Abstract
Two similar 4 K 22-pole trapping machines have been recently completed in our laboratory. As a first experimental test in one of them, CH +5 and H3O+ ions have been trapped in the presence of helium gas at 3.8 K and the kinetics and temperature dependence of He tagging investigated. A first shell closure with 5 and (3 + 2) attached He atoms, respectively, is observed for both species. Applying IR radiation in the 3 μm region, helium attachment to CH +5 is hindered by exciting CH-stretches of CH +5 prior to attachment. The resulting spectroscopic signal shows that the kinetic temperature of the stored CH +5 ensemble is below 12 K.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 From Paul traps to cryogenic multipole traps
Wolfgang Paul is the father of the quadrupole mass selector [1] and the quadrupole ion trap (Paul trap) [2, 3] (a nice summary of his activities is given in his Nobel lecture [4]) which are standard constituents in many of today's experiments in ion physics, physical chemistry, atmospheric chemistry, astrochemistry and biochemistry. In particular, the Paul trap (in its axially symmetric or linear form) enabled the exciting development of laser-cooled stored ions, leading to the observation of single ionic atoms [5], the formation of Coulomb crystals [6], as well as the advent of optical clocks [7].
In astrochemistry and atmospheric chemistry, trap experiments turned out to be perfect tools to investigate ion-molecule reactions. So for long time, these experiments (e.g., ion-cyclotron resonance (ICR) or Penning traps) have been used to examine ion-molecule reactions close to room temperature. In particular for astrochemistry, the knowledge of the speed of ion-molecule reactions at low temperatures is of utmost importance, as many astronomical objects (dark molecular clouds) have temperatures as low as 6 K (see e.g., [8]), and extrapolating from room temperature to low temperature is not straightforward due to subtle quantum mechanical effects. Applying a Paul trap, the quadrupolar field will heat the ensemble of ions (when applying simple buffer gas cooling) and the ions will never adapt to the nominal temperature of the trap. This is in particular true for light ions whose mass is close to that of the helium atoms [9] of the buffer gas. In this special case, the use of a multipolar trap is thus mandatory. Dieter Gerlich pioneered the field of low temperature multipole traps [10], culminating in the development of the 22-pole ion trap [11, 12], which is now in use in many laboratories worldwide. In the 1990s, with a 22-pole ion trap mounted on a 10 K cryogenic cooler, many (astrophysically motivated) experiments were performed, as for example radiative association [13, 14], deuteration [15] or clustering of H2 to H +3 at 10 K [16] (note: The first author of the last paper is a different Wolfgang Paul).
For floppy molecules as CH +5 [17, 18] and complex biological molecules [19–21], it is mandatory to go to even lower temperatures. For this reason, two new generation 4 K 22-pole machines have been recently completed in our laboratory. These siblings are named COLTRAP and FELion. While COLTRAP will be mainly used for 4 K-spectroscopy of small molecules in Cologne, FELion will be transferred permanently to the Free-Electron Laser for Infrared eXperiments (FELIX) [22] facility in Nijmegen, the Netherlands, for the investigation of more complex species. Besides their low temperature range down to a nominal 3.8 K, they feature a bent geometry [23] which allows to couple external modules (e.g., THz radiation or molecular beam sources) to the vicinity of the 22-pole trap. In this paper, we take the opportunity to present a thorough technical description of COLTRAP, while special features of FELion will be given in a future publication. We show first results obtained with COLTRAP on the attachment of helium atoms to stored ionic molecules at 3.8 K. In particular, this clustering can be exploited to obtain spectral features of the parent molecular ion by hindering He-attachment.
2 COLTRAP: 4 K 22-pole trap machine
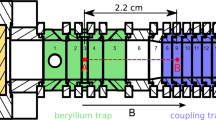
Figure 1 shows a sketch of the newly built machine. In the following sections, the most important parts of COLTRAP are described, paying attention to newer technical developments and routes followed in Cologne.
Setup of the Cologne-built 4 K ion trap machine COLTRAP. It comprises an ion storage source, a first quadrupole mass selector (QP1), an electrostatic quadrupole bender (QB), the 22-pole ion trap mounted on a 4 K coldhead, the second quadrupole mass selector (QP2) and finally a Daly-type ion detector. While both end flanges (close to QB and detector) can be used for laser access, the flange closer to the 22-pole trap is especially suited for attaching a THz radiation source or a molecular beam which can traverse the trap
2.1 Vacuum system
Highly reactive ions need ultra-high vacuum conditions if stored on the order of seconds. Therefore, all flanges in COLTRAP are copper gasket sealed (Conflat). COLTRAP is equipped with oil-free magnetically levitated turbopumps on the trap chamber (HiPace 300M, 255 l/s) and on the chamber of QP1 (TMU 200MP, 180 l/s), together backed by a turbomolecular drag pump (TPD011, 10 l/s) and a 15 l/min oil-free membrane pump (all Pfeiffer Vacuum). To measure the pressure in these chambers, COLTRAP is equipped with hot cathode ion gauges read out by two NGC2 gauge controllers (Arun Microlectronics Ltd.). With this setup, pressures in the range of 10−9 mbar are reached without using the cold head. A self-made interlock system assures that all high voltages inside the vacuum chamber are switched off when the measured pressure surmounts a set limit. Thus, it is safe to leave all power supplies switched on over night, leading to a stable operation.
2.2 Ion source
Different ion sources can be coupled to the source chamber of the machine. At the moment, COLTRAP operates with a Gerlich-type storage ion source [10] (as shown in Fig. 1). This source is mainly a stack of eight molybdenum plates (80 × 50 mm) in which a double H-shaped structure is cut and 2 endplates (also molybdenum) at the top and bottom of the stack. The four plates in the middle of the stack have a circular outlet for the ions on both front sides. An RF voltage (f ≈ 6.3 MHz, powered by self-made RF generators of the push–pull-type [24, 25]) is connected alternately to the eight inner plates, while a positive DC voltage is applied to the endplates. The neutral precursor gas is ionized in one of the side arms of the double H-structure by electron impact. Electrons are generated from a resistively heated filament above one endplate and are accelerated (typically 15–24 V) through a wide slit (50 × 2 mm) into the source volume. The created ions are stored by the RF field and may thus drift around in the storage volume. During this storage period, the ions can undergo elastic and inelastic collisions, electron transfer and chemical reactions with the neutral gas in the source. In this way, secondary ions can be produced which are difficult to produce otherwise, e.g., CH +5 from CH4 precursor gas. Those ions reaching the middle channel of the double H-structure may be formed into a short ion pulse (duration some ms) by applying a negative pulse to the exit lens (aperture 4 mm).
2.3 22-pole trap with pulsed valve
The heart of the machine is the 22-pole ion trap. Its copper housing harbors two monolithic stainless steel electrode sets (each with 11 thin long extruding rods) to which the RF is connected. This new design is thoroughly documented in [12]. The trap is mounted onto a 4 K Sumitomo coldhead (RDK-408E2) with a refrigeration capacity of 1 Watt at 4 K. The temperature can be increased by applying a maximum of about 40 W (40 V/1 A) to a kapton thermofoil heater (Minco, R = 37.3 \(\Upomega, 11 \times 150\) mm) which is firmly attached to the neck of the coldhead. The resulting temperature is measured with a Lakeshore DT-470-CU-13 diode attached to the trap housing. In the whole trap assembly, the usage of indium is avoided. The trap is driven by a RF power supply consisting of a commercial 20 MHz DDS (direct digital synthesis) circuit followed by a 10 W amplifier [12].
For the relaxation of the incoming ions and also for He tagging, a pulsed valve is connected to the trap via a Teflon tube. This piezo valve is of the Gerlich-type [14] and contains a small piezo element (12 × 10 × 0.5 mm) with a small glued silicon sealing. Applying a voltage to the piezo element (up to 300 V) bends the piezo element in the range of several tens of \(\upmu\)m, so that a polished nozzle is opened by the sealing. Short (some 10 \(\upmu\)s) and dense (exceeding 1015 cm−3) pulses can be generated with this type of valve. The short voltage pulses for the piezo excitation are produced in a push–pull amplifier with built-in high voltage modules (ISEG APp0425512, 400 V, 2.5 mA).
The electrostatic quadrupole bender (QB in Fig. 1) is directly attached to the cold shield of the trap assembly and adjusted so that it is aligned with the 22-pole trap at low temperature. It consists of four quarter cylinders with radius 10.2 mm. It allows to bend the ions coming from QP1 into the 22-pole trap, while its horizontal aperture of 6.6 mm allows to easily let pass long-wavelength radiation or a molecular beam coming from the nearest flange toward the 22-pole trap.
2.4 Quadrupoles I and II
The two quadrupole assemblies have been built in our in-house mechanical workshop. QP1 of COLTRAP has a length of 250 mm, and its rods are parts of a cylinder with an inner radius of curvature of r = 5 mm. Inside the quadrupole assembly, the radius inscribed by these four rods is r 0 = 4.3 mm. At the end of the quadrupole assembly, a fourfold segmented Einzel lens is attached which allows for focusing and steering the ion beam through the bender into the ion trap. The quadrupole assembly is mounted on the source vacuum chamber so that it can be slid out of the quadrupole vacuum chamber for easy maintenance. The quadrupole QP1 (as well as the storage ion source, Sect. 2.2) is powered by a self-made RF generator of the push–pull-type [24, 25] using QQE06/40 double tetrode tubes. For the mass range of interest (1–100 u), the frequency of the RF generator has been set to 1.6 MHz. The RF generator has been optimized in Cologne to be contained in one single 19′′ unit including all necessary power supplies. The RF amplitude as well as the overlayed DC voltage necessary for mass filtering is adjusted by hand, as the mass of the first quadrupole is usually not scanned during the experiments.
QP2 of COLTRAP is built from four 260-mm-long polished stainless steel rods with diameter d = 18 mm. According to d/2 = 1.148 r 0 (see e.g., [10]), the inscribed radius of the quadrupole is 2 r 0 = 15.678 mm. The four quadrupole rods are fixed via circular insulating Marcor pieces to two massive outer stainless steel rings (visible in Fig. 1). These rings have slides which just let the assembly fit into the middle of the vacuum chamber. At the end of QP2, there is a doubly segmented Einzel lens with a wide 20 mm aperture, guiding the ions into the Daly detector (see below). As the mass adjustment of QP2 has to be changed and controlled in a typical experiment, a commercial RF power supply (Balzers QMH511, mass range 1–100 u) is used. The mass spectrometer is controlled by an analog voltage (0–10 V) coming from the data acquisition system (see below). The RF power supply had to be adapted to the capacity of the self-made quadrupole assembly (which is typically in the range 50 pF). This has been achieved by decreasing the original 2 MHz operation frequency of the QMH511 power supply to 1.64 MHz (by changing the quartz oscillator) and fine adjustment with additional Mica HF-capacitors. The QMH511 RF generator is powered by a self-made supply, which is an FPGA-based replica of Balzers QHS511.
2.5 Daly detector
For ion detection, we use an old-fashioned Daly-type detector [26], in which the incoming cations are smashed onto a high voltage knob (−30 to 40 kV), releasing electrons, which are accelerated in the same field in the opposite direction toward a scintillator (Saint-Gobain, BC-400, 0.5 mm, cut to circles with diameter 16 mm and sputtered in-house with 200 nm Al on one side), after which the generated light is detected in a photomultiplier tube (PMT Hamamatsu R647-01 driven by a Hamamatsu C4900-series power supply unit). Although the conversion processes (cations \(\rightarrow\) electrons \(\rightarrow\) photons \(\rightarrow\) electrons) seems somewhat cumbersome, the Daly detector is still one of the most sensitive ion counting detectors, and it does not deteriorate with time or with bad vacuum. Of course, for counting anions in future experiments, different detector schemes (MCPs, channeltron) have to be used. The short (7 ns) pulses from the PMT enter an amplifier discriminator (Phillips Scientific model 6906, 300 MHz) and are finally counted with a 100 MHz counter (Ortec model 996) and read out via RS232. The count rate is currently limited to about 30 MHz, the limitation most probably being the current drawn from the Hamamatsu C4900 power supply.
The high voltage part of the detector (In Fig. 1 only the PMT side is visible while the HV-part is cut away) is shielded by a cylindrical stainless steel container for security. To simplify the setup, the HV converter (Spellman model UM40N4, −40 kV, 100 \(\upmu\)A max) has been included inside this container. Due to the small distance between the converter and the high voltage feedthrough, the interspace has been filled with machined Plexiglas parts to avoid arcing which could damage the converter.
2.6 Data acquisition system
We use National Instruments Labview 2012 to control the measurement PC as well as a NI cRIO-9022 controller attached to an 8-slot cRIO-9114 chassis. This chassis features a VIRTEX-5LX50 (Xilinx) FPGA (field-programmable gate array). Such an FPGA is a semiconductor device that can be hardware programmed after manufacturing, allowing for flexible programming and fast execution times. Our FPGA-chassis contains four modules (NI9263, 2 × NI9264, NI9401) which permits output of a total of 36 analog voltage channels (±10 V, 16 bit) and 8 channels of TTL-input/output, which are all synchronized on the \(\upmu\)s time scale. The analog outputs used for ion beam control are directly connected to the electrodes and lenses of the machine.
The FPGA-based system not only minimizes the space and power requirement for the DAQ system considerably, it also makes it possible to put arbitrary voltage waveforms on the electrodes. Figure 2 shows a typical scheme of analog and digital signals as used in our trapping cycles. The flexibility of the waveforms allows more advanced ion manipulations, as for example minimizing RF heating [9] by lowering the RF power and the trap electrode voltages some ms after the trapping begins. Similarly, a soft ramp of the extraction voltage at the end of the trapping cycle as shown in Fig. 2 is important to reduce collision-induced dissociation (CID) of weakly bound helium clusters and to avoid saturation of the ion detector.
Sequence of analog and digital signals as typically used for a single trapping cycle. The two upper TTL-signals control the pulse for the piezo valve (the generated helium pulse has to fill the trap before the ions arrive) and the gate, which, after completion of the trapping cycle, enables counting in the ORTEC 996 counter. The lower five signals are analog voltages. The trap entrance and exit electrodes control the trapping itself. As can be seen, the FPGA-based DAQ system allows for more advanced waveforms, e.g., at the end of the trapping cycle the voltages can be slowly changed. This can be used for example to avoid saturation of the detector. Lowering the trap RF amplitude after the first milliseconds of trapping is not implemented yet
3 First results
3.1 He tagging at 4 K
As a first test of the machine performance and in particular the cooling of the stored ions, pulses of mass selected H3O+ (ubiquitous in the ion source) or CH +5 (our favorite guinea pig [18, 27, 28]) have been sent toward the ion trap and some thousand ions stored in the presence of helium gas (Linde gas helium 5.0). Helium has been introduced either by the pulsed piezo valve at the beginning of the trapping cycle or constantly via a leakage valve through a small channel in the trap baseplate. In general, the pulsed valve generates a higher instantaneous density with which more He atoms can be attached to the stored ionic molecules. This can be seen in Fig. 3, where CH +5 or H3O+ have been cooled and tagged by a strong helium pulse leading to ternary collision processes of the type
and the formed species extracted from the trap after 1,000 ms. For both injected ions a yield of the singly attached species \(\hbox{CH}_5^+ \cdot\hbox{He}\) and \(\hbox{H}_3\hbox{O}^+ \cdot\hbox{He}\), respectively, in the range of at least 10 % of the parent ions counts is feasible. For CH +5 , up to 5 helium atoms can easily be attached, indicating a shell closure at which the 5 atoms are connected to the 5 protons of CH +5 , while the attachment of an additional helium atom (signal at mass 41 u) is suppressed. For H3O+, the attachment of three helium atoms has been expected (bound to the three protons), but the experiment shows that even two more helium atoms are attached, most probably to the oxygen center on the C3 axis, and a shell closure is reached. The attachment of a sixth He atom (mass 43 u) is again unprobable.
The attachment of helium atoms can be used as a sensitive temperature probe, as shown in Fig. 4. In this measurement, helium gas has been admitted constantly, leading to a lower yield in species with more than one helium atom attached. The nominal trap temperature has been increased in steps from 3.8 K to about 12 K and mass spectra taken. At temperatures higher than 12 K, H2, which is a contamination in helium, is no longer effectively frozen out onto the trap walls and the inlet channel, and thus, the number of detected \(\hbox{CH}_5^+ \cdot\hbox{H}_2\) clusters (mass 19 u) increases considerably. As can be seen, increasing the temperature of the helium bath leads to a substantial decrease in clusters with one helium atom.
In Fig. 5, the time development of the cluster formation (and destruction) is shown as a function of trapping time. As mentioned above, with helium constantly admitted, the instantaneous helium density is relatively low so that clusters with predominantly one helium atom are formed.
Time development of clustering of He atoms to CH +5 at a nominal temperature of 3.8 K. Helium was constantly leaked into the trap with an estimated number density n ≈ 5 × 1014 cm−3. Under these conditions, an equilibrium with a maximum number of \(\hbox{CH}_5^+ \cdot\)He ions is reached after about 350 ms. This trapping time can be used for spectroscopic experiments (see next section). With a series of such experiments at different number densities n the ternary rate coefficient k 3 for the first attachment step can be estimated (see inset and text)
Under the chosen conditions, more than 1,000 \(\hbox{CH}_5^+ \cdot\)He clusters are formed within the first 350 ms of trapping time when an equilibrium between formation and destruction is established. The inset in Fig. 5 shows the effective rate coefficient extracted for the first attachment step (three-body reaction (1)) at different helium number densities n, from which the ternary rate coefficient is estimated k 3 ≈ 4 × 10−30 cm6 s−1. We conservatively assume this value to be valid within a factor of four, as (1) the calibration of the trap helium density was estimated within a factor of less than two, and (2) the pressure reading at the lowest cold head temperature of 3.8 K varied strongly with the cold head cycle, leading to the scatter of data at higher pressures as visible in the inset of Fig. 5. Similarly, simulating measurements such as shown in Fig. 5, we obtain for collision-induced dissociation (the reverse of reaction (1)) k CID ≈ 5 × 10−15 cm3 s−1 (within a factor of two). With a proper calibration of the helium number density, the ternary rate coefficient k 3 and in particular its temperature dependence k 3(T) will be addressed in a future publication.
3.2 First spectroscopic results: laser-induced inhibition
Attaching (noble) gases to ionic species can be exploited for spectroscopy. Usually, in ‘tagging’ or ‘messenger’ experiments argon, neon or even molecules such as hydrogen, nitrogen or water are used to label the ionic species of interest, in cases where the spectra of the naked species are difficult to obtain [29–33]. This is the case for molecules with covalent bonds which cannot be ruptured by a single IR photon. A single IR photon does not have enough energy to fragment the covalent bond of a bare ion, but it can dissociate the tagged one. Of course, the tagged transition frequencies are shifted, but one gets a good idea of the vibrational band positions of the naked ion which can be compared to ab initio calculations. In particular, Boo and Lee [34, 35] obtained the first spectroscopic information on CH +5 by solvating it in hydrogen and observing the detachment of H2 by a pulsed IR laser. If one wants to reduce the influence of the messenger, helium is probably the best choice, as it is much less strongly bound (the helium binding energy is typically <1 kJ mol−1 ≃ 83.6 cm−1 and thus one order of magnitude smaller than other tags [36, 37]) so that much less distortions of the molecular structure and thus lower changes and shifts in the spectrum can be expected. Traditionally, He-tagged clusters are directly produced in the ion source [37, 38], but for more complex species (like biological samples produced in an electrospray ion source), it can be more favorable to produce them in a cold ion trap filled with the messenger gas [36, 39].
On the other side, it is possible to excite the parent ions under investigation so that the attachment of the loosely bound messenger atom is suppressed. While the hindering effect of rotational excitation on the association reaction \(\hbox{CO}^+ + 2\hbox{CO} \rightarrow\, \hbox{(CO)}_2^+ + \hbox{CO}\) has been shown more than 20 years ago [40], the similar effect due to electronic or vibrational excitation has not been exploited until recently [41, 42]. Suggested by Dieter Gerlich and termed ’laser-induced inhibition of complex growth’ (LIICG), experiments in the group of John Maier in Basel allow to measure electronic spectra of N +2 at a nominal temperature of 5 K by inhibiting helium attachment [41]. Interestingly, they do not observe optical transitions of the \(\hbox{N}_2^+ \cdot\)He complex.
To test the potential for IR spectroscopy in our experiment, a commercial cw IR optical parametric oscillator (OPO) operating in the region 2,560–3,130 cm−1 (Aculight Argos Model 2400) was coupled to our 4 K-machine. The ∼1 W beam entered the machine on the trap side (at QB in Fig. 1) and left the machine at the detector side using differentially pumped windows (of the same type as documented in [43]) with 5-mm-thick CaF2 window pieces. Great care has been taken on good adjustment through the 6.6 mm aperture of the 22-pole trap to avoid its heating by the IR laser. Additionally, a laser baffle has been mounted on the entrance window to protect the trap. After leaving the machine, the laser beam was blocked by a power meter (Coherent PowerMax PM10). The frequency of the IR laser was measured with a wavemeter (Bristol Instruments Model 621A-IR), which has an accuracy of 30 MHz (if well aligned). The laser frequency has been controlled by the 0\(\ldots\)10 V output of the NI 9263 module followed by a voltage amplifier, which allowed to make scans 2.5 cm−1 wide.
We started the test experiments by storing about 10,000 CH +5 ions in the trap at a nominal temperature of 3.8 K, constantly colliding with cold He buffer gas and being irradiated by the narrow-band OPO. The density of helium was high, ∼5 × 1014 cm−3, corresponding to about 1 collision per microsecond. A trapping time of about 400 ms is sufficient with the chosen conditions, as an equilibrium of the \(\hbox{CH}_5^+ \cdot\)He counts is reached, see Fig. 5. When adjusting QP2 to mass 21 (\(\hbox{CH}_5^+ \cdot\)He) and scanning the OPO, signals as shown in Fig. 6 were obtained after some time of search. We choose to depict this particularly strong line due to its good signal-to-noise ratio, although it is quite broad with a full width half maximum (FWHM) of about 65 MHz. More typically, most of the hitherto ∼80 observed lines are less saturated and have FWHM values scattering around 54 MHz. In the regime we are operating, pressure broadening is still negligible (on the order of 1 MHz) and Doppler broadening dominates together with power broadening and saturation of the signal. Attributing the typical observed linewidth of 54 MHz only to thermal broadening (neglecting for the moment power broadening) and using a simple Gaussian for the fit yields an upper limit of the kinetic temperature of about 12 K for an ion ensemble with mass 17 u. Usually, in cryogenic buffer gas RF multipole trapping experiments, the ions are RF-heated beyond the nominal trap temperature [9, 19, 44–47], and as the contribution of power broadening is unclear at the moment, we just bracket the kinetic temperature of the ion ensemble between 4 and 12 K. Also, as effective clustering sets in below 12 K (see Fig. 4), we probably only capture all ions falling below that temperature limit during the initial cooling process in the trap.
Spectrum of a single rovibrational line obtained by injecting about 10,000 CH +5 ions into a cloud of cold and dense helium (\(3.8 \, \hbox{K}, \sim\!\!10^{15}\, \hbox{cm}^{-3}\)) and irradiation with a 1.2 W cw laser beam during 450 ms trapping time. By the resonant excitation, the number of ion counts on mass 21 u (\(\hbox{CH}_5^+ \cdot\)He) is decreased. As detailed in the text, the signal is most probably due to laser-induced inhibition of helium attaching to CH +5 and not destruction of \(\hbox{CH}_5^+ \cdot\)He. The accuracy of the used wavemeter is about 30 MHz
The resonance in Fig. 6 shows a 50 % reduction in the \(\hbox{CH}_5^+ \cdot\)He counts after 450 ms of trapping. Due to this reduction, it is tempting to assume that the signal originates from a depletion of the \(\hbox{CH}_5^+ \cdot\)He formed in the trap. However, the signal can also stem from the inhibition of \(\hbox{CH}_5^+ \cdot\)He formation, i.e., LIICG [41] as discussed above. Experimental evidence shows that the latter process is prevailing for our spectra taken so far: (1) the majority of the hitherto observed ∼80 lines coincide with lines from a highly accurate experimental CH +5 linelist within <30 MHz (the accuracy of the wavemeter in the current experiment). The mentioned CH +5 linelist has been obtained in our group with a rather different laser-induced reaction method described in [28]. Those lines cannot stem from \(\hbox{CH}_5^+ \cdot\)He since they are formed at higher temperatures (about 25 K) and thus no \(\hbox{CH}_5^+ \cdot\)He has been found in respective mass spectra. Furthermore, (2) optimizing the experimental conditions to destroy \(\hbox{CH}_5^+ \cdot\)He (fast production of \(\hbox{CH}_5^+ \cdot\)He within 100 ms using the pulsed valve and long irradiation time over 3 s) lead to a weaker signal, and (3) optimizing experimental conditions for LIICG (slow production of \(\hbox{CH}_5^+ \cdot\)He with low density of continuous helium and 500 ms trapping time) improved the S/N ratio.
At this point, it should be noted that single rovibrational lines of CH +5 as shown in Fig. 6 cannot be assigned yet [17, 28] to quantum numbers and thus a further analysis of the signal intensity is impeded. But the fact that LIICG allows us to obtain spectra of bare CH +5 at a nominal trap temperature of 4 K can be considered a breakthrough for the spectroscopy of CH +5 since the number of rovibrational transitions is reduced substantially which shall help to ultimately assign the spectrum and thus determine the rotational structure of this elusive molecule.
As mentioned, we do not have any evidence for the destruction of \(\hbox{CH}_5^+ \cdot\)He complexes, similar to the work in Basel [41]. This is somewhat confusing as Boo and Lee [34, 35] observed rupture of \(\hbox{CH}_5^+ \cdot \hbox{H}_2\). So what is different? It could be possible that the loosely bound helium atom acts as a spectator when the cluster is excited so that the vibration of the inside core is rapidly redistributed and heats the inside core, but does not lead to detachment. But a more probable reason is that the short lifetime of the predissociating complex makes the signal quite broad so that it is simply not seen with our high-resolution laser (intrinsic linewidth ≤ 100 kHz).
4 Discussion and outlook
The recent developments in storing molecular ions in 4 K helium open up new possibilities for spectroscopy. In contrast to the method of laser-induced reaction (LIR) [18, 44, 45, 48–51] which always requires a suitable neutral reaction partner, LIICG is a more general spectroscopy method with no apparent requirements to the ionic species, although restricted to very low temperatures. Other ionic molecular species will be tested soon (in particular ions with known IR spectra), as well as the extension to somewhat higher temperatures using hydrogen, argon or neon. In contrast to LIR, LIICG does not come with a chemical selection, thus all ions of the same selected mass admitted simultaneously to the 22-pole ion trap will be probed. Hence, it is well possible that some of the LIICG-signals not attributable to CH +5 in the present work are due to C13H +4 (mass 17 u). It will be also interesting to test to what extent LIICG will be applicable at longer wavelengths in the far infrared, a limitation LIR can suffer from (see e.g., Fig. 2 of [18]). In particular, as cluster formation is dependent on the rotational state of the reactants [40], LIICG could be exploited for rotational spectroscopy in cold ion traps [51–53].
But, to become a powerful general method able to compete with other schemes like LIR (which is in principle background-free), the signal-to-noise ratio has to be considerably improved down to the Gaussian noise limit. For example, a current problem seems to be the 1 Hz temperature variation of the coldhead on the order of 0.2 K, which also leads to a variation of the number of formed \(\hbox{CH}_5^+ \cdot\)He. Also the admission scheme of helium has to be improved, in a way that the incoming ions are first cooled without producing clusters and without laser, and then the cluster production is enabled by a higher He density, while the laser is inhibiting. This is somewhat challenging, as helium is sticking to the surface of the trap below 4 K, and thus, fast density variations cannot be made. Also higher powers of the laser will be explored soon using a reflector at the detector side. Again, greatest care in adjustment has to be exercised, and laser baffles are needed to avoid IR-heating of the trap. Using a back-reflected laser beam with a comb-stabilized frequency will probably enable Doppler-free spectroscopy limited only by the bandwidth of the laser.
References
W. Paul, M. Raether, Das elektrische Massenfilter. Z. Phys. 140, 262–273 (1955)
W. Paul, O. Osberghaus, E. Fischer, Ein Ionenkäfig. Forsch. Ber. d. Wirtsch. minist. Nordrh. Westfal. Nr. 415, 1–42 (1955)
E. Fischer, Die dreidimensionale Stabilisierung von Ladungsträgern in einem Vierpolfeld. Z. Phys. 156, 1–26 (1959)
W. Paul, Electromagnetic Traps for charged and neutral particles, 1989. Nobel Lecture, available on http://www.nobel.se.
W. Neuhauser, M. Hohenstatt, P. Toschek, H. Dehmelt, Localized visible Ba+ mono-ion oscillator. Phys. Rev. A 22, 1137–1140 (1980)
D.J. Wineland, J.C. Bergquist, W.M. Itano, J.J. Bollinger, C.H. Manney, Atomic-ion Coulomb crystal in an ion trap. Phys. Rev. Lett. 59, 2935–2938 (1987)
T. Rosenband, D.B. Hume, P.O. Schmidt, C.W. Chou, A. Brusch, L. Lorini, W.H. Oskay, R.E. Drullinger, T.M. Fortier, J.E. Stalnaker, S.A. Diddams, W.C. Swann, N.R. Newbury, W.M. Itano, D.J. Wineland, J.C. Bergquist, Frequency ratio of Al+ and Hg+ single-ion optical clocks; metrology at the 17th decimal place. Science 319(5871), 1808–1812 (2008)
J. Harju, M. Juvela, S. Schlemmer, L.K. Haikala, K. Lehtinen, K. Mattila, Detection of 6 K gas in Ophiuchus D. Astron. Astrophys. 482, 535–539 (2008)
O. Asvany, S. Schlemmer, Numerical simulations of kinetic ion temperature in a cryogenic linear multipole trap. Int. J. Mass Spectrom. 279, 147–155 (2009)
D. Gerlich, Inhomogeneous RF fields: a versatile tool for the study of processes with slow ions. In: C.Y. Ng, M. Baer (eds) Adv. Chem. Phys.: State-Selected and State-to-State Ion-Molecule Reaction Dynamics, vol. LXXXII, (Wiley, New York, 1992) pp. 1–176.
D. Gerlich, Ion-neutral collisions in a 22-pole trap at very low energies. Phys. Scr. T 59, 256–263 (1995)
O. Asvany, F. Bielau, D. Moratschke, J. Krause, S. Schlemmer, New design of a cryogenic linear rf multipole trap. Rev. Sci. Instrum. 81, 076102 (2010)
D. Gerlich, S. Horning, Experimental investigations of radiative association processes as related to interstellar chemistry. Chem. Rev. 92, 1509–1539 (1992)
D. Gerlich, Recent progress in experimental studies of ion-molecule reactions relevant to interstellar chemistry. In: I. Nenner (eds) Molecules and Grains in Space, (AIP Press, New York, 1994) pp. 489–500.
D. Gerlich, S. Schlemmer, Deuterium fractionation in gas-phase reactions measured in the laboratory. Planet. Space Sci. 50, 1287–1297 (2002)
W. Paul, B. Lücke, S. Schlemmer, D. Gerlich, On the dynamics of the reaction of positive hydrogen cluster ions (H +5 to H +23 ) with para and normal hydrogen at 10K. J. Mass Spectrom. Ion Process. 150, 373–387 (1995)
E.T. White, J. Tang, T. Oka, CH +5 : the infrared spectrum observed. Science 284, 135–137 (1999)
O. Asvany, P.P. Kumar, B. Redlich, I. Hegemann, S. Schlemmer, D. Marx, Understanding the infrared spectrum of bare CH +5 . Science 309, 1219–1222 (2005)
O.V. Boyarkin, S.R. Mercier, A. Kamariotis, T.R. Rizzo, Electronic spectroscopy of cold, protonated tryptophan and tyrosine. J. Am. Chem. Soc. 128, 2816–2817 (2006)
X. Chen, M. Tirado, J.D. Steill, J. Oomens, N.C. Polfer, Cyclic peptide as reference system for b ion structural analysis in the gas phase. J. Mass Spectrom. 46(10), 1011–1015 (2011)
J. Grzetic, J. Oomens, Spectroscopic evidence for an oxazolone structure in anionic b-type peptide fragments. J. Am. Soc. Mass Spectrom. 23(2), 290–300 (2012)
D. Oepts, van der A.F.G. Meer, van P.W. Amersfoort, The free-electron-laser user facility felix. Infrared Phys. Technol. 36, 297–308 (1995)
D. Gerlich, The study of cold collisions using ion guides and traps. In: I.W.M. Smith (eds) Low Temperatures and Cold Molecules, chap. 3 (Imperial College Press Distributer World Scientific Publishing Co. Pte. Ltd., Singapore, 2008) pp. 121–174.
R.M. Jones, D. Gerlich, S.L. Anderson, Simple radio-frequency power source for ion guides and ion traps. Rev. Sci. Instrum. 68(9), 3357–3362 (1997)
R.M. Jones, S. L. Anderson, Simplified radio-frequency generator for driving ion guides, traps, and other capacitive loads. Rev. Sci. Instrum. 71(11), 4335–4337 (2000)
N.R. Daly, Scintillation type mass spectrometer ion detector. Rev. Sci. Instrum. 31, 264–267 (1960)
S.D. Ivanov, O. Asvany, A. Witt, E. Hugo, G. Mathias, B. Redlich, D. Marx, S. Schlemmer, Quantum-induced symmetry breaking explains infrared spectra of CH +5 isotopologues. Nat. Chem. 2, 298–302 (2010)
O. Asvany, J. Krieg, S. Schlemmer, Frequency comb assisted mid-infrared spectroscopy of cold molecular ions. Rev. Sci. Instrum. 83, 093110 (2012)
M. Okumura, L.I. Yeh, J.D. Myers, Y.T. Lee, Infrared specta of the solvated hydronium ion: vibrational predissociation spectroscopy of mass-selected H3O+(H2O) n (H2) m . J. Phys. Chem. 94, 3416–3427 (1990)
E.J. Bieske, O. Dopfer, High-resolution spectroscopy of cluster ions. Chem. Rev. 100, 3963–3998 (2000)
J.M. Headrick, E.G. Diken, R.S. Walters, N.I. Hammer, R.A. Christie, J. Cui, E.M. Myshakin, M.A. Duncan, M.A. Johnson, K.D. Jordan. Spectral signatures of hydrated proton vibrations in water clusters. Science 308(5729), 1765–1769 (2005)
A.M. Ricks, G.E. Douberly, P.V.R. Schleyer, M.A. Duncan, Infrared spectroscopy of gas phase C3H +3 ions: the cyclopropenyl and propargyl cations. J. Chem. Phys. 132(5), 051101 (2010)
M.Z. Kamrath, R.A. Relph, T.L. Guasco, C.M. Leavitt, M.A. Johnson, Vibrational predissociation spectroscopy of the H2-tagged mono- and dicarboxylate anions of dodecanedioic acid. Int. J. Mass Spectrom. 300(23), 91–98 (2011)
B.D. Wan, Z.F. Liu, A.G. Suits, J.S. Tse, Y.T. Lee, Dynamics of carbonium ions solvated by molecular hydrogen: CH +5 (H2) n (n = 1,2,3). Science 269, 57–59 (1995)
D.W. Boo, Y.T. Lee, Infrared spectroscopy of the molecular hydrogen solvated carbonium ions, CH +5 (H2) n (n = 1–6). J. Chem. Phys. 103, 520–530 (1995)
J. Jašík, J. Žabka, J. Roithová, D. Gerlich, Infrared spectroscopy of trapped molecular dications below 4 K. Int. J. Mass Spectrom. (2013). doi:10.1016/j.ijms.2013.06.007
A. Patzer, M. Schütz, T. Möller, O. Dopfer, Infrared spectrum and structure of the adamantene cation: direct evidence for Jahn-Teller distortion. Angew. Chem. Int. Ed. 51, 4925–4929 (2012)
E.J. Bieske, A. Soliva, M.A. Welker, J.P. Maier, The B \(\leftarrow\) X electronic spectrum of N +2 -He. J. Chem. Phys. 93(6), 4477–4478 (1990)
M. Brümmer, C. Kaposta, G. Santambrogio, Knut R. Asmis, Formation and photodepletion of cluster ion–messenger atom complexes in a cold ion trap: infrared spectroscopy of VO+,VO +2 , and VO +3 . J. Chem. Phys. 119(24), 12700–12703 (2003)
D. Gerlich, T. Rox, Association reactions with state selected ions at meV collision energies: CO+ (v=0,j) + 2CO \(\rightarrow \hbox{(CO)}_2^+ + \hbox{CO}\). Z. Phys D Atoms Mol. Clust. 13(3), 259–268 (1989)
S. Chakrabarty, M. Holz, A. Banerjee, D. Gerlich, J.P. Maier, A novel method to measure electronic spectra of cold molecular ions. J. Phys. Chem. Lett. (2013) (submitted)
S. Schlemmer, O. Asvany, S. Brünken, Low temperature trapping: from reactions to spectroscopy. In International Symposium on Molecular Spectroscopy 68th Meeting, Columbus, Ohio, (2013)
O. Asvany, E. Hugo, S. Schlemmer, CF16 differentially pumped window for ultrahigh vacuum applications. J. Vac. Sci. Technol. A 25, 628 (2007)
J. Glosík, P. Hlavenka, R. Plašil, F. Windisch, D. Gerlich, A. Wolf, H. Kreckel, Action spectroscopy of H +3 and D2H+ using overtone excitation. Philos. Trans. R. Soc. A, 364, 2931–2942 (2006)
J. Mikosch, U. Frühling, S. Trippel, D. Schwalm, M. Weidemüller, R. Wester, Evaporation of buffer-gas-thermalized anions out of a multipole rf ion trap. Phys. Rev. Lett. 98, 223001 (2007)
O. Asvany, E. Hugo, F. Müller, F. Kühnemann, S. Schiller, J. Tennyson, S. Schlemmer, Overtone spectroscopy of H2D+ and D2H+ using laser induced reactions. J. Chem. Phys. 127, 154317 (2007)
R. Otto, A. von Zastrow, T. Best, R. Wester, Internal state thermometry of cold trapped molecular anions. PhysChemChemPhys 15, 612 (2013)
S. Schlemmer, T. Kuhn, E. Lescop, D. Gerlich, Laser excited N +2 in a 22-pole ion trap: experimental studies of rotational relaxation processes. Int. J. Mass Spectrom. 185, 589–602 (1999)
S. Schlemmer, E. Lescop, J.V. Richthofen, D. Gerlich, Laser induced reactions in a 22-pole trap: \(\hbox{C}_2\hbox{H}_2^+ + h\nu_3 + \hbox{H}_2 \rightarrow \hbox{C}_2\hbox{H}_3^+\) + H. J. Chem. Phys. 117, 2068–2075 (2002)
O. Asvany, T. Giesen, B. Redlich, S. Schlemmer, Experimental determination of the ν5 cis-bending vibrational frequency in ground state \((\hbox{X}^2\Uppi_u) \hbox{C}_2\hbox{H}_2^+\) using laser induced reactions. Phys. Rev. Lett. 94, 073001 (2005)
O. Asvany, O. Ricken, H.S.P. Müller, M.C. Wiedner, T. Giesen, S. Schlemmer, High-resolution rotational spectroscopy in a cold ion trap: H2D+ and D2H+. Phys. Rev. Lett. 100, 233004 (2008)
J. Shen, A. Borodin, M. Hansen, S. Schiller, Observation of a rotational transition of trapped and sympathetically cooled molecular ions. Phys. Rev. A 85, 032519 (2012)
S. Gärtner, J. Krieg, A. Klemann, O. Asvany, S. Brünken, S. Schlemmer, High-resolution spectroscopy of CH2D+ in a cold 22-pole ion trap. J. Phys. Chem. A 117, 9975–9984 (2013)
Acknowledgments
This work has been financially supported by the Deutsche Forschungsgemeinschaft (DFG) via SCHL 341/6-1. S.B. and St.S. acknowledge support from DFG SPP 1573 grant BR 4287/1-1. The authors gratefully acknowledge the work done over the last years by the electrical and mechanical workshops of the I. Physikalsiche Institut, as well as our engineers Frank Bielau, Peter Stölzgen and Henning Adams. The authors thank Sabrina Gärtner, Sven Fanghänel, Alexander Stoffels and Alexey Potapov for setting up components of the instrument, as well as Stefan Wulff for Al-coating the scintillators. Most of all we want to thank Dieter Gerlich for sharing his ideas with us.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Asvany, O., Brünken, S., Kluge, L. et al. COLTRAP: a 22-pole ion trapping machine for spectroscopy at 4 K. Appl. Phys. B 114, 203–211 (2014). https://doi.org/10.1007/s00340-013-5684-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-013-5684-y