Abstract
We have demonstrated the photon-stimulated surface spectroscopy using broadband vacuum ultraviolet (VUV) emissions from a laser-produced plasma, in which adsorbed atoms or molecules on material surfaces were to be desorbed and dissociated as a result of absorption of the wavelength-selected VUV photons. Desorbed and dissociated atoms or molecules were then detected by a mass spectrometer. Mass spectra of polyethylene and polyvinyl chloride samples were obtained as a function of the irradiation wavelength. We have found sufficient characteristic differences of the mass spectra in the irradiation wavelength shorter than 200 nm in each sample. The desorption and dissociation of atoms or molecules from material surfaces depended on bond energy or molecular structure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Short-wavelength photons in the vacuum ultraviolet (VUV) spectral region would induce interesting photochemical reactions, since they have high photon energies. In certain photochemical interactions such as surface treatment of soft materials, low-power, incoherent lamp emission, rather than intense laser emission, may be adequate. Discharge-excited lamps using rare gas excimers, Ar2*, Kr2*, and Xe2* are practical VUV emission sources for scientific or industrial fields. VUV emissions from the rare gas excimer lamps have been utilized for many applications [1–3]. Photochemistry of polymers was reported by using VUV emission sources such as rare gas resonance lamps, deuterium lamps, and so forth [4–6]. Emission spectra of these VUV lamps are, however, almost monochromatic with their emission bandwidths of approximately between 0.1 and 10 nm, which may not be applicable to certain spectroscopy that requires continuous VUV emissions. In contrast to these monochromatic light sources, a synchrotron radiation source is presently one of the most versatile continuous emission sources in the VUV and X-ray spectral regions. It provides completely continuous emission with some degree of coherence, which can be used for surface physics and chemistry, advanced spectroscopy, and even for materials processing. Photodissociation processes of several simple molecules such as H2, O2, N2, and CO have been investigated by using such radiation [7]. This radiation source is, however, usually large and has limited use.
It has been well known that optically induced plasmas are created in gases by focusing a compact high-intensity laser. Such a plasma in gas can be approximated as a blackbody radiator, which produces continuum emission in the VUV [8]. We have demonstrated a spectrally continuous VUV emission source in the wavelength between 115 and 200 nm in an Ar laser-produced plasma (LPP) as a spectroscopic emission source to measure VUV absorption of exotic solid-state materials [9]. Despite lower brightness and coherence compared with that of synchrotron radiation, the LPP emission source can be easily operated in a compact size in a small-scale laboratory. We then have proposed the surface spectroscopy using such broadband VUV emissions from a LPP, in which adsorbed atoms or molecules on material surfaces are to be desorbed and dissociated as a result of absorption of the wavelength-selected VUV photons. In addition, the photon-stimulated surface spectroscopy should have superior characteristics to conventional thermal desorption spectroscopy in terms of energy and spatial resolutions with minimum heat effect. This method should have potentials in scientific and industrial fields such as basic surface physics and evaluation of surface contamination. Basic photochemical reactions of various organic materials in gas phase, aiming to the photon-stimulated surface spectroscopy applications, have been investigated using rare gas excimer lamps [10]. In this paper, we report the photon-stimulated surface spectroscopy using broadband VUV emissions from a LPP. The irradiation wavelength dependence of mass spectra of polyethylene (PE) and polyvinyl chloride (PVC) samples was demonstrated. We have found that the sufficient characteristic differences of the mass spectra were obtained in the wavelength shorter than 200 nm in each sample. The dissociation of atoms or molecules from material surfaces depended on bond energy or molecular structure.
2 Experimental setup
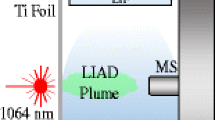
Figure 1a shows a schematic diagram of the experimental apparatus. Our experimental setup for surface spectroscopy utilized the broadband VUV emissions from a LPP in an Ar gas cell as an energetic photon source to induce surface interaction as shown in Fig. 1b. A Q-switched Nd:YAG laser at the wavelength of 1064 nm produced an output energy of 400 mJ with a pulse width of 10 ns (FWHM). The laser was operated at a repetition rate of 10 Hz. The laser beam was focused inside a gas cell filled with 1-atm Ar gas to produce a LPP as a broadband VUV emission source. The continuous VUV emission shown in Fig. 1b was measured by using a VUV spectrometer with a spectral resolution of approximately 1 nm (FWHM). The broadband emissions were introduced into a grating cell, in which certain wavelengths of the emission source were selected. The wavelength-selected VUV emissions were then introduced onto a sample placed in a sample cell. The wavelength scanning was obtained by a computer-controlled rotating grating. The typical resolution of the irradiation wavelength was approximately 10 nm (FWHM). All of the wavelength-dependent mass spectra shown in this paper have not been corrected with the resolution of the instrumental function. The grating cell, the Ar gas cell, and the sample cell were all separated by MgF2 windows. The shortest irradiation wavelength was thus limited to 115 nm because of the cutoff wavelength of these windows as shown in Fig. 1b.
A sample was prepared and introduced inside the sample cell via a load-lock chamber. The typical pressure of the sample cell with a sample was <2.5 × 10−7 Pa. PE and PVC were chosen as samples since they have similar chemical structures. These samples were thin films with a thickness of approximately 10 μm, which were commercial products being used to wrap food products. Bond dissociation energies with corresponding wavelengths relevant to PE and PVC are shown in Table 1. A quadruple mass spectrometer (QMS) was attached into the sample cell to detect desorbed and dissociated atoms or molecules, when the sample was irradiated by the VUV emissions. Since both the grating and QMS were computer controlled, wavelength-scanned two-dimensional mass spectra were thus obtained. The ultimate sensitivity of the system depended on that of the QMS.
To clarify background mass signals during measurements, we have measured mass spectra of the sample cell itself with and without VUV irradiation. Figure 2a shows a mass spectrum without a sample and VUV irradiation with respect to the irradiation time. Mass signals with mass numbers of 1 (H), 2 (H2), 16 (O), 19 (F), 28 (CO), and 44 (CO2) were observed with no time dependence, which were mainly caused by outgassing from the sample cell. Figure 2b shows a mass spectrum observed with VUV irradiation with no sample. Obtained mass signals were same as those in Fig. 2a. Note that the fluorine (M/z = 19) mass signal coming from the sample cell showed wavelength dependence. These background QMS signals were carefully analyzed to assign real mass signals caused by photo-stimulated desorption from a sample.
3 Results and discussion
Figure 3a and b show typical wavelength-dependent mass spectra of PE and PVC, respectively. Note that the mass signals of PE and PVC appeared in the wavelength longer than 200 nm were same as those without VUV irradiations. These signals may thus mainly come from outgassing in the sample cell. In contrast, the characteristic differences of the mass spectra were obtained in the wavelength shorter than 200 nm. In this wavelength region, the mass signals of 35Cl (M/z = 35), 37Cl (M/z = 37), and HCl (M/z = 36) were found in PVC at the center wavelength of approximately 170 nm, which were not detected in PE. The mass spectra indicate that Cl atoms in PVC were dissociated by absorbing VUV photons with wavelength shorter than 200 nm.
The H2 (M/z = 2) mass signal in PE was enhanced at the center wavelength of 150 nm, which was clearly separated from signals originated from outgassing. The H2 signal in PE was caused by VUV emissions with the peak energy of approximately 8 eV (150 nm), indicating the direct dissociation of H atoms from C–H bond matrices. The higher photodissociation energy compared with bond dissociation energy of C–H bond (4.29 eV) was reasonable, since the excitation from the bonding orbital to the antibonding orbital was necessary to induce the direct photodissociation of H atoms from C–H matrices. On the other hand, there was no wavelength dependence of H2 signal in PVC, despite the existence of hydrogen atoms in the sample. The irradiated VUV photons in the wavelength shorter than 200 nm were thus expensed selectively to dissociate Cl atoms from C–Cl matrices, not H atoms from C–H ones in PVC. In addition, weak wavelength-dependent signals of dissociated species including C atoms such as CO (M/z = 28) and CO2 (M/z = 44) appeared in both samples in the wavelength shorter than 200 nm. Detailed will be discussed later with Fig. 4. Note that the rather strong fluorine mass signal (M/z = 19) looked also wavelength dependent, which, however, was caused from outgassing of the sample cell as shown in Fig. 2b.
Figure 4 shows the wavelength dependence of the signal intensities of 35Cl (M/z = 35), 37Cl (M/z = 37), and HCl (M/z = 36) in PVC. This figure was traced from Fig. 2b with higher resolution. All of the signal intensities increased at the emission wavelength shorter than 200 nm and peaked at approximately 170 nm. The corresponding photon energy was 7.3 eV, which was higher than the bond dissociation energy of C–Cl (3.52 eV) [11]. The increase in the photodissociation energy of Cl atoms from PVC matrices was explained by similar reasons to those of H2 from PE matrices. The quantitative analysis of these photodissociation energies is, however, under investigation. The signal peaks at approximately 110 nm appeared shorter than the cutoff wavelength of MgF2 (115 nm) may be artifact caused by the VUV irradiation system with a low spectral resolution of approximately 10 nm (FWHM). In addition to these wavelength-depended signal behaviors, the signal intensity ratio of 35Cl and 37Cl was found to be approximately 3:1, which agreed with the natural isotopic ratio of chlorine (3.1:1).
Figure 5 shows the comparison of wavelength-dependent CO (M/z = 28) and CO2 (M/z = 44) signal intensities in both PE and PVC. Oxygen atoms reacted with dissociated C atoms were assumed to come from background outgassing in the sample cell, since the O (M/z = 16) signals observed in Fig. 2a and b were wavelength independent. Furthermore, signal intensities of C atoms (M/z = 12) in Fig. 2a and b were also independent of the irradiation wavelength, indicating majority of C atoms coming from outgassing of the sample cell. The wavelength-dependent CO and CO2 signals, therefore, indicate that dissociation of C atoms in PVC and PE was also wavelength dependent. The signal intensities were found to be lower than those of dissociated Cl, HCl, and H2, leading to a lower dissociation rate of C atoms. This may be explained by the molecular structures of PE and PVC, in which chemical bonding of C atoms in the matrices of PE and PVC was rather stronger compared with those of Cl and H atoms.
4 Summary
We have demonstrated the photon-stimulated surface spectroscopy using broadband vacuum ultraviolet (VUV) emissions from a laser-produced plasma, in which adsorbed atoms or molecules on material surfaces were to be desorbed and dissociated as a result of absorption of the wavelength-selected VUV photons. Mass spectra of polyethylene and polyvinyl chloride samples were obtained as a function of the irradiation wavelength. We have found sufficient characteristic differences of the mass spectra in the irradiation wavelength shorter than 200 nm in each sample. The desorption and dissociation of atoms or molecules from material surfaces depended on bond energy or molecular structure.
References
B. Gellert, U. Kogeschatz, Appl. Phys. B 52, 14 (1991)
A. Yokotani, N. Takezoe, K. Kurosawa, T. Igarashi, H. Matsuno, Appl. Phys. Lett. 69, 1399 (1996)
T. Ohtsubo, T. Azuma, M. Takaura, T. Higashiguchi, S. Kubodera, W. Sasaki, Appl. Phys. A 76, 139 (2003)
Y. Hatano, Phys. Rep. 313, 109–169 (1999)
V. Skurat, Nucl. Instrum. Methods Phys. Res. B 208, 27–34 (2003)
C. Duca, G. Imoberdorf, M. Mohseni, Photochem. Photobiol. 90, 238–240 (2014)
C. Decker, M. Balandier, Eur. Polym. J. 18, 1085–1091 (1982)
P. Laporte, N. Damany, H. Damany, Opt. Lett. 12, 987 (1987)
M. Kaku, T. Yamaura, T. Higashiguchi, S. Kubodera, W. Sasaki, Jpn. J. Appl. Phys. 42, 3458 (2003)
M. Wasamoto, M. Katto, M. Kaku, S. Kubodera, A. Yokotani, Appl. Surf. Sci. 255, 9861 (2009)
G.W.C. Kaye, T.H. Laby (eds.), Table of physical and chemical constants (Longman, London, 1973)
Acknowledgments
This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 25870565.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaku, M., Kai, D., Katto, M. et al. Surface analysis by photo-stimulated desorption using tunable VUV radiation. Appl. Phys. B 119, 427–433 (2015). https://doi.org/10.1007/s00340-015-6093-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-015-6093-1