Abstract
We report on experimental measurements of the refractive index of twelve organic solvents at five different wavelengths (450, 532, 632.8, 964 and 1,551 nm) and a temperature of 300 K. Based on these new data visible to near-infrared dispersion relations are constructed. Group-velocity dispersion (GVD) is theoretically calculated. Zero- and negative-GVD situations are identified for two common solvents in near-infrared wavelengths. Via comparison with refractive index data available in bibliography, estimated values of thermo-optic coefficients are also presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Refractive index of organic liquids, along with its wavelength dependence, is an important parameter for various photonics applications that include, but are not limited to the (a) design of novel optofluidic devices [1, 2], (b) analysis of the guiding characteristics in liquid core, or liquid immersed, optical and photonic crystal fibers [3–6], (c) supercontinuum generation and filamentation in liquid media [7, 8], (d) excitation of plasmon-resonance effects and control of the structural properties of nano-solutions/dispersions/colloids [9–12] and (e) optimization of the emission characteristics of dye lasers and fluorescent molecules [13–15]. Additionally, refractive index dispersion of organic liquids is used for the production of theoretical structure–property relation models, as well as for correlation with other material properties [16, 17].
Despite the widespread need for refractive index data of solvents, till date bibliography on this field remains relatively poor. In specific, refractive index of liquids is typically measured and reported either at a single wavelength (most commonly corresponding the Fraunhofer D-line) or just a few wavelengths in the visible spectral range. As a result of this situation, in the cases of most solvents (a) index data in the near-infrared are nonexistent, and (b) wavelength dispersion fits are unavailable, thus even in the visible spectral range prediction of index values at wavelengths other than those of measurement is not possible.
In this article, we present experimental measurements of the refractive index of twelve important organic solvents, namely 1,4-dioxane, 1,5-pentanediol, 1-butanol, 1-propanol, acetonitrile, amyl alcohol, benzene, carbon tetrachloride, isoamyl alcohol, isobutanol, methanol and toluene. Analytical grade liquid samples (with purities ranging between >99 and >99.99 %) were purchased from standard suppliers and used without further purification. Refractive index measurements were obtained at a constant temperature of 300 K and at five different wavelengths covering the entire visible spectral range, as well as the near-infrared spectrum up to the conventional telecommunications window. To the best of our knowledge, this is the first time that refractive index data are presented in such a broadband spectral region (that includes the near-infrared) for eight out of the twelve liquids. For the remaining four fluids under investigation (namely benzene, carbon tetrachloride, methanol and toluene), broadband index data and dispersion equations have been previously presented by other authors at temperatures other than 300 K.
Based on the available experimental data, dispersion relations are constructed and consequently group-velocity dispersion (GVD) is theoretically calculated. Zero- and negative-GVD conditions are predicted—for the first time to the best of our knowledge—for infrared wavelengths exceeding ~1,320 nm (1,515 nm), in the cases of methanol (1-propanol), respectively. This observation may be of particular interest for dispersion management and pulse compression applications. Finally, discrete experimental data and (when available) alternative dispersion equations at different temperatures are located in bibliography and consequently used for the estimation of the thermo-optic coefficients (TOC).
2 Refractive index measurements and dispersion relations
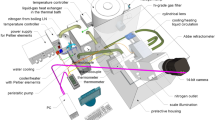
The experimental setup used for refractive index measurements is based on the prism-coupling scheme that has been previously used with solid samples [18], with the addition of a homemade liquid holder. The sample under investigation is brought in direct contact with the base of a gadolinium gallium garnet (GGG) reference prism of known index n p . Radiation from a continuous-wave laser is directed upon the base of the prism at an angle of incidence that may be controlled via a rotary table. A photodetector monitors the beam that is reflected at the interface between the prism and the sample. The angle of light incidence eventually reaches a critical value (θ c ) at which frustrated total internal reflection at the prism base leads to abrupt decrease in photodetector reading. This critical angle θ c directly relates to the unknown index n of the sample via:
This apparatus is equipped with five independent laser sources, thus permitting measurement of refractive index at five different wavelengths with a typical resolution of fourth decimal digit and an accuracy equal to (or better than) ±2 × 10−4. Available laser sources include three diode lasers emitting radiation at 450, 964 and 1,551 nm, as well as a helium–neon laser and a frequency-doubled YAG laser emitting radiation at 632.8 and 532 nm, respectively. Finally, a thermocouple is employed for reading the temperature at the prism base, which is expected to be equal to the temperature of the liquid sample.
Several sets of measurements were obtained for each solvent, exhibiting excellent reproducibility. Table 1 presents measured indices for the twelve solvents under a constant temperature of 300 K (with an accuracy of ±0.5 K). Lower (higher) index value of 1.3173 (1.5144) is observed for methanol (benzene) at 1,551 nm (450 nm), respectively. Following a comparative evaluation of various semi-empirical dispersion equations, the following extended-Cauchy relation was opted for fitting the wavelength dependence of refractive index (λ in μm):
Calculated Cauchy coefficients (A 0–A 4) for the twelve organic liquids are shown in Table 2. It should be pointed out that theoretical index values from Eq. (1) reproduce measured data up to the fourth decimal digit (that is, with an accuracy equal to the resolution of the experimental setup). Therefore, fitting error may be assumed to be practically zero. Theoretical dispersion curves, along with corresponding experimental data, are cumulatively depicted in Fig. 1.
3 Group-velocity dispersion calculations
Group-velocity dispersion is an important parameter associated with the propagation of ultrashort laser pulses through transparent media. Ignoring higher-order dispersion effects (which is a valid approximation for pulse durations exceeding ~50 fs), GVD may be considered exclusively responsible for changes in the shape of the temporal pulse envelope, typically leading to pulse broadening and peak intensity reduction. By the use of the extended-Cauchy relations presented in the previous section, GVD of the twelve solvents may be straightforwardly calculated (see for example [19]) via:
where λ is the central wavelength of the spectrum of the pulse, and c is the speed of light in vacuum.
Figure 2 depicts theoretical GVD values in the spectral band of measurement (450–1,550 nm) for the various liquids. Typically, GVD in the visible range is in the order of ~100 fs2/mm and decreases down to less than ~50 fs2/mm toward the conventional telecommunications window in the near-infrared. Abnormal behavior is observed in the case of methanol (1-propanol) which exhibits GVD ≤ 0 in the near-infrared for wavelengths λ ≥ 1,320 nm (λ ≥ 1,515 nm), respectively. In particular, negative-GVD values reaching −13.4 fs2/mm at 1,551 nm are calculated for methanol. To the best of our knowledge, this is the first prediction of zero- and negative-GVD in such common and inexpensive liquids, indicating their potential use in dispersion compensation applications.
Theoretical values of GVD for the twelve solvents (solid lines). For comparison, experimental values of GVD for three solvents (from Ref. [20]) are also shown (open circles)
Recently, two reports on direct experimental determination of GVD in liquid samples have been published [20, 21]. Most relevantly to the present work, authors in Ref. [20] have determined GVD of various solvents (including methanol, carbon tetrachloride and toluene) at various wavelengths within the 700–900 nm range. Experimental GVD data from Ref. [20] are also shown for comparison in Fig. 2. Excellent agreement between our theoretical values and the experimentally determined ones is observed. Indicatively, at a wavelength of 800 nm, present calculations result in GVD values of 103.7, 61.2 and 30.0 fs2/mm for toluene, carbon tetrachloride and methanol, respectively. Corresponding experimental values from Ref. [20] are 105.7, 63.1 and 30.4 fs2/mm.
4 Thermo-optic coefficients estimates
The temperature dependence of refractive index, typically quantified through the thermo-optic coefficient, is a necessary parameter for the calculation of refractive index at temperatures other than those of the actual measurement. Estimating TOC values for the twelve solvents under investigation requires comparison between the present data (obtained at 300 K) and previously published data obtained at different temperatures. For most of these organic liquids, bibliographic index data are available either at only one wavelength (typically corresponding to the Fraunhofer D-line, as is the case with 1,5-pentanediol) or just a few wavelengths in the visible spectral range (as is the case with amyl alcohol, isobutanol, etc.).
In this direction, index data (n T D ) from various sources, measured at the Fraunhofer D-line (589.2 nm) and temperatures (T) other than 300 K, were located in the literature and are presented in Table 3. Corresponding theoretical index values at 300 K (n 300K D ) produced by the extended-Cauchy equations of this work are also shown in Table 3. From this compilation of data, the magnitude of the TOC (dn/dT) may be straightforwardly estimated. As is observed, TOC acquires systematically negative values (ranging between −2.3 × 10−4 and −8.3 × 10−4 K−1 for isoamyl alcohol and benzene, respectively), indicating a decrease in refractive index as temperature increases.
It should be stated that TOC estimates presented here are associated with relatively large error margins. In an effort to estimate this effect, one may neglect other sources of error (such as those related to the experimental index determination and the purity of the samples) and account only for the propagation of temperature error. Assuming that temperature T is measured by others with an accuracy similar to that of the present study (±0.5 K) leads to the conclusion that relative TOC error is in the order of \({\raise0.7ex\hbox{${\sqrt 2 \cdot 0.5}$} \!\mathord{\left/ {\vphantom {{\sqrt 2 \cdot 0.5} {\varDelta T}}}\right.\kern-0pt} \!\lower0.7ex\hbox{${\varDelta T}$}}\) (ΔT denotes the difference between 300 K and T). Therefore, typical TOC errors are expected to be in the range of 10, 15, 25 and 35 % for calculations based on bibliographic data obtained at a temperature of 293, 295, 303 and 298 K, respectively. It is worth noting, however, that TOC estimates presented in Table 3 exhibit reasonable agreement with TOC values reported sparsely in literature. For example, TOC for amyl alcohol (−3.9 × 10−4 K−1) and methanol (−4.0 × 10−4 K−1) compares well with values of −3.82 × 10−4 and −3.9 × 10−4 K−1 reported in [32] and [33], respectively.
To the best of our knowledge, previous reports on refractive index dispersion relations covering the visible and near-infrared spectral range exist only for four out of the twelve organic solvents under investigation; more specific, for methanol (405–2.09 μm, at 298 K) in [31]; for benzene and toluene (300–2,500 nm at 293 K) in [27]; for carbon tetrachloride and toluene (500–1,600 nm, at 293 K) in [28]. By the use of these relations, as well as the equations of the present work, wavelength dispersion of TOC was calculated and plotted in Fig. 3. In general, the absolute value of TOC reduces as wavelength increases, approaching a nearly zero value for methanol in the near-infrared. Local extrema are observed for all solvents, either in the visible (for benzene and toluene) or in the boundary between visible and infrared light (for methanol and carbon tetrachloride). This effect is not fully understood and thus requires further investigation. Finally, it is worth noting that two curves were computed for toluene, based on comparison with two different dispersion relations that are available in bibliography. These two curves nearly overlap in the infrared portion of the spectrum, while exhibiting only small discrepancies in the visible.
5 Conclusion
This study presented the experimental determination of the refractive index of twelve organic solvents at five different wavelengths (450, 532, 632.8, 964 and 1,551 nm) and a constant temperature of 300 K. Liquid samples included 1,4-dioxane, 1,5-pentanediol, 1-butanol, 1-propanol, acetonitrile, amyl alcohol, benzene, carbon tetrachloride, isoamyl alcohol, isobutanol, methanol and toluene. Extended-Cauchy equations were constructed for modeling the wavelength dependence of refractive index; group-velocity dispersion was theoretically computed, and zero-crossing points were observed for methanol (1-propanol) in near-infrared wavelengths of 1,320 nm (1,320 nm), respectively. The existence of negative-GVD possibilities in the spectral region of 1.55 μm may be of significant interest for pulse compression applications. Finally, the thermo-optic properties of the twelve solvents were investigated. Consistently, negative TOC values indicate decrease in refractive index with increasing temperature.
References
D. Psaltis, S.R. Quake, C. Yang, Nature 442, 381 (2006)
C. Monat, P. Domachuk, B.J. Eggleton, Nat. Photonics 1, 106 (2007)
F.M. Cox, A. Argyros, M.C.J. Large, Opt. Express 14, 4135 (2006)
Y. Zhu, X. Chen, Y. Xu, Y. Xia, J. Lightwave Technol. 25, 3051 (2007)
R. Zhang, J. Teipel, X. Zhang, D. Nau, H. Giessen, Opt. Express 12, 1700 (2004)
T. Gissibl, M. Vieweg, M.M. Vogel, M. Aboud Ahmed, T. Graf, H. Giessen, Appl. Phys. B 106, 521 (2012)
L. Guyon, K.M. Hajek, F. Courvoisier, V. Boutou, R. Nuter, A. Vincotte, S. Champeaux, L. Berge, J.-P. Wolf, Appl. Phys. B 90, 383 (2008)
J. Liu, H. Schroeder, S.L. Chin, R. Li, Z. Xu, Opt. Express 13, 10248 (2005)
S. Leekumjorn, S. Gullapalli, M.S. Wong, Phys. Chem. B 116, 13063 (2012)
J.J. Mock, D.R. Smith, S. Schultz, Nano Lett. 3, 485 (2003)
J.H. Chen, L. Shao, K.C. Woo, T. Ming, H.-Q. Lin, J. Wang, J. Phys. Chem. C 113, 17691 (2009)
L.A. Gomez, C.B. de Araujo, A.M. Brito-Silva, A. Galembeck, Appl. Phys. B 92, 61 (2008)
M.E. Lusty, M.H. Dunn, Appl. Phys. B 44, 193 (1987)
H. El-Kashef, R. Shalaby, Opt. Laser Technol. 44, 71 (2012)
D. Toptygin, J. Fluoresc. 13, 201 (2003)
A.R. Katritzky, S. Sild, M. Karelson, J. Chem. Inf. Comput. Sci. 38, 840 (1998)
N. Tekin, C. Tarimci, Opt. Laser Technol. 38, 498 (2006)
K. Moutzouris, I. Stavrakas, D. Triantis, M. Enculescu, Opt. Mat. 33, 812 (2011)
C. Rullière, Femtosecond Laser Pulses: Principles and Experiments (Springer, New York, 2005)
P. Devi, V.V. Lozovoy, M. Dantus, AIP Adv. 1, 032166 (2011)
T.D. Scarborough, C. Petersen, C.J.G.J. Uiterwaal, New J. Phys. 10, 103011 (2008)
M. Gupta, I. Vibhu, J.P. Shukla, Phys. Chem. Liq. 48, 415 (2010)
E.W. Flick, Industrial Solvents Handbook, 5th edn. (Noyes Data Corporation Publ, Westwood, NJ, USA, 1998)
A. Rodriguez, J. Canosa, J. Tojo, J. Chem. Eng. Data 46, 1506 (2001)
J.P. Hawranek, N. Michniewicz, W. Wrzeszcz, M. Pajdowska, J. Non-Cryst. Solids 353, 4555 (2007)
I.Z. Kozma, P. Krok, E. Riedle, J. Opt. Soc. Am. B 22, 1479 (2005)
A. Samoc, J. Appl. Phys. 94, 6167 (2003)
S. Kedenburg, M. Vieweg, T. Gissibl, H. Giessen, Opt. Mater. Express 2, 1588 (2012)
M.N. Roy, R.S. Sah, P. Pradhan, Int. J. Thermophys. 31, 316 (2010)
S. Chen, Q. Lei, W. Fang, J. Chem. Eng. Data 47, 811 (2002)
M.H. Frosz, A. Stefani, O. Bang, Opt. Express 19, 10471 (2011)
I. Akihiko, G. Akikazu, Trans. Jpn. Soc. Mech. Eng. B 576, 2875 (1994)
J.E. Bertie, Z. Lan, J. Chem. Phys. 103, 10152 (1995)
Acknowledgments
For the supply of liquid samples and their advice, the authors wish to thank: Prof. E. M. Papamichael of the University of Ioannina, as well as Prof. D. Vattis and Dr. C. Fountzoula of the Technological Educational Institution of Athens.
Author information
Authors and Affiliations
Corresponding author
Additional information
Myrtia Papamichael and Sokratis C. Betsis have contributed equally to this article.
Rights and permissions
About this article
Cite this article
Moutzouris, K., Papamichael, M., Betsis, S.C. et al. Refractive, dispersive and thermo-optic properties of twelve organic solvents in the visible and near-infrared. Appl. Phys. B 116, 617–622 (2014). https://doi.org/10.1007/s00340-013-5744-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-013-5744-3