Abstract
The dynamics of the excited states on a laser-ablated Mo plume was studied, both in air and in vacuum, by emission spectroscopy along the plume expansion axis. The emission related to ionized atoms occurs in the beginning of the plume expansion, near the metal surface, and is predominantly ultraviolet emission. In the middle of the plasma plume, it takes place the electron transitions between excited states of neutral atoms, and in the end of the plume, the visible emission is to transitions to the ground state of neutral molybdenum atoms. It was possible to determine plume parameters such as plasma expansion velocity of (5.0 ± 0.7) km/s at atmospheric pressure and (4.0 ± 0.7) km/s in vacuum, and the plasma duration that was (160 ± 14) ns at atmospheric pressure and (138 ± 18) ns in vacuum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Molybdenum has been object of many studies because its significance on industry, agriculture, medicine and building. One of its possible applications is the use of 99 molybdenum isotope, the only source of 99m technetium, which is applied in Medicine as tracer in scintigraphy examinations for medical diagnostics [1–3]. An alternative route for the generation of 99Mo is the irradiation of 98Mo with neutrons. This process, however, has a typical low yield (1 part per million), and an enrichment process in the production line would be highly desirable [4]. Since the 99Mo has a lifetime of about 66 h, the separation methods must be very quick and should avoid intermediate chemical processes, which eliminates the processes based on MoF6, such as centrifuges or molecular laser isotope separation. The combination of laser ablation with atomic vapor laser isotope separation (AVLIS) reveals to be a promising method [5].
In order to generate a neutral jet of Mo for AVLIS purpose, it is necessary to study its plume generation and expansion. However, there is no information in abundance about laser ablation of Mo in the literature. Rodrigues et al. [6] presented studies on the threshold of vapor and electric charges generation for laser ablation of Mo with HyBrID copper laser. Heise et al. [7] presented results of Mo thin film removal from transparent substrate using a ps laser. Samad et al. [8] studied laser ablation threshold for Mo illuminated by fs laser in air and in gas, and recently, we presented preliminary spectroscopic study of ablated plumes of Mo with 355 and 532 nm Nd:YAG ns lasers [9].
This paper presents results of a space-resolved study of Mo laser-ablated plume emission at atmospheric pressure and in vacuum (1.5 × 10−2 Torr), where it was possible to determine directly the plume expansion velocity and the dynamics of the atomic population in the expanding plume.
2 Experimental setup
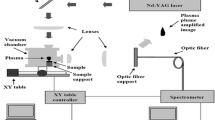
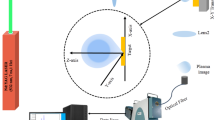
A schematic diagram of the experimental setup is shown in Fig. 1. A coherent AVIA 355-X Nd:YAG laser emitting pulses with 0.205 mJ energy and 25 ns duration (FWHM) in 355 nm had its beam focused to a molybdenum target, with a laser fluence of 6 J/cm2. The target was placed inside a vacuum chamber, which is fixed to a XY table controlled by computer to avoid cratering. The plume was imaged by a 2× telescope, and the light emission was collected at the image position by a fiber optic on a XY positioner and coupled to an Ocean Optics HR4000 spectrometer. Wavelength calibration was made for all experimental spectra using a Newport Hg lamp model 6035. For laser light scattering experiments, the emission of the plasma plume was attenuated by a neutral density filter and the spectrometer time integration was reduced to avoid saturation of the laser scattered light signal. Laser pulse duration was measured by a Newport Model 818-BB-21A phototransistor attached to a JC Instruments Model JC 2000 oscilloscope.
3 Results and discussion
For the determination of plasma expanding velocity, it was measured the laser light scattered by Mo plume along its expansion axis (Fig. 2) and registered the duration of the laser pulse (Fig. 3). It is important to note that the plasma image was two times magnified, and all calculations must take this effect into account.
Table 1 indicates the length of scattered light, the laser pulse duration and the calculated plasma expansion velocity for the both conditions. Within the experimental uncertainties, the expansion velocity is basically the same both for experiments in air and in vacuum. With these velocities, it is possible to have time scales for the space-resolved experiments that follow.
Figure 4 shows emission spectra from different distances along plasma plume expansion axis performed in air. Spectra acquired in vacuum condition have the same feature, except that the emission line intensities are lower than at atmospheric pressure. Some lines that can be assigned on spectra acquired at atmospheric pressure cannot be assigned on spectra acquired in vacuum. The emission line around 355 nm refers to laser scattering by the plume. It can be seen that spectrum migrates from ultraviolet emission lines in the base of the plume to visible emission lines at the top of the plume.
Dashed line in Fig. 5 refers to experimental spectrum. In Fig. 5a, the spectrum was acquired at 0.5 mm from the surface and in Fig. 5b at 0.75 mm. The solid line represents theoretical spectrum [10, 11]. This way Fig. 5 shows a typical method to assignment of the experimental emission lines and was applied to spectra acquired along the plume expansion axis and for different wavelength regions. In order to help to assign lines and to identify those that are adequate for our purposes (intense and insulated from the other lines), it was plotted together every experimental and synthetic spectra considering only neutral and singly ionized states.
When experimental and theoretical spectra are compared, it can be concluded that the spectrum emitted from the plasma near the surface of the target is, mainly, due to de-excitation of molybdenum-ionized atoms. As the plasma expands, many collisions occur between the atoms and the end of light emitted by the plasma is due to transitions of neutral atoms to the ground state.
Despite the linewidth limitation of the experimental apparatus, the chosen lines could be attributed to a theoretical line. In Fig. 5, the emission lines that can be isolated from others were chosen based on its well-defined wavelength and its Einstein coefficient. Table 2 presents the data of the chosen lines. Italicized values are related to transitions between ionized states (ultraviolet emission), normal values are related to transitions of neutral excited states, and bold values are related to transitions to the ground state (visible emission).
The most important results of this work are the understanding of plasma expansion dynamics. Figure 6 shows the light intensity along the expansion axis of plasma plume, which indicates the dynamics of plasma expansion at atmospheric pressure. Emission related to ionic transitions occurs in the beginning of the plume expansion, near metal surface, and, as it was previously described, is predominantly in the UV. In the middle of the plasma plume, electronic transitions between excited states of neutral atoms takes place, and in the end of the plume, the emission, mainly in the visible, is due to transitions to the ground state of neutral molybdenum atoms. At the end of light emission, it can be concluded that all atoms decay to a permanent (or long duration) state and the state distribution is practically frozen for the rest of the expansion.
Figure 7 shows the line intensities along the plume expanding pathway for low-pressure experiments. Basically, the behavior is the same, with the ion line coming first, followed by the line emission of transitions between excited neutral states and, at last, the line with the transition ending in the ground state. Other sets of emission lines were analyzed along the plume expansion axis, and both for air and for vacuum, it confirms the behavior of plume dynamic, shown in Figs. 6 and 7.
By comparing the results in Figs. 6 and 7, two aspects called attention: the overall plume duration and the relative ion line duration.
It was expected that the plasma in atmosphere would last shorter than in vacuum because in the first case, the collisions would accelerate the excited states relaxation, reducing the overall light emission lifetime. In fact, it was observed the contrary, in atmosphere light emission lasted about (160 ± 14) ns, whereas in vacuum, it lasted about (138 ± 18) ns.
The second interesting difference between the two experiments is that, in the case of ablation in air, the ion emission vanished much before the light emission dropped down, whereas in vacuum, ion emission lasted until the end of the plume emission.
4 Conclusions
Space-resolved spectroscopic studies of laser ablation of molybdenum were performed in air and in vacuum.
Measurement of laser light scattering in the plume against the distance from the target gave rise to a time scale, which allowed study spectra with respect to time behavior.
For both experiments in air and in vacuum, first emission due to ion transitions appeared, followed by emission of transitions between excited atomic states and later emission due to transitions down to the ground state.
The overall plume emission in air lasted longer than for ablation in vacuum. In air, the ion emission finished just after the excited atomic transition started, whereas, for vacuum, ion emission lasted the whole time the plume emitted light.
If one assumes that the plume population freezes somewhere near the place where the plume stops emitting light, it is very likely that, for distances from the target larger than the plume length, the plume would have a higher ion population for ablation in vacuum than in air.
References
International Molybdenum Association. www.imoa.info. Accessed 25 Feb 2013
D. Soonawala, T. Amin, K.P. Ebmeier, J.D. Steele, N.J. Dougall, J. Best, O. Migneco, F. Nobili, K. Scheidhauer, NeuroImage 17, 1193 (2002)
R.E. Boyd, Int. J. Appl. Radiat. Isot. 33, 801 (1982)
J. R. Maiorino, J. A. Perrotta, R. Frajndlich: The 1998 International Reduced Enrichment for Test Reactor Conference, São Paulo (1998)
J.B. Matos, M.L. Oliveira, E.M.A. Sternberg, M.G. Destro, R. Riva, N.A.S. Rodrigues, J. Aerosp. Technol. Manag. 4, 413 (2012)
N.A.S. Rodrigues, M.A.P. Gião, C.A.B. Silveira, R. Riva, C. Schwab, Appl. Surf. Sci. 200, 68 (2002)
G. Heise, M. Englmaier, C. Hellwig, T. Kuznicki, S. Sarrach, H.P. Huber, Appl. Phys. A 102, 173 (2011)
R. E. Samad, J. T. Vidal, W. Rossi, N. D. Vieira, The Latin America Optics & Photonics Conference, Recife, Brazil (2010)
E.M.A. Sternberg, N.A.S. Rodrigues, J. Amorim, M.E. Sbampato, J. Phys: Conf. Ser. 370, 012052 (2012)
National Institute of Standards and Technology. http://www.nist.gov/physlab/data/asd.cfm. Accessed 21 Nov 2012
P. Palmeri, J.F. Wyart, Phys. Scr. 58, 445 (1998)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sternberg, E.M.A., Rodrigues, N.A.S., Sbampato, M.E. et al. Excited states time evolution on a laser-ablated molybdenum plume. Appl. Phys. B 116, 985–989 (2014). https://doi.org/10.1007/s00340-014-5786-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-014-5786-1