence studies were carried out in both solutions and thin films as shown in the corresponding spectra in Figs. 4a and 5b, respectively. The visible absorption spectra can be interpreted as an allowed \(\pi \to \pi^{*}\) interband transition yielding electrons and holes in the conduction and valence bands, respectively [28, 29]. With ultraviolet or visible light, fluorophores in the dye molecule are excited to higher vibrational levels of the first or second singlet energy state. Therefore, the fluorescence emission occurs from the singlet electronic excited states to an allowable vibrational level in the electronic ground state [30]. Correlating the electronic absorption peaks to the emission peaks, a shift to longer wavelength can be observed. In other terms, the fluorescence emission peaks in both solution and thin film samples are red-shifted compared to those of the UV–Vis spectra. Although there is a similarity between the solution and thin film absorption spectra, a considerable difference between their fluorescence emission spectra can be observed. With increasing absorption, that is, with a decreasing energy difference between the excited and ground state, the number of options to get rid of the excited-state energy by radiationless deactivation increases. Hence, most known stable and bright fluorophores in the dye chromophore absorb and emit in the wavelength range between 300 and 700 nm [31]. Upon excitation to higher excited singlet states, molecules relax through internal conversion to higher vibrational levels of the first excited singlet state. If return transitions to the ground state (S 0) usually occur to a higher vibrational level, which subsequently reaches thermal equilibrium, then both vibrational relaxation and internal conversion cause heating of the solvent as the dye molecules in a high vibrational level of the triplet state can lose energy through collisions with solvent molecules [31, 32], which emission response would differ from that of thin film where the solvent is evaporated. Moreover, the extra peaks may arise from the host polymer which could blend the dye donor and acceptor systems, leading to the enhancement of the emission.
3.3 Linear optical parameters determination
Figure 5 shows spectroscopic ellipsometry data from the spin-coated thin films of laccaic acid dye–PMMA composite of 2000, 4000, 6000 and 8000 rpm. The corresponding thicknesses are of 51.52, 49.42, 27.26, 10.71 nm. The refractive index (n) and extinction coefficient (k), commonly called optical constants [33] were evaluated using Drude-Lorentz oscillator models in which the complex dielectric function is described by [33–35] :
where
and
where \(\varepsilon_{\infty }\) is the high-frequency dielectric constant. P is the Cauchy principal part of the integral. E j is the peak transition energy, E g is the optical band gap, C j is the brooding term, and A j is proportional to the transmission probability matrix element. The derived dispersion relations of the linear refractive index and the extinction coefficient as a function of the wavelength are displayed in Fig. 5. It can be seen that the refractive index (n) continuously decreases with the increased wavelength. However, the extinction coefficient for Tf1 increases from 345 up to 500 nm and becomes constant above 500 nm. For thin films Tf2, Tf3 and Tf4, the extinction coefficients are dominated by a peak in the near-visible/blue region of the spectrum. A closer inspection shows that the extinction coefficient spectra for Tf2 and Tf3 are the same, while their refractive indices spectra at longer wavelength differ. Above 600 nm, we can observe a relative increasing extinction coefficient (k) (except in Tf1), whereas no absorption peak is observed in the UV–Vis absorption spectra. All these observations suggest that the electron-donating character of auxochromes of the laccaic acid dye and, hence the steric effects do not prevent the electronic π-stacking interactions with the backbone [36]. Moreover, there should also be a contribution of increased reflection losses caused by the changes in the refractive index. Another possible explanation could be associated with the substrate transparency. As the substrate is transparent, then it is possible for some of the light to be reflected from the back surface of the substrate into the detector [37], hence influencing the extinction coefficient results. From the plot of refractive index and coefficient of extinction versus the thickness (not shown here), the minimum and maximum values are 1.32 and 1.43 for the refractive index and 0.06 and 0.15 for the coefficient of extinction, respectively. This shows that these parameters are thickness dependent.
3.4 Nonlinear optical absorption
The nonlinear absorption parameters were evaluated through the open-aperture Z-scan technique. Different processes such as two-photon absorption (TPA), saturable absorption (SA), reverse saturable absorption (RSA), transient absorption, free carrier interband absorption and nonlinear scattering are reported to be operative in the nonlinear absorption response. In the open-aperture Z-scan measurements, the Z-scan data exhibiting a peak shape will describe saturable absorption (SA) effect, while those showing a valley will be a signature of a reverse saturable absorption (RSA) process. Figure 6 shows the open-aperture Z-scan measurements on laccaic acid dye samples in solution and dye–PMMA composite thin film. It can be seen that the value of transmittance decays from the maximum absorbance value when the sample moves away from the pump beam focus. It can also be noticed that, as the input energy of the pumping beam increases, the transmittance value exhibits saturation far from the focus, in both negative and positive Z-position [38]. Therefore, one can accordingly deduce that the RSA process is likely to describe the observed nonlinear absorption of the laccaic acid dye. To confirm two or more photon absorption as main driving physical process, the experimental Z-scan data were fitted using the relation established by Sutherland [39]. The experimental data and numerical simulations are shown in Fig. 6. The NLA coefficient (β 2), the imaginary part of the third-order susceptibility (Im[χ (3)]) and the second-order hyperpolarizability (γ) were evaluated, which the numerical are summarized in Table 1.
Based on the fitting model [39–41], 2PA is found to be more consistent. Therefore, we propose 2PA as main physical mechanism process involved in the nonlinear response of our dye material. From the interesting NLA results observed, laccaic acid dye could be potential for applications such as optical limiters and optical data storage devices.
4 Conclusion
We have investigated the linear and nonlinear optical properties of laccaic acid dye. From the absorption spectroscopy analysis, it was found that the linear properties are concentration and thickness dependent. Nonlinear optical parameters β 2, Im[χ (3)] and γ calculated through the open-aperture (OA) Z-scan technique were of 10−10, 10−11 and 10−32 order, respectively. The achieved values of γ in the solutions were of 10-1 order of magnitude compared to that of anionic 3-dicyanomethylen-5,5-dimethyle-1-[2-(4-hydroxyphenyl)ethenyl)]-cyclohexene (1) in Ref. [42]. The study also reveals two-photon absorption to be consistent physical mechanism describing the nonlinear optical response of the laccaic acid dye. These results suggest that our material could be interesting candidate for photonic nonlinear applications.
References
Y.P. Sun, J.E. Riggs, Organic and inorganic optical limiting materials, from fullerenes to nanoparticles. Int. Rev. Phys. Chem. 18, 43–90 (1999)
A. Thankappan, S. Thomas, V.P.N. Nampoori, Solvent effect on the third order optical nonlinearity and optical limiting ability of betanin natural dye extracted from red beet root. Opt. Mater. 35, 2332–2337 (2013)
A. Sarkar, A. Thankappan, V.P.N. Nampoori, Effect of silver nanoparticles on fluorescence and nonlinear properties of naturally occurring betacyanin dye. Opt. Mater. 39, 211–217 (2015)
K.H. Drexhage, Structure and properties of laser dyes, in Dye Lasers, Topics in Applied Physics, vol. 1, ed. by F.P. Schäfer (Springer, Berlin, 1973), pp. 144–179
T.J. Bunning, I.V. Natarajan, M.G. Schmitt, B.L. Epling, R.L. Crane, Optical limiting in solutions of diphenyl polyenes. Appl. Opt. 30, 4341 (1991)
M. Anandi, Two-photon pumped unconverted lasing in dye doped polymer waveguides. Appl. Phys. Lett. 62(26), 3423–3425 (1993)
G.S. He, J.D. Bhawalkar, C.F. Zhao, P.N. Prasad, Optical limiting effect in a two-photon absorption dye doped solid matrix. Appl. Phys. Lett. 67, 2433–2435 (1995)
R. Chari, S.R. Mishra, H.R. Rawat, S.M. Oak, Reverse saturable absorption and optical limiting in indanthrone dyes. Appl. Phys. B 62, 293 (1996)
J.E. Ehrilich, X.L. Wu, I.Y.S. Lee, Z.Y. Hu, H. Rockel, S.R. Marder, J.W. Perry, Two-photon absorption and broadband optical limiting with bis-donor stilbenes. Opt. Lett. 22(24), 1843–1845 (1997)
G.S. He, G. Xu, P.N. Prasad, B.A. Reinhardt, J.C. Bhatt, A.G. Dillard, Two-photon absorption and optical-limiting properties of novel organic compounds. Opt. Lett. 20(5), 435–437 (1995)
H.S. Nalwa, S. Miyata, Nonlinear Optics of Organic Molecules and Polymers (CRC Press, Boca Raton, 1997)
D.A. Parthenopoulos, P.M. Rentzepis, Three-dimensional optical storage memory. Science 245(4920), 843–845 (1989)
M. Sheik-Bahae, A.A. Said, E.W. Van Stryland, High-sensitivity, single-beam n2 measurements. Opt. Lett. 14(17), 955 (1989)
M. Sheik-Bahae, A.A. Said, T.H. Wei, D.J. Hagan, E.W. Van Stryland, Sensitive measurement of optical nonlinearities using a single beam. IEEE J. Quantum Electron. 26(4), 760–769 (1990)
Y.P. Sun, J.E. Riggs, Organic and inorganic optical limiting materials from fullerenes and nanoparticles. Int. Rev. Phys. Chem. 18(1), 43–90 (1999)
T. Kindahl, J. Öhgren, C. Lopes, B. Eliasson, Synthesis, optical power limiting, and DFT calculations of triplet–triplet absorption of three novel Pt(II)-diacetylide chromophores. Tetrahedron Lett. 54, 2403–2408 (2013)
A. Tuhl, H. Manaa, S. Makhseed, N. Al-Awadi, J. Mathew, H.M. Ibrahim, T. Nyokong, H. Behbehani, Reverse saturation absorption spectra and optical limiting properties of chlorinated tetrasubstituted phthalocyanines containing different metals. Opt. Mater. 34, 1869–1877 (2012)
B. Kouissa, K. Bouchouit, S. Abed, Z. Essaidi, B. Derkowska, B. Sarhaoui, Investigation study on the nonlinear optical properties of natural dyes: chlorophyll a and b. Opt. Commun. 293, 75–79 (2013)
A.A. Jose, P. Prakash, A. Thankappan, S. Thomas, V.P.N. Nampoori, Study of regulation of anthocyanin production from confederate rose by spectroscopic method and their nonlinear optical characterization. Int. J. Sci. Nat. 4(2), 294–298 (2013)
S. Zongo, A.P. Kerasidou, B. Sone, A. Diallo, P. Mthunzi, K. Iliopoulos, M. Nkosi, M. Maaza, B. Sahraoui, Nonlinear optical properties of poly(methyl methacrylate) thin films doped with Bixa orellana dye. Appl. Surf. Sci. 340, 72–77 (2015)
I. Iosub, F. Kajzar, M. Makowska-Janusik, A. Meghea, A. Tane, I. RauRau, Electronic structure and optical properties of some anthocyanins extracted from grapes. Opt. Mater. 34, 1644–1650 (2012)
A. Diallo, S. Zongo, P. Mthunzi, S. Rehman, S.Y. Alqaradawi, W. Soboyejo, M. Maaza, Z-scan and optical limiting properties of Hibiscus sabdariffa dye. Appl. Phys. B 117(3), 861–867 (2014)
S. Pramodini, P. Poornesh, Effect of conjugation length on nonlinear optical parameters of anthraquinone dyes investigated using He–Ne laser operating in CW mode. Opt. Laser Technol. 62, 12–19 (2014)
S. Zafar, Z.H. Khan, M.S. Khan, MS, Experimental and theoretical investigations of nonlinear optical properties of 1,4-diamino-9,10-anthraquionone. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 164–169 (2013)
S. Dokmaisrijan, A. Payaka, V. Tantishaiyakul, M.C. Piyarat Nimmanpipug, V.S. Lee, Conformations and spectroscopic properties of laccaic acid A in the gas phase and in implicit water. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 105, 125–134 (2013)
G. Saraswathy, S. Pal, C. Rose, T.P. Sastry, A novel bio-inorganic bone implant containing deglued bone, chitosan and gelatin. Bull. Mater. Sci. 24, 415 (2001)
R.M. Silverstein, F.X. Webster, Spectrometric Identification of Organic Compounds, 6th edn. Wiley, New York, p. 81 (1998)
T. Peter, C. So, C.Y. Dong, Fluorescence: spectrophotometry. Massachusetts Institute of Technology, Cambridge, Massachusetts, USA, Macmillan Publishers Ltd, Nature Publishing Group (2002)
B. Valeur, Molecular Fluorescence: Principles and Applications. ISBNs: 3-527-29919-X, Wiley-VCH Verlag GmbH (2001)
B. Herman, J.R. Lakowicz, D.B. Murphy, Fluorescence Excitation and Emission Fundamentals, Olympus Microscopy. America In. (2012)
M. Sauer, J. Hofkens, J. Enderlein, Handbook of Fluorescence Spectroscopy and Imaging. ISBNs: 978-3-527-31669-4. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim (2011)
R.J. Woods, A.K. Pikaev, Applied Radiation Chemistry Process: Radiation Processing. Wiley (1994)
J.H. Qiu, P. Zhou, X.Y. Gao, J.-N. Yu, S.-Y. Wang, J. Li, Y.-X. Zheng, Y.-M. Yang, Q.-H. Song, L.-Y. Chen, Ellipsometric study of the optical properties of silver oxide prepared by reactive magnetron sputtering. J. Korean Phys. Soc. 46, S269–S275 (2005)
G.E. Jellison Jr, F.A. Modine, Erratum: Parameterization of the optical functions of amorphous materials in the interband region. Appl. Phys. Lett. 69, 371 (1996)
M. Campoy-Quiles, G. Heliotis, R. Xia, M. Ariu, M. Pintani, P. Etchegoin, Ellipsometric characterization of the optical constants of polyfluorene gain media. Adv. Funct. Mater. 15, 925–933 (2005)
N.B.S. Cowan, E. Vauthey, A.J. Heeger, Ultrafast relaxation of the poly(3-hexylthiophene) emission spectrum. J. Phys. Chem. C 115, 9726–9739 (2011)
H.G. Tompkins, E.A. Irene, Handbook of Ellipsometry, William Andrew, Inc. Springer-Verlag GmbH & Co. KG (2005)
T. Omatsu, N. Hayashi, H. Watanabe, A. Hasegawa, M. Tateda, Tunable, visible phase conjugator with a saturable-amplifier polymer laser dye. Opt. Lett. 23, 1432 (1998)
R.L. Sutherland, Handbook of Nonlinear Optics (Marcel Dekker, New York, 1996)
J. Wang, B. Gu, X.-W. Ni, H.-T. Wang, Z-scan theory with simultaneous two- and three-photon absorption saturation. Opt. Laser Technol. 44, 390–393 (2012)
D.S. Correâ, L. De Boni, L. Misoguti, I. Cohanoschi, F.E. Hernandez, C.R. Mendonc, Z-scan theoretical analysis for three-, four- and five-photon absorption. Opt. Commun. 277, 440–445 (2007)
Ts. Kolev, I.V. Kityk, J. Ebothe, B. Sahraoui, Intrinsic hyperpolarizability of 3-dicyanomethylene- 5,5-dimethyl-1-[2-(4-hydroxyphenyl)ethenyl]-cyclohexene nanocrystallites incorporated into the photopolymer matrices. Chem. Phys. Lett. 443, 309–312 (2007)
Acknowledgments
This research program was generously supported by grants from the German Academic Exchange Service (DAAD, A/14/090080), the National Research Foundation of South Africa (NRF), iThemba LABS, the UNESCO-UNISA Africa Chair in Nanosciences and Nanotechnology, and the Abdus Salam ICTP via the Nanosciences African Network (NANOAFNET) as well as the African Laser Centre (ALC) to whom we are grateful. We gratefully acknowledge the Department of Physics and Chemistry, MOLTECH-Anjou, as well as the French Government for the financial support through the international mobility grant (No. 810581B). The authors are thankful to Prof. T. Nyokong for the Z-scan unit access.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zongo, S., Dhlamini, M.S., Kerasidou, A.P. et al. Linear and nonlinear optical absorption characterization of natural laccaic acid dye. Appl. Phys. B 120, 389–396 (2015). https://doi.org/10.1007/s00340-015-6148-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-015-6148-3