Abstract
This paper compares three of the tracers most commonly used for laser-induced fluorescence in gaseous flows, toluene, naphthalene, and acetone. Additionally, anisole (methoxybenzene, CH3OC6H5) is included in the comparison. Each tracer is employed to image the scalar field in the same nonreacting transient impinging turbulent jet. The jet fluid is seeded with tracer vapor in a bubbler, excitation is at 266 nm, and both air and nitrogen are used as bath gases. Measured signals are compared to theoretical predictions based on fluorescence quantum yield, absorption cross-section, and vapor pressure. We find that anisole shows the highest total signal intensity of all investigated species, while naphthalene features the highest signal per molecule. Acetone has the advantage of being insensitive to quenching by oxygen and that its fluorescence is partly at visible wavelengths. In addition to this volatility-limited scenario at room temperature, we also compare the expected relative signals for elevated temperatures and for a hypothetical case in which the amount of admissible tracer seeding is limited.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Laser-induced fluorescence (LIF) is a powerful technique for nonintrusive visualization of concentrations in mixing of gaseous [1–4] and liquid [5] flows and for gas-temperature imaging [6–9]. It can be used in industrially relevant applications like internal combustion (IC) engines [10–13]. In many cases, a so-called fluorescent “tracer” is added to a nonfluorescing base fluid such that fluorescence is from a well-defined species with known fluorescence properties. Typical organic tracers for gas-phase diagnostics include aromatics, ketones, and less frequently, amines and ethers. In 2005, applications of tracer LIF were surveyed in a review article [14], but since then the knowledge of the photophysical properties of tracers as well as the choice of substances has expanded significantly. Therefore, instead of an extended introduction, a brief summary of the relevant properties of the tracers considered in this work will be given, followed by a discussion of the factors that influence the detectable LIF signal in typical experiments.
For the current tracer comparison, toluene, naphthalene, and acetone were selected because, as the references in the next section show, they are among the most commonly used species for gas-phase imaging, yet are very different from each other in spectroscopic and physical properties. Additionally, anisole (methoxybenzene, CH3OC6H5) was included because recent spectroscopic investigations in [15] and [16] concluded that it has promising photophysical properties as a fluorescent tracer for LIF imaging. Thus, in the current work, anisole was compared in an imaging experiment to the better-known tracers toluene, naphthalene, and acetone to develop practical guidelines for the choice of tracers for specific experimental conditions.
2 A brief overview of the tracers’ relevant properties
Fluorescent organic species are the most commonly used tracer group for imaging applications via laser-induced fluorescence in, e.g., gaseous flows or IC engines during the compression stroke. Among them, the most common subgroups are aromatics and ketones that are often used as an admixture to nonfluorescing hydrocarbon fuels. Aromatics—typical representatives are toluene and naphthalene—are often chosen because of their chemical similarity to commercial fuels, their high fluorescence quantum yield, or their strong dependence of the LIF signal intensity on O2 concentration. On the other hand, ketones—typical representatives are acetone and 3-pentanone—are sometimes preferred because their fluorescence emission is in the visible part of the spectrum, their insensitivity with respect to O2 quenching, and their nontoxicity. Table 1 summarizes the main physical and photophysical properties of the tracers used here, toluene, anisole, naphthalene, and acetone.
Aromatic species are often used because of their high fluorescence quantum yield (FQY, ϕ), with toluene and naphthalene being preferred in the majority of cases [1, 2, 6, 10, 14, 17–21] because of the availability of photophysical data and the accumulated experience documented in the literature. Note that no exhaustive screening has been documented; therefore, it is well possible that for any given applications, better tracers than previously explored ones exist. For example, anisole (methoxybenzene, C6H5OCH3), a single-ring aromatic hydrocarbon [22], has the potential to provide up to 100 times stronger signal compared to toluene in some experiments because of a higher FQY, a larger absorption cross-section, and a larger Stokes shift [15]. The latter is important in spectrally separating the desired fluorescence from the undesired elastically scattered light of the exciting laser with reduced signal losses.
A typical representative of the ketone group of tracers is acetone, with 3-pentanone the most often used species. The fluorescence quantum yield of acetone in nitrogen at 300 K is about three orders of magnitude lower than that of aromatics because intersystem crossing into the nonfluorescent triplet state strongly reduces the excited-state lifetime. However, for the same reason, quenching by O2 is much less efficient than for aromatics. In first approximation at low pressure, the influence of O2 can be neglected. Depending on the application, this insensitivity can be a major advantage. For species other than O2, collision-induced vibrational relaxation dominates over quenching and has a negligible dependence on the actual colliding species (such as CO2 and N2). Note, however, that collision-induced thermalization can influence the FQY both in a positive and in a negative way depending on the respective species and the temperature. Yet, all these effects are small compared to collisional quenching by O2.
In many applications, the tracer concentration is limited by the vapor pressure at the lowest occurring temperature to avoid condensation. For example, in an IC engine, gas temperatures during intake and early compression stroke are typically below the coolant temperature. Generic flow experiments are often performed at room temperature to keep the experimental apparatus simple. Therefore, the vapor pressure of the tracer in connection with the design temperature can become limiting for the achievable fluorescence intensity. In other cases, it is important to limit the total concentration of the tracer to avoid attenuation of the laser beam. Finally, in experiments where unwanted interaction of the tracer with its surroundings is of concern, tracers with a maximum fluorescence per molecule are of interest to allow minimizing the tracer concentration. Thus, the requirements can be different depending on the system under study, demanding tracers with high vapor pressure, high fluorescence quantum yield, or combined large absorption cross-section and quantum yield, respectively. These aspects are discussed in the following section.
3 Implications for tracer selection
For weak incident laser intensity I Laser, the detected LIF signal I LIF depends on a number of parameters, grouped by their macroscopic effects in Eq. 1 (equation and interpretation taken from [14, 33, 46, 47]
In many applications, the detection is shot-noise limited; hence, increasing I LIF means increasing measurement precision. The absorption cross-section, σ, and fluorescence quantum yield, ϕ, always depend on the tracer, while the number density n of the fluorescing molecules may depend on the tracer. σ, ϕ, and n may be directly linked to the quantity of interest, or they may contribute unwanted cross-sensitivity. Although less obvious, I Laser, detection quantum efficiency, η, and the solid angle of collection, Ω, also depend on the tracer. Therefore, in the following, a brief discussion is given of how the choice of tracer influences each factor in Eq. 1.
-
Laser intensity I Laser: Powerful laser sources are available only at few wavelengths within the absorption spectrum of typical tracers. KrF*-excimer lasers at 248 nm and frequency-quadrupled Nd:YAG lasers at 266 nm can excite the aromatics and the ketone listed in Table 1; XeCl*-excimer lasers at 308 nm can be used for acetone (and other ketones). Raman shifters or dye lasers can access other wavelengths, but in practice they are only useful in specific applications [48, 49]. For weak excitation, as assumed by Eq. 1, LIF intensity scales linearly with laser intensity. For high laser intensities, saturation occurs. As the onset of saturation depends on the number of photons per time interval, laser intensity and hence LIF intensity can be increased when using long-pulsed laser sources like excimer lasers with typical pulse widths of ~20 ns compared to Nd:YAG lasers with typically ~8 ns pulse width.
-
Number density n: As long as the tracer number density is low enough to allow self-quenching to be neglected, the LIF intensity scales linearly with n. In many applications, there is a minimum gas temperature, such that the tracer concentration must be kept below a certain level to avoid condensation. Therefore, the vapor pressure of the tracer can become limiting for the achievable fluorescence intensity. In reacting flows, e.g., combustion in an IC engine, the tracer may disturb the reaction chemistry of the parent fuel [50, 51]. This may pose another limit on the tracer concentration that can be used.
-
Absorption cross-section σ of the tracer molecule: The larger the σ, the more the light is absorbed, increasing the number of molecules in the upper electronic state from which fluorescence can occur. In general, σ is a function of temperature and excitation wavelength. For some tracers (here, toluene, acetone, and naphthalene), σ is known for typical laser wavelengths as a function of temperature; for other tracers (here, anisole), there is still a lack of data. In imaging experiments, it is often desirable to limit the attenuation of the laser sheet as it travels across the field of view. Then, since attenuation depends on the product of absorption cross-section and number density, nσ, the only advantage of larger σ is that proportionally less tracer can be used.
-
Fluorescence quantum yield (FQY) ϕ: This property depends on excitation wavelength, temperature, total pressure, and quenching by bath gas species (primarily O2). Each of these dependences could constitute an unwanted cross-sensitivity, or it could be the exploited property for diagnostics purposes. In either case, this multiparameter dependence must be known. For many tracers, the parameter field of ϕ is widely explored, especially for toluene, but the data base for naphthalene, acetone, and anisole is now also sufficient for typical diagnostics. There are, however, still environmental conditions that are not investigated yet, especially at simultaneously elevated temperatures and pressures, which are characteristics of many practical systems.
-
Collection solid angle Ω and quantum efficiency of detection η: While η and Ω are usually constants for a given experiment and thus can easily be calibrated, they can vary between different tracers. Visible fluorescence can be imaged using mass-produced camera lenses with excellent resolution at large apertures, while UV lenses are far more expensive, yet not as capable as lenses for the visible. The situation for sensors is similar; only very few CCDs with high UV sensitivity are available, while the development of high-performance imaging sensors for visible light has profited from the consumer market and biological applications.
In summary, to increase the detected fluorescence signal, ϕ, Ω, and η need to be as large as possible, while I Laser, n, and σ can be subject to trade-offs specific to a given experiment, such that increasing their values can actually be disadvantageous.
Beyond these parameters that influence the LIF signal intensity, additional considerations may influence the choice of tracer. Physical and chemical properties must match those of the parent fuel as closely as possible if the tracer is meant to track that fuel, especially in experiments open to atmosphere, toxicity, carcinogenicity (e.g., benzene), and smell (e.g., biacetyl and triethylamine) must be taken into account. Some tracers dissolve polymers commonly used in seals, and some tracers are known to exhibit low thermal stability [52]. Last but not least, the costs of the tracer can be important when large amounts are necessary. The four tracers considered in this paper pose relatively minor health hazards and are inexpensive, but acetone attacks many commonly used sealing materials.
Among the properties discussed above, the fluorescence quantum yield ϕ of organic tracers tends to exhibit the greatest sensitivity to parameters that are variable in applications like combustion engines: pressure, temperature, and bath gas composition. Physically, this is because ϕ depends on the rotational-vibrational manifolds in both the upper and lower electronic state and nonradiative de-excitation processes. For the tracers and excitation wavelength considered here, ϕ generally (but not always) decreases with increasing temperature. Strong temperature dependence is on the one hand undesirable, because the LIF signal is low at high temperatures. On the other hand, it can be advantageous when the temperature is the quantity of interest. Figure 1 displays the relative quantum yield as a function of temperature in N2 as a bath gas for several tracers. The ketones and naphthalene exhibit rather low temperature dependence, enabling measurements at high temperatures with comparable high quantum yield. Data for 3-pentanone are included here to demonstrate that tracers with very different molecular structure can exhibit nearly the same temperature dependence. Toluene LIF is very temperature sensitive, which can be exploited for precise temperature measurements [10, 53], although at high temperatures the low signal requires efficient detection.
In many cases, LIF measurements with N2 as bath gas can deliver valuable information on heat transfer and mixing. However, when combustion is of importance to the physical effects to be measured, air must be used as bath gas. The quantum yield of aromatics in air is in general one to two orders of magnitude lower than in N2, i.e., the LIF signal is much weaker. Figure 2 shows that in air, the temperature dependence of ϕ is significantly different. For aromatic tracers, the temperature dependence is negligible up to about 600 K, followed by a small decrease. This is advantageous in cases where measurements are performed in an environment with inhomogeneous temperature; conversely, it is disadvantageous for temperature measurements. The quantum yield of ketones is much lower than that of aromatics in N2, but it is only slightly affected by the presence of O2. Thus, ketones can be used with similar sensitivity in both air and N2 as bath gas. In air, ketone LIF can be more or less temperature sensitive than that of aromatics, as demonstrated by the strong temperature dependence exhibited by 3-pentanone.
Temperature dependence of the fluorescence quantum yield of selected tracers for excitation at 266 nm in 1 bar air normalized to the value at the lowest investigated temperature. Note that the ordinate extends over only one order of magnitude, while that of Fig. 1 spans three. Data taken from [15, 28, 43, 55]
In the following, we compare the four tracers in a simple experiment, demonstrating the impact of the properties discussed before. For this comparison, we use an experiment that has the original purpose to visualize turbulent mixing in a transient, impinging gas jet. In the present context, results of interest are the total signal that can be reached with a simple single-stage bubbler system and possible deviations in measured flow structure because all investigated tracers show differences in boiling point and vapor pressure and hence might differ in gas transport properties. For comparison, relative signal intensities per molecule can be calculated from the known absorption cross-section [23–26] and the FQY [15, 23, 27, 28] of the tracers. Taking into account the differences in number density (in this experiment due to different vapor pressures), it is possible to calculate the relative signal intensity per volume of tracer-seeded fluid.
4 Experiment and data evaluation
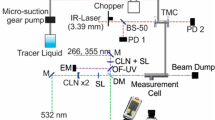
The experimental arrangement is shown in Fig. 3. A mass-flow controller and a single-stage bubbler delivered a mixture of carrier gas and tracer to a 25-l storage tank at about 16 mbar above atmospheric pressure. The storage tank fed into a convergent nozzle immediately downstream of a fast-acting gas valve (Hoerbiger GV 50). The exit diameter of the nozzle was 10 mm. A transient turbulent jet with an exit Reynolds number of about 20,000 was formed in a rectangular vessel below by opening the valve for 65 ms. The jet impinged orthogonally on a flat plate 200 mm (20 nozzle diameters) downstream of the nozzle exit. After each injection, the vessel was flushed with the bath gas. For optical access, the vessel was equipped with large quartz windows arranged at right angles.
In the bubbler, toluene, anisole, and acetone were used as pure substances. Naphthalene is solid at room temperature and thus was dissolved in dodecane, which is nonfluorescent. All three aromatics were studied in both N2 and air as bath gas. Acetone was studied in N2 only, because due to the high vapor pressure at room temperature, a saturated air/acetone mixture may be explosive, and the experiment was not designed for this hazard. However, it is known that quenching by O2 is minor for this tracer [30].
The optical arrangement, also depicted in Fig. 3, was typical for gas-phase LIF imaging. The output of a double-pulse frequency-quadrupled Nd:YAG laser at 266 nm was formed into a light sheet of about 200 mm height and a thickness of 1 mm by two cylindrical lenses. The repetition rate was 12.5 Hz with a pulse width of 10 and a 50 ns delay between the two pulses, which is short enough to consider the two pulses a single event for the current flow. A double pulse was used here merely as a means of increasing the total excitation energy without also increasing the instantaneous power, thereby avoiding problems with ground-level depletion (saturation), as for example reported by [10] for toluene LIF. The total excitation energy was about 50 mJ per pulse pair, corresponding to 10 mJ/cm2 for each pulse, which was below the saturation limit of all tracers used here [15, 47]. Laser attenuation was below 10 % across the observed field (see below). After passing through the region of interest, the laser beam was absorbed by a beam dump inside the vessel. In all but one experiment, fluorescence emission was collected perpendicular to the laser sheet by an f = 45 mm, f # = 1.8 UV camera lens and imaged on an intensified CCD camera (S20 photocathode, effective detector area 238 mm2). A dielectric long-pass filter suppressed scattered laser light. Based on a previously recorded photon transfer curve of the CCD detector, the gain of the intensifier was adjusted for each experimental condition such that shot-noise contributed more than 90 % to the total noise for mixture fractions greater than ξ = 0.1. This resulted in about 1,500 digital counts in the brightest sections of the image. In the case of acetone, an alternative detection scheme was explored. In addition to intensified imaging with the UV-transparent lens described above, we also used a nonintensified interline-transfer CCD camera (PCO SensiCam QE, effective detector area 59.5 mm2) with a standard camera lens of a focal length f = 50 mm, f # = 1.2. This lens/camera combination was only sensitive to visible light.
In terms of fluid physics, the purpose of the experiment is to visualize jet propagation and wall impingement, but here it serves as a simple demonstration of the impact of tracer selection on the quality of single-shot imaging of turbulent mixing. For this purpose, we compare images from single timing of the image capture with respect to the injection event, which was at 58 ms after start of injection (aSOI). For this timing, a set of 50 images was acquired phase-locked over 50 injections, i.e., each single-shot image was taken in a separate injection event. Thus, differences in instantaneous jet structure are unavoidable due to the stochastic nature of the turbulent jet.
For each combination of tracer and bath gas, an average background image was recorded with the laser firing and pure bath gas inside the vessel. Equally, for each condition, the vessel was filled with a homogeneous mixture of tracer and bath gas to record an average “flat field” to account for spatial inhomogeneities in the laser sheet and in detection efficiency. From each jet image, the background was subtracted, and the result divided by the corresponding background-corrected flat field. This yields instantaneous images of the jet mixture fraction relative to that of the flat field. The relative mixture fraction fields were equalized across tracers by dividing each image by the spatial mean of the ensemble mean of the 50-image series. It is thereby presumed that in a phase-locked spatial mean sense, each tracer represents the underlying gas jet to the same degree. The result of this equalization was then pegged to the image intensity for toluene in nitrogen a few millimeters below the nozzle, where the mixture fraction ξ of the tracer-laden injected gas flow was presumed to be unity. This quantification procedure neglected single-shot variations in the total laser pulse energy and in the vertically transverse energy distribution, each amounting to 4 % (one standard deviation).
The above procedure translates camera “counts” into mixture fraction without the need to know the tracer concentration or the efficiency of collection and detection. However, we also wanted to investigate how well the relative fluorescence signal level could be predicted a priori on the basis of the photophysical data in Table 1. Therefore, we further quantified tracer concentration and the detection system.
The intensifier’s relative gain factor was determined experimentally, while the spectral quantum efficiency of detection, plotted in Fig. 4, was derived from the component manufactures’ transmission and sensitivity curves. Figure 4 also shows the peak-normalized fluorescence spectra of each tracer.
As expected, the direct comparison of calculated and measured signals showed that the jet gas was not saturated with tracer vapor after leaving the bubbler. Therefore, the actual evaporation rate of all tracers was determined at the relevant flow rate of the carrier gas by weighing the bubbler system in suitable time intervals. The resulting tracer number densities were calculated based on the ideal-gas law. In the case of the solution of naphthalene in dodecane, the real behavior of this two-component system was not considered and equilibrium gas-phase concentrations were calculated based on the vapor pressure of pure naphthalene. This assumption may not be valid.
Table 2 shows that for all tracers, the experimentally determined tracer number densities reached with the bubbler are about a factor of two below equilibrium. In fact, we find it surprising that is so similar among the tracers, given that no effort was made to control the size of the bubbles, which is correlated with the surface-to-volume ratio and thus mass transfer. While the bubbler could be improved to approach the saturation limit more closely, an evaporator based on positive displacement (like a syringe pump-driven injector or a HPLC pump combined with a temperature-controlled evaporation chamber and a controlled carrier gas flow) would be preferable.
5 Results
Figure 5 compares mixture fraction imaging measurements using the four tracers in both N2 and air as a bath gas (acetone only N2). The images show a single realization of the jet at 58 ms aSOI with a high-magnification inset to give a rough indication of the impact of noise on the image. Mixture fraction ξ = 1 represents areas where the tracer-laden flow is not mixed with ambient gas. Because of the stochastic nature of turbulent flows, Fig. 5 cannot serve as a stringent comparison of the achievable measurement precision and spatial resolution. It does, however, provide results from a typical target application of LIF imaging, nonreacting turbulent mixing.
The main finding from Fig. 5 is that at the coarse resolution provided by the scaled-down images in this paper, all cases deliver images suitable for single-shot wide-field visualization, even naphthalene in air with its low vapor pressure at room temperature and its strong quenching by O2. Thus, all four tracers are in principle suitable for imaging gas mixing processes at temperatures much below the tracers’ boiling points, despite the wide range of physical and photophysical properties and tracer number densities spanning more than three orders of magnitude.
The magnified insets in Fig. 5 are positioned to show a fixed-size region with high scalar gradients in the outer mixing layer of the jet. Here, differences between tracers are noticeable. The images from the aromatic tracers have better signal-to-noise ratio (SNR) in N2 than in air, particularly in the case of naphthalene. Acetone shows rather weak signal due to its small absorption cross-section and low FQY. In air, however (not directly tested here), acetone becomes competitive due to its insensitivity to quenching by O2. Acetone LIF detection with the nonintensified camera (right image) yields visibly better spatial resolution than with the intensifier (left image). Resolution can be traded for improved SNR by spatial filtering, which can result in significantly better precision when detecting with a nonintensified camera [45].
The visual appearance of the images in Fig. 5 is essentially the result of the corresponding LIF signal level.
Table 3 compares the experimentally achieved LIF signal with the “theoretically” expected one. The experimental signal is calculated by spatial averaging over the imaged domain of the average over 50 images captured with the same image timing. This spatially averaged ensemble mean was then corrected for image intensifier gain, which for this purpose is just a multiplicative factor in the number of detector counts. For acetone, only intensified imaging was considered.
The theoretically expected signal was calculated based on the laser pulse energy, measured rates of evaporation and carrier gas flow in the bubbler, multiplied with the FQY, the absorption cross-section, σ, and the quantum efficiency of detection, η. The latter was determined by integrating the fluorescence spectrum weighted by the detection quantum efficiency at each wavelength (i.e., by the data from Fig. 4). In other words, with the exceptions noted below, the calculation modeled the chain of fluorescence excitation and detection: Tracer concentration multiplied by probabilities of laser absorption, fluorescence emission, and photon detection. Since only intensified imaging was considered, the (geometric) collection efficiency Ω was the same for all experiments compared here and was therefore not explicitly taken into account. Signal losses due to reflection on the window surfaces (typically 4 % on each of the two surfaces) were assumed to be independent of wavelength and thus identical for all tracers. For all four tracers, absorption and emission spectra partially overlap. Therefore, the florescence signal emitted in the light sheet can be attenuated through absorption by tracer molecules in the detection path. This is true both in the actual jet experiment and in the corresponding flat-field calibration. In our analysis, potential differences between tracers with respect to attenuation were neglected because they are insignificant for the relatively small dimensions of our experiment.
All experimental signal levels were normalized with respect to that of toluene in N2; all theoretical signals to the theoretical one of that same tracer/bath gas combination. The results are collected in Table 3 and visualized in Figs. 6 and 7. Table and graphs display LIF signal strengths in two different frames of reference, per volume of tracer-doped fluid, and per molecule of tracer. The former is the number relevant to the current experiment, in which the gas temperature is below the boiling point, and thus, condensation (or limited evaporation in the bubbler) limits the maximum tracer concentration. The latter allows a direct comparison with photophysical data and can be relevant to choose tracers with maximum fluorescence signal, e.g., in a scenario in which the tracer concentration must be kept low, for example, to limit the impact on combustion chemistry.
Measured and calculated signals per volume in N2 and air (acetone only in N2) relative to those of toluene in N2 at room temperature. The comparison is based on intensities obtained with a bubbler system, thus with strongly varying number densities for the different tracers (see Table 2). Numerical values can be found in Table 3
Figure 6 shows that calculated and measured signal levels agree well in the case of air as bath and carrier gas, but less so in N2. In the case of the aromatic tracers, quenching by O2 from air contamination of the nitrogen in the vessel may contribute to the disagreement. A contribution to the measurement uncertainty in both N2 and air is the tracer evaporation rate, in particular in case of naphthalene and anisole, for which the weight-based evaporation measurement lasted several days before a sufficient quantity had been transported from the bubbler by the carrier gas stream. Additionally, the calculated values are based on a chain of literature values, each with its own uncertainty, which also may explain the discrepancies. For acetone, with the filters used, phosphorescence is detected together with fluorescence, but the short intensifier gate (50 ns) should discriminate effectively against phosphorescence, which has a lifetime of ~200 μs. Whatever the cause of the disagreement between calculation and measurement, the fact that the measured spatially integrated intensities vary less between tracers than the calculated ones is consistent with the finding that all four tracers yield images with sufficient quality to easily discern large-scale structures in the turbulent jet. To some extent, we may conclude from Fig. 6 and the discussion above that predicting even the relative signal a priori is not simple.
As expected, fluorescence from the aromatic tracers is much lower in air than in N2 due to quenching by O2. Anisole provides very strong signal even in air, confirming that it is a tracer with high potential in LIF diagnostics. In the experiment, the greatest difference is between anisole in N2 and naphthalene in air. The former yields about 200 times stronger signal, which implies 14 times better precision in a shot-noise limited measurement, all else being equal. However, in this experiment, LIF from the aromatic tracers in N2 was so bright that in order to avoid saturating the CCD detector, the image intensifier had to be operated with gains that were two orders of magnitude lower than for naphthalene in air. The noise characteristics of image intensifiers can deteriorate significantly at low gains [56]. Hence, the visible differences in “image quality” between the tracers and bath gases in the corresponding magnified insets in Fig. 5 are likely to underestimate the difference in precision that would be encountered in a low-light experiment or with an unintensified, UV-sensitive camera.
The results are in good agreement with the qualitative results of Hirasawa et al. [22], especially showing that anisole exhibits a very strong signal per volume. There are some discrepancies between the results in this paper and those of Rossow [33], especially for toluene and acetone, with Rossow reporting less signal from acetone than we do. Beyond the possible sources of error discussed above, there are some differences between the two experiments. Here, the experiment was performed at room temperature (296 K) while Rossow’s was at 450 K. Also, as opposed to Rossow’s detection scheme, in the present case toluene LIF is not detected from the entire spectrum due to the long-pass filter necessary to reject elastic scattering at 266 nm. For toluene, this would be the typical scenario when exciting with a frequency-quadrupled Nd:YAG laser, which is the most commonly used type of laser for this diagnostics.
Figure 7 compares the tracers’ LIF signals for two scenarios, an experiment limited by tracer vapor pressure versus one in which only a certain amount of tracer is admissible, i.e., in cases, where the tracer concentration affects chemical reactions such as in fuel/air auto ignition [50, 51]. Then, oxygen is present, and data with air as a bath gas are more relevant. In the first case, photon-limited measurement precision is determined by the signal per volume of tracer-doped gas in evaporative equilibrium, and in the second one by signal per molecule. In each scenario, toluene was taken as a point of reference. For each bath gas, the factor between the signal on a per volume and that on a per molecule basis is inversely proportional to the vapor pressure of the tracer. Thus, acetone has the smallest difference between solid and cross-hatched bars, naphthalene the largest. As expected, in N2, on a per molecule basis, the aromatic compounds can yield much higher LIF intensity than acetone, with the signal from naphthalene exceeding that from acetone by five and a half orders of magnitude. In the case of air as bath gas, the advantage of the aromatics diminishes somewhat, with toluene at room temperature being only one order of magnitude better than acetone, but naphthalene still providing far more signal.
In most industrially relevant applications, interesting parts of the flow are not at the simple thermodynamic conditions of the experiment discussed here, but at elevated temperature and pressure. An exhaustive discussion of the influence of these parameters on tracer LIF is beyond the scope of this paper, but for completeness we include a brief comparison of the influence of elevated temperatures on the tracers discussed here.
Figure 8 shows the calculated LIF signal intensities per volume for a tracer concentration corresponding to liquid–vapor equilibrium at room temperature and pressure. A corresponding experimental scenario could be a flow that is seeded to vapor saturation at room temperature, but then heats up inside of a device in which we want to perform LIF imaging. The graph is based on the relative values of theoretically detectable LIF signals from Table 3 combined with literature data on the temperature-dependent FQYs and absorption cross-sections. For anisole, the temperature dependence of the absorption cross-section is presently unknown; thus, for all temperatures, it was set to the value at room temperature [24]. Such temperature independence is the case for naphthalene upon 266 nm excitation and toluene upon 248 nm excitation; especially, the latter case has some photophysical similarities to LIF from anisole using 266 nm excitation [15]; hence, this rather crude assumption may not be completely wrong. Future work must confirm whether this assumption is justified or not. For acetone, values in air were set to be identical to those in nitrogen because quenching by O2 is minor here [30, 57]. Note that the effect of total pressure, which is of great interest in, e.g., IC engines or gas turbines, is not considered in Fig. 8.
Extrapolated LIF signal intensities per volume for a tracer concentration corresponding to liquid–vapor equilibrium at room temperature and pressure (Table 3; Fig. 6) and the literature-reported temperature dependences of the fluorescence quantum yield (Figs. 1, 2) and absorption cross-section. See text for more details
Figure 8 shows that in N2, naphthalene becomes the “brightest” tracer at temperatures above about 750 K because its FQY decreases relatively little with increasing temperature. Within the available range of base data, anisole LIF is stronger than that of toluene and acetone, the latter being of low relative intensity for all temperatures. Figures 1 and 8 indicate that the relative signal advantage of naphthalene over acetone does not change significantly with increasing temperature, while toluene and anisole lose much of their advantage at high temperature.
In air as a bath gas, the signal levels of the aromatics are much lower than in N2, but still above that of acetone. Note that this comparison is based on identical detection systems, while in reality more efficient detection of acetone LIF may be possible because about half of it is in the visible region of the spectrum. The naphthalene LIF signal is always below the levels of anisole and toluene due to strong quenching by O2, while the anisole LIF signal is always the strongest. In air, LIF from the aromatic compounds increases slightly (naphthalene and anisole) or moderately (toluene) for intermediate temperatures, but decreases at high temperatures.
Finally, maximizing the detected signal may not be the crux of a given experiment. Minimizing the influence of sensitivity to ill-known parameters like (in many cases) temperature may be much harder. In the current constant pressure scenario, naphthalene LIF has very little temperature dependence up to 700 K in air, anisole LIF up to 600 K. In N2, only acetone LIF decreases by less than one order or magnitude over the temperature range considered here. Again, in many technologically relevant devices, pressure and temperature are coupled and an a priori assessment of the expected signal strength would be more complicated than the current example, in particular in air.
6 Conclusions
Several organic species have been used as fluorescing tracers for LIF imaging in the past two decades. For almost all of the frequently used tracers, the photophysical properties are now available in the literature (at least over some range of conditions) and can be compared. The challenge in choosing the optimal tracer for a given application is that many different parameters of both tracer and experiment must be considered. As a demonstration, we performed semi-quantitative LIF imaging of the mixture fraction in a turbulent jet at room temperature and pressure using both nitrogen and air as parent fluids. The tracer concentrations were those that could be reached with a single-stage bubbler system at room temperature, which for all tracers turned out to be about half the vapor–liquid equilibrium concentration.
Based on the actual quantity of tracer delivered into the jet’s gas by the bubbler, anisole yields the best signal per volume in both N2 and air. In terms of signal alone, anisole would be a good (though not always the best) choice in the application scenarios considered here, in N2 as well as in air. Thus, anisole is an attractive candidate for the visualization of gas flow mixing in devices such as internal combustion engines. However, not much practical experience exists in the use of this tracer, and some basic data, such as the temperature dependence of the absorption cross-section, its thermal stability, and its interaction with combustion chemistry, are still lacking.
It is also remarkable how sensitively naphthalene fluorescence in N2 can be detected. Despite a concentration of only about 20 ppm in the jet gas, reasonable single-shot images could be obtained. With both anisole and naphthalene, the low concentration required can be advantageous in cases where high tracer concentrations may disturb thermodynamic or chemical properties of the fluid of interest.
In N2 at room temperature, the aromatic tracers are clearly advantageous. In air, anisole provided the strongest signal on a per volume level. Acetone, despite its much higher vapor pressure and its negligible quenching by O2, provided slightly weaker signal. In many applications, however, the gas is at elevated temperatures. Fluorescence from aromatic tracers strongly decreases with increasing temperature. If concentration or mixture fraction were to be measured, both the decrease in signal and the resulting sensitivity to local variations in temperature negatively impact measurement precision and accuracy.
In the current experiment, even a relative prediction of the signal with respect to the measured fluorescence from toluene in N2 was accurate only within one order of magnitude. This indicates that a selection of tracers for a potential experiment solely on the basis of literature data may be insufficient. While it is certainly possible to improve various experimental aspects that the signal prediction was based on, the findings of this publication still give some benchmark to consider when choosing a suitable tracer for a given application.
References
B.H. Cheung, R.K. Hanson, Appl. Phys. B 98, 581 (2010)
S.A. Kaiser, M.B. Long, Proc. Combust. Inst. 30, 1555 (2005)
W. Koban, J. Schorr, C. Schulz, Appl. Phys. B 74, 111 (2002)
M. Orain, F. Grisch, E. Joudanneau, B. Rossow, C. Guin, B. Trétout, C. R. Mecanique 337, 373 (2009)
T. Hagemeyer, M. Hartmann, M. Kühle, D. Thévenin, K. Zähringer, Exp. Fluids 52, 361 (2012)
M. Cundy, P. Trunk, A. Dreizler, V. Sick, Exp. Fluids 51, 1169 (2011)
M. Orain, P. Baranger, B. Rossow, F. Grisch, Appl. Phys. B 100, 945 (2010)
J. Yoo, D. Mitchell, D.F. Davidson, R.K. Hanson, Exp. Fluids 49, 751 (2010)
M. Luong, W. Koban, C. Schulz, J. Phys. Conf. Series 45, 155 (2006)
J.E. Dec, W. Hwang, SAE Int. J. Engines 2, 421 (2009)
M. Luong, R. Zhang, C. Schulz, V. Sick, Appl. Phys. B 91, 669 (2008)
W. Koban, J.D. Koch, V. Sick, N. Wermuth, R.K. Hanson, C. Schulz, Proc. Combust. Inst. 30, 1545 (2005)
V.M. Salazar, S.A. Kaiser: SAE Technical Paper 2009-01-2682 (2009)
C. Schulz, V. Sick, Progr. Energy. Combust. Sci. 31, 75 (2005)
S. Faust, T. Dreier, C. Schulz, Appl. Phys. B 112, 203 (2013)
K.H. Tran, C. Morin, M. Kühni, P. Guibert, Appl. Phys. B. doi:10.1007/s00340-013-5626-8 (2013)
R. Devillers, G. Bruneaux, C. Schulz, Appl. Phys. B 96, 735 (2009)
W. Koban, C. Schulz: SAE Technical paper 2005-01-2091 (2005)
K. Mohri, M. Luong, G. Vanhove, T. Dreier, C. Schulz, Appl. Phys. B 103, 707 (2011)
M. Orain, P. Baranger, B. Rossow, F. Grisch, Appl. Phys. B 102, 163 (2011)
C. Strozzi, J. Sotton, A. Mura, M. Bellenue, Meas. Sci. Technol. 20, 125403 (2009)
T. Hirasawa, T. Kaneba, Y. Kamata, K. Muraoka, Y. Nakamura, J. Vis. 10, 197 (2007)
W. Koban, J.D. Koch, R.K. Hanson, C. Schulz, Phys. Chem. Chem. Phys. 6, 2940 (2004)
T. Etzkorn, B. Klotz, S. Sörensen, I.V. Patroescu, I. Barnes, K.H. Becker, U. Platt, Atmos. Environ. 33, 525 (1999)
M. Suto, X. Wang, J. Shan, L.C. Lee, J. Quant. Spectrosc. Radiat. Transfer 48, 79 (1992)
J.D. Koch, J. Gronki, R.K. Hanson, J. Quant. Spectrosc. Radiat. Transfer 109, 2037 (2008)
N. Nijegorodov, R. Mabbs, D.P. Winkoun, Spectrochim. Acta A 59, 595 (2003)
S. Faust, T. Dreier, C. Schulz, Chem. Phys. 383, 6 (2011)
S. Faust, G. Tea, T. Dreier, C. Schulz, Appl. Phys. B 110, 81 (2013)
M.C. Thurber, R.K. Hanson, Appl. Phys. B 69, 229 (1999)
A. Ehn, O. Johansson, A. Arvidsson, M. Aldén, J. Bood, Opt. Exp. 20, 3043 (2012)
W. Koban, J.D. Koch, R.K. Hanson, C. Schulz, Appl. Phys. B 80, 777 (2005)
B. Rossow: Photophysical processes of organic fluorescent molecules and kerosene - application to combustion engines, Institut des Sciences Moléculaires d’Orsay, Université Paris-Sud 11, Paris (2011)
J. Savard, Ann. Chim. 10, 287 (1929)
F.P. Zimmermann, W. Koban, C.M. Roth, D.-P. Herten, C. Schulz, Chem. Phys. Lett. 426, 248 (2006)
J.K. Marsh, J. Chem. Soc. Trans. 125, 418 (1924)
F. Ossler, T. Metz, M. Aldén, Appl. Phys. B 72, 465 (2001)
E. Wiedemann, G.C. Schmidt, Ann. Phys. 56, 18 (1895)
A. Braeuer, F. Beyrau, A. Leipertz, Appl. Opt. 45, 4982 (2006)
G.H. Damon, F. Daniels, J. Am. Chem. Soc. 55, 2363 (1933)
M. Loeffler, F. Beyrau, A. Leipertz, Appl. Opt. 49, 37 (2010)
A. Lozano, B. Yip, R.K. Hanson, Exp. Fluids 13, 369 (1992)
F. Ossler, M. Aldén, Appl. Phys. B 64, 493 (1997)
M.C. Thurber, F. Grisch, B.J. Kirby, M. Votsmeier, R.K. Hanson, Appl. Opt. 37, 4963 (1998)
V.M. Salazar, S.A. Kaiser, F. Halter, SAE Int. J. Fuels Lubr. 2, 737 (2009)
J.D. Koch, Fuel Tracer Photophysics for Quantitative Planar Laser-Induced Fluorescence (Stanford University, California, USA, 2005)
M.C. Thurber, Acetone Laser-Induced Fluorescence for Temperature and Multiparameter Imaging in Gaseous Flows (Stanford University, California, USA, 1999)
D.A. Rothamer, J.A. Snyder, R.K. Hanson, R.R. Steeper, Appl. Phys. B 99, 371 (2010)
S. Einecke, C. Schulz, V. Sick, Appl. Phys. B 71, 717 (2000)
M. Fikri, L.R. Cancino, M. Hartmann, C. Schulz, Proc. Combust. Inst. 34, 393 (2013)
M. Hartmann, I. Gushterova, M. Fikri, C. Schulz, R. Schießl, U. Maas, Combust. Flame 158, 172 (2011)
C. Schulz, J. Gronki, S. Andersson: SAE Technical paper 2004-01-1917 (2004)
S.A. Kaiser, M. Schild, C. Schulz, Proc. Combust. Inst. 34, 2911 (2013)
J.D. Koch, R.K. Hanson, Appl. Phys. B 76, 319 (2003)
S.M. Faust, Characterisation of Organic Fuel Tracers for Laser-Based Quantitative Diagnostics of Fuel Concentration, Temperature, and Equivalence Ratio in Practical Combustion Processes (University of Duisburg-Essen, Duisburg, 2013)
S.E. Moran, B.L. Ulich, W.P. Elkins, R.L. Stritttmatter, M.J. DeWeert, Proc. SPIE 3173, 430 (1997)
F. Grossmann, P.B. Monkhouse, M. Ridder, V. Sick, J. Wolfrum, Appl. Phys. B 62, 249 (1996)
Acknowledgments
This work was funded by the Rückkehrerprogramm of the NRW Ministry for Innovation, Science, and Research and by the Deutsche Forschungsgemeinschaft (DFG). Additionally, the authors would like to thank IFPEN (France) for lending the hardware for the jet experiment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Faust, S., Goschütz, M., Kaiser, S.A. et al. A comparison of selected organic tracers for quantitative scalar imaging in the gas phase via laser-induced fluorescence. Appl. Phys. B 117, 183–194 (2014). https://doi.org/10.1007/s00340-014-5818-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-014-5818-x