Abstract
Due to their large trap depths (∼1 eV or 10,000 K), versatility, and ease of construction, Paul traps have important uses in high-resolution spectroscopy, plasma physics, and precision measurements of fundamental constants. An ion-neutral hybrid trap consisting of two separate but spatially concentric traps [a magneto-optic trap (MOT) for the neutral species and a mass-selective linear Paul trap for the ionic species] is an ideal apparatus for sympathetic cooling. However, over the past few years, hybrid traps have proven most useful in measuring elastic and charge-exchange rate constants of ion-neutral collisions over a wide temperature range from kilo-Kelvin to nano-Kelvin. We report some initially surprising results from a hybrid trap system in our laboratory where we have loaded the Paul trap with Ca+ ions in the presence of a Na MOT (localized dense gas of cold Na atoms). We find a strong loss of Ca+ ions with MOT exposure, attributed to an exothermic, non-resonant ion-neutral charge-exchange process with an activation barrier, which leads to the formation of Na+ ions. We propose a detailed mechanism for this process. We obtain an estimated measure of the rate constant for this charge exchange of ∼2 × 10−11 cm3/s, much less than the Langevin rate, which suggests that the Langevin assumption of unit efficiency in the reaction region is not correct in this case.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Background
The long-range interactions between neutral atoms play an important role in understanding the properties of quantum gases. Typical neutral van der Waals interactions have cross sections of a few atomic units (a.u.) over a wide energy range. Ion-neutral reactions are of intermediate range between ion–ion and neutral–neutral collisions and can have much larger total cross sections, especially in the temperature range below 1 mK. These intermediate range interactions are dominated by universal long-range polarization potentials, with the principal term proportional to −α /R 4. Here, α is the dipole polarizability of the neutral collision partner, and R is the internuclear distance in a binary ion-atom collision. In the ion-neutral case, the R −4 polarization potential leads to enormous elastic scattering cross sections (∼106 a.u. at 1 mK), charge-exchange cross sections, and the possibility of orbiting collisions [1], in the case of a singly charged atomic ion and an alkali atom neutral [2–4].
Historically, the ultracold or low-energy ion-neutral collisions have been relatively unexplored experimentally. However, in the past few years, interest in this area has blossomed, since the introduction of the hybrid trap by our group [2, 5]. A hybrid trap apparatus consists of a neutral atom trap and a separate ion trap, which are concentric and capable of spatially overlapping two or more species. The hybrid trap in one form or another has become a popular apparatus for precise measurements of ion-neutral charge-exchange, elastic scattering rate constants, and demonstrating sympathetic cooling [6–18]. Two of these papers, Refs. [13, 14], even involve hybrid trap experiments with a single ion in a Bose–Einstein condensate. A recent review of cold ion-neutral collision theory and experiment can be found in [19], and there is also a report of low-temperature elastic collisions between Ca+ and Li atoms using an optical trap rather than a MOT [20].
In general, ion-neutral cold collision studies show great promise for investigating both cold atomic and molecular ions, especially studies of internal state selection, internal cooling mechanisms [2, 6, 10], quantum control reactions [21], quantum information (including error and decoherence rates due to patch fields and surface charges) [22], precision spectroscopy, and ion-molecule interactions important in astrophysics [23–25] or plasma physics.
Although the temperature of interstellar space is a relatively warm ∼3 K, there are cold ion-neutral exothermic processes in the interstellar medium [23–25]. Low-energy ion-neutral interactions are also known by astrophysicists to be important in the upper atmosphere, e.g., secondary ions from solar wind collisions can undergo further ion-molecule reactions.
We believe that such charge-exchange studies will serve as valuable tests for screening possible combinations of atomic or molecular ion and neutral species with the potential for being used in ion-based qubits. Charge exchange could lead to undesirable loss pathways in quantum information schemes, so knowledge of the rate coefficient’s dependence on collision energy and excited-state fraction of the collision partners should prove useful in choosing species for quantum information applications.
Interest in cold ion-neutral charge exchange has been bolstered by new theoretical studies. For example, Tacconni et al. [26] have calculated the total cold charge-exchange cross section in the (CaRb)+ system for various final channels, starting with the entrance channel Ca+(4s) + Rb (ground state). At 1 mK, charge exchange to Ca(4s4p3P) + Rb+ has a calculated cross section ∼4 × 103 a.u., which is fairly large. Experimental results obtained using an ion-neutral hybrid trap have shown good agreement [15]. Comparisons between theory and experiment are critical in evaluating the current scattering models in this relatively unexplored energy regime [19].
2 Na + Ca+ charge-exchange reactions
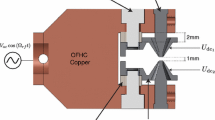
Our second-generation hybrid trap consists of a sodium (Na) magneto-optic trap (MOT) [27] concentric within a linear rf quadrupole ion Paul trap [28]. An illustration of our hybrid apparatus is shown in Fig. 1, and the apparatus is also discussed in Refs. [17, 18]. The ion trap can hold a large number of atomic or molecular ions of many types. Therefore, the hybrid apparatus allows us to spatially overlap practically any ionic species with Na and study their interactions experimentally in a highly controllable manner. Because ion traps use the Coulomb force to trap ions, the trap depths can be rather large: at least ∼1 eV or 10,000 K for our trap, which can allow for very long storage times [29].
Schematic of our hybrid trap apparatus. The three retro-reflected 589-nm laser beams and anti-Helmholtz coils form the Na MOT, concentric with the linear segmented ion Paul trap (LPT). The number of trapped ions can be measured destructively using the Channeltron electron multiplier (CEM). An electron gun can be used to create Ca+ ions, which in future experiments will be laser cooled with slightly off-axis 397 and 866 nm laser beams
For the experiments presented here, the ions are not laser cooled. In future experiments, we plan to laser cool the Ca+ ions as illustrated in Fig. 1. Typically, we use the Na Type II MOT with a peak density of ∼1010 cm−3 at ≈1 mK. The Ca+ ions are created from electron-impact ionization of a background Ca gas, and the Na+ ions are created by resonance-enhanced multiphoton ionization (REMPI) of a background Na gas. Both the Na gas and the Ca gas are produced via getters or small ovens, which create a hot ∼1,000 K background gas. We estimate an initial ion cloud that contains ∼103 ions. This estimate is based on a crude calibration of our CEM discussed in Ref. [18].
When we tried to sympathetically cool Ca+ ions experimentally with the Na MOT, we had expected from our simulations [17] (which considered only elastic scattering and not charge exchange) that the sympathetically cooled ions should have longer trap lifetimes. Ion lifetime increased when we sympathetically cooled Na+ with our Na MOT [18] or when Rb+ was cooled in an Rb MOT [16]. Furthermore, because Ca+ is more massive than Na, there will be less atom-ion rf heating [30, 31], which should result in lower equilibrium temperatures [17]. Figure 2 shows semi-log fits to the fractional number of Ca+ ions remaining in the Paul trap as a function of time delay after loading, with the Na MOT on (square points) and off (circles). We found that the trapped Ca+ lifetime decreased when exposed to the MOT in the hybrid trap.
Single-exponential decay curves in the hybrid trap for Ca+ ions. The square points (black) show a semi-log plot of the fraction of ions remaining vs. time with the MOT on, and the circles (red) show a similar plot of ions remaining vs. time with the MOT off. The fitted lifetime with the cold Na atoms (MOT) present is 1.6 ± 0.2 s, and with the MOT turned off is 5.6 ± 0.9 s. The slight curvature in the lower decay curve (MOT on) is due to a small constant background included in the semi-log fit. We infer that the difference in the two decay rates approximates the net charge transfer rate from Na to Ca+ under these experimental conditions to be ≈0.5 Hz
While conducting this experiment, we used mass-selective resonant quenching (MSRQ) [32] to completely remove Na+ formed via photodissociation of Na +2 created by associative ionization from Na(3p) atoms within the MOT [33]. We believe that the loss of Ca+ ions from the trap is due to a low-energy non-radiative charge-exchange process, \(\hbox{Ca}^{+}+\hbox{Na}(3\hbox{p})\rightarrow\hbox{Ca}(4\hbox{s}^2\,\hbox{or}\,4\hbox{s}4\hbox{p})+\hbox{Na}^{+}\), whose product Na+ ions are also removed as a consequence of the same MSRQ.
Recall that there was no laser cooling of the Ca+ ions, only collisional interaction with the cold Na atoms from the MOT. We believe that sympathetic heating between Ca+ and Na is not the cause of the observed trap loss, since our simulations [17] of sympathetic cooling of Ca+ ions by Na MOT collisions showed effective cooling when only elastic Ca+ on Na collisions were considered.
For the data in Fig. 2, the geometrical center of the Paul trap coincides quite accurately with the position of the Na MOT, and the ion cloud is likely to be much larger than the MOT, so we believe that the overlap between the ion cloud and the MOT is satisfactory. By taking the difference between the fitted reciprocal lifetime of the lower curve in Fig. 2 (squares—MOT on) and the upper curve (circles—MOT off), we estimate the net charge-exchange rate for our particular MOT and trapped Ca+ ion density to be \(\Upgamma \approx 0.5\,\hbox{Hz}\).
In Fig. 3, the number of ions extracted as a fraction of the number of ions that remain after 8 s of trapping with no MOT exposure is plotted as a function of the percent of the trapping time that the MOT is present. The ions were held for 8 s, then extracted, so a MOT exposure of 25 % means that the MOT was on for the first 2 s of the trapping time and was then turned off for the final 6 s of the trapping time.
Measured ratio of the number of ions remaining after a fixed trapping time to the number of ions remaining after the same time when not exposed to the MOT vs. % of the fixed trapping time exposed to the Na MOT. Squares (orange): Trapped Na+ ions, error bars are smaller than the size of the points. Circles (Blue): Trapped Ca+, with any product Na+ ions selectively quenched, suggesting a charge-exchange reaction of Ca+ with cold Na MOT atoms
Since the dependent variable is the number of ions extracted as a fraction of the number of ions remaining after 8 s with no MOT exposure, the Na+ curve is a measure of the increase in the number of surviving ions due to the cooling by the MOT. For instance, exposure to the MOT for the full 8 s trapping time increases the number of sodium ions remaining by a factor of almost three. Contrast that with the case of Ca+, where increased exposure to the MOT causes a smaller fraction of the ions to remain at the extraction time, compared to no MOT interaction. In both cases, the background ions created by the MOT were suppressed using MSRQ.
The increase in the number of Na+ ions in the trap with MOT exposure is due to the fact that both elastic and charge-exchange collisions result in a lower energy ion, which on average will remain trapped longer. Elastic collisions may reduce the energy of the ion by some fraction, while charge-exchange collisions replace a fast moving ion with an ultracold ion.
In the case of the Ca+ ions experiment, elastic collisions cool the ions, but charge-exchange collisions cause an ion to be lost from the trap. The charge-exchange product Na+ ions are lost from the trap because, while we cool Ca+, we quench the Na+ created by the MOT using MSRQ. The charge-exchange rate is high enough that this process significantly reduces the number of trapped Ca+ ions, as Fig. 3 shows.
The charge-exchange pathway \(\hbox{Ca}^{+}+\hbox{Na}(3\hbox{p})\rightarrow\hbox{Ca}(4\hbox{s}^2\,\hbox{or}\,4\hbox{s}4\hbox{p})+\hbox{Na}^{+}\) was unexpected because the lowest potential curves we originally published for the (NaCa)+ system are ∼1 eV apart and do not provide a good low-energy pathway [3]; the dominant radiative association rate to the ground state of (NaCa)+ was calculated to be very low (∼1 reaction per 15 min in the MOT, per Ca+ ion). Unfortunately, it is not straightforward to isolate and measure the trapped Na+ ions produced by the charge exchange alone, since the MOT produces an additional source of Na+ ions, as previously discussed.
The Côté group has done preliminary calculations on excited states in the NaCa+ quasimolecular system with asymptotes involving excited Ca, Na, and Ca+(4p). Figure 4 shows first six \(\Upsigma\) states of the NaCa+ quasimolecule. The ground and low-lying excited states in NaCa+ were calculated by using the equation of motion coupled cluster (EOM-CCSD) method, as implemented in the MOLPRO 2010.1 [34] suite of quantum chemistry programs. In the case of NaCa+, the valence space consists of two electrons, and CCSD theory is equivalent to full configuration interaction (FCI). The core electrons have been efficiently described by an effective core potential (ECP) along with a core polarization potential (CPP). The ECP + CPP approach has been used previously with much success for the description of core-valence correlations, in Ca +2 molecular ions [35, 36]. Basis sets corresponding to the ECP for Na were obtained from [37], while those for Ca were taken from [38].
It appears that the most plausible mechanism for Ca+ ion depletion that we observed in our hybrid trap uses the entrance channel Ca+(4s) + Na(3p) (the state closest to the top of Fig. 4), which has only a small collision barrier (∼0.17 eV or 1371 cm -1) at large internuclear distance R ∼ 16 Bohr. The state with the asymptote Na+ + Ca(1P) (the second state from the top in Fig. 4) can be populated exothermically via an avoided crossing (near 12 Bohr) from the entrance quasimolecular state. Since this entrance channel requires a target of excited Na(3p) atoms, an accurate model of the excited-state fraction within the MOT is required to make a quantitative measurement of the charge-exchange rate. Non-zero excited-state population has been shown to have a significant effect on charge-exchange reactions in some other cases [7].
A charge-exchange rate constant k can be extracted from the measured rate, \(\Upgamma = 0.5\,\hbox{Hz}\), using the equation \(\Upgamma=k\langle n \rangle\), which requires knowledge of the average atomic density experienced by the ions, \(\langle n \rangle\) [11]. A measurement of \(\langle n \rangle\) is typically made by imaging the overlap between the MOT and the ion cloud, which cannot be done directly with our current apparatus because it lacks the necessary lasers for exciting Ca+. Instead, we estimate the overlap.
We sampled the ion cloud’s cumulative distribution function by suddenly reducing the r.f. trap depth just before ion extraction, using the technique presented in a previous paper [18]. This allows us to estimate the mean initial Ca+ ion cloud’s energy/ion to be ∼0.70 eV, corresponding to ∼5,300 K. The initial ion temperature is larger than the neutral background gas temperature because most of the ions are not born along the Paul trap’s nodal line and have a nonzero initial potential energy due to their position in the trapping potential [17]. A significant fraction of the ions has an energy greater than the activation barrier height (∼0.17 eV), so the reaction can proceed. From this energy measurement and the spring constant of the trapping potential, we can estimate the volume of the ion cloud [39].
The average atomic density is then found using \(\langle n\rangle\approx N_A(3\hbox{p})\times\rho_I\), where N A (3p) is the number of excited atoms in the MOT [40] and ρ I is the average density of the ions with an energy greater than the activation barrier. This equation assumes that the ion cloud is larger than the MOT.
From this, we extract a rate constant of ∼2 × 10−11 cm3/s, which is two orders of magnitude smaller than the Langevin rate of \(\pi\sqrt{\frac{C_4}{\mu}}=2 \times 10^{-9}\,\hbox{cm}^{3}/\hbox{s}\) [41]. The discrepancy is comparable to that found by others for non-resonant charge exchange [15]. This discrepancy is not surprising, since based on the potential curves the Langevin assumption of unit efficiency in the reaction region is certainly too high. In the future, we plan to measure the rate constant’s dependence on collision energy, as well as on ionic and/or atomic excited-state populations.
3 Conclusion
The hybrid ion-neutral trap system is ideal for measuring sympathetic cooling rates and properties of cold charge-exchange reactions. The example of Na + Ca+ charge exchange is discussed in some detail, including measurements we report here that suggest this exothermic reaction is strongly enhanced when there is a large fraction of the Na target atoms in the 3p excited state, as in a typical MOT.
To test the effect of the barrier in the entrance channel more quantitatively, in a future experiment trapped Ca+ ions could be initially laser cooled to varying degrees using a 397-nm diode laser on the 4s-4p resonance line of Ca+, along with the 866-nm repump laser on the 3d-4p resonance line of Ca+, as seen in Fig. 1. Since our Ca+ ions are initially hot, we can change the collision energy of the Ca+ + Na interaction by laser cooling the Ca+ to explore the size of the small barrier in the Ca+(4s) + Na(3p) entrance channel. We might also be able to suppress the charge-exchange reaction altogether when the Ca+ ions have been laser cooled below the barrier. Furthermore, by turning off the 866 nm repumper, which would optically pump the Ca+ into the 3d state, we could isolate and study any possible contribution from the metastable Na + Ca+(2D) entrance channel.
References
C. Tapalian, W.W. Smith, Resonant collisional dissociation of Na +2 by Na(3p) in an effusive beam. Phys. Rev. A 49(2), 921–926 (1994)
W.W. Smith, O.P. Makarov, J. Lin, Cold ion-neutral collisions in a hybrid trap. J. Mod. Opt. 52(16), 2253–2260 (2005)
O.P. Makarov, R. Côté, H. Michels, W.W. Smith, Radiative charge-transfer lifetime of the excited state of NaCa+. Phys. Rev. A 67(4), 042705 (2003)
R. Côté, A. Dalgarno, Ultracold atom-ion collisions. Phys. Rev. A 62(1), 012709 (2000)
W.W. Smith, E. Babenko, R. Côté, H.H. Michels, On the collisional cooling of co-trapped atomic and molecular ions by ultracold atoms: Ca+ + Na and Na +2 (v*,J*) + Na. In: N.P. Bigelow, J.H. Eberly, C.R. Stroud, I.A. Walmsley (eds) Coherence and quantum optics VIII (No.8)., (Kluwer Academic/Plenum, UK, 2003) pp. 623–624.
W.G. Rellergert, S.T. Sullivan, S.J. Schowalter, S. Kotochigova, K. Chen, E.R. Hudson, Evidence for sympathetic vibrational cooling of translationally cold molecules. Nature 495, 490 (2013)
S.T. Sullivan, W.G. Rellergert, S. Kotochigova, E.R. Hudson, Role of electronic excitations in ground-state-forbidden inelastic collisions between ultracold atoms and ions. Phys. Rev. Lett. 109(22), 223002 (2012)
W.G. Rellergert, S.T. Sullivan, S. Kotochigova, A. Petrov, K. Chen, S.J. Schowalter, E.R. Hudson, Measurement of a large chemical reaction rate between ultracold closed-shell 40Ca atoms and open-shell 174Yb+ ions held in a hybrid atom-ion trap. Phys. Rev. Lett. 107(24), 243201 (2011)
S.T. Sullivan, W.G. Rellergert, S. Kotochigova, K. Chen, S.J. Schowalter, E.R. Hudson, Trapping molecular ions formed via photo-associative ionization of ultracold atoms. Phys. Chem. Chem. Phys. 13(42), 18859 (2011)
E.R. Hudson, Method for producing ultracold molecular ions. Phys. Rev. A 79(3), 032716 (2009)
A.T. Grier, M. Cetina, F. Oručević, V. Vuletić, Observation of cold collisions between trapped ions and trapped atoms. Phys. Rev. Lett. 102(22), 223201 (2009)
C. Zipkes, S. Palzer, L. Ratschbacher, C. Sias, M. Köhl, Cold heteronuclear atom-ion collisions. Phys. Rev. Lett. 105(13), 133201 (2010)
C. Zipkes, S. Palzer, C. Sias, M. Köhl, A trapped single ion inside a Bose-Einstein condensate. Nature 464, 388–391 (2010)
S. Schmid, A. Härter, J.H. Denschlag, Dynamics of a cold trapped ion in a Bose-Einstein condensate. Phys. Rev. Lett. 105(13), 133202 (2010)
F.H.J. Hall, M. Aymar, N. Bouloufa-Maafa, O. Dulieu, S. Willitsch, Light-assisted ion-neutral reactive processes in the cold regime: radiative molecule formation versus charge exchange. Phys. Rev. Lett. 107(24), 243202 (2011)
K. Ravi, S. Lee, A. Sharma, G. Werth, S.A. Rangwala, Cooling and stabilization by collisions in a mixed ion-atom system. Nat. Commun. 3, 1126 (2012)
D.S. Goodman, I. Sivarajah, J.E. Wells, F.A. Narducci, W.W. Smith, Ion-neutral-atom sympathetic cooling in a hybrid linear rf Paul and magneto-optical trap. Phys. Rev. A 86(3), 033408 (2012)
I. Sivarajah, D.S. Goodman, J.E. Wells, F.A. Narducci, W.W. Smith, Evidence of sympathetic cooling of Na+ ions by a Na magneto-optical trap in a hybrid trap. Phys. Rev. A 86(6), 063419 (2012)
F.H.J. Hall, E. Pascal, G. Hegi, M. Roault, M. Aymar, O. Dulieu, S. Willitsch, Ion-neutral chemistry at ultralow energies: dynamics of reactive collisions between laser cooled Ca+ ions and Rb atoms in an ion-atom hybrid trap. arXiv:1302.4682 [physics.atom-ph] (2013)
S. Haze, S. Hata, M. Fujinaga, T. Mukaiyama, Observation of elastic collisions between lithium atoms and calcium ions. arXiv, p. 1305.3346v1 (2013)
L. Ratschbacher, C. Zipkes, C. Sias, M. Kohl, Controlling chemical reactions of a single particle. Nat. Phys. Lett. 8(9), 649–652 (2012)
J.I. Cirac, P. Zoller, Quantum computations with cold trapped ions. Phys. Rev. Lett. 74(20), 4091–4094 (1995)
A. Dalgarno, M.R.H. Rudge, Cooling of interstellar gas. Astrophys. J. 140, 800 (1964)
N. Balakrishnan, A. Dalgarno, Chemistry at ultracold temperatures. Chem. Phys. Lett. 341(56), 652–656 (2001)
P.C. Stancil, S. Lepp, A. Dalgarno, The lithium chemistry of the early universe. Astrophys. J. 458, (1996)
M. Tacconi, F.A. Gianturco, A.K. Belyaev, Computing charge-exchange cross sections for Ca+ collisions with Rb at low and ultralow energies. Phys. Chem. Chem. Phys. 13(42), 19156–19164 (2011)
E.L. Raab, M. Prentiss, A. Cable, S. Chu, D.E. Pritchard, Trapping of neutral sodium atoms with radiation pressure. Phys. Rev. Lett. 59(23), 2631–2634 (1987)
J.D. Prestage, G.J. Dick, L. Maleki, New ion trap for frequency standard applications. J. Appl. Phys. 66(3), 1013–1017 (1989)
W. Neuhauser, M. Hohenstatt, P.E. Toschek, H. Dehmelt, Localized visible Ba+ mono-ion oscillator. Phys. Rev. A 22(3), 1137–1140 (1980)
F.G. Major, H.G. Dehmelt, Exchange-collision technique for the rf spectroscopy of stored ions. Phys. Rev. 170(1), 91–107 (1968)
S. Schwarz, Simulations for ion traps buffer gas cooling. in Trapped Charged Particles and Fundamental Interactions, volume 749 of Lecture Notes in Physics. (Springer, Berlin / Heidelberg, 2008), pp. 1–21
A. Drakoudis, M. Sllner, G. Werth, Instabilities of ion motion in a linear Paul trap. Int. J. Mass Spectrom. 252(1), 61–68 (2006)
P.S. Julienne, R. Heather, Laser modification of ultracold atomic collisions: theory. Phys. Rev. Lett. 67(16), 2135–2138 (1991)
H.-J. Werner et al. version 2010.1, http://www.molpro.net
S. Banerjee et al., Chem. Phys. Lett., 542, 138 (2012)
S. Banerjee, Electronic structure calculations and properties of alkaline-earth molecular ions, April 2013. Ph.D. thesis, see http://digitalcommons.uconn.edu/dissertations/26/
M. Aymar, O. Dulieu, Journal of Chemical Physics, 122, 204302 (2005)
M. Krosnicki, E. Czuchaj, H. Stoll, Theo. Chem. Accts., 110, 28 (2003)
S. Lee, K. Ravi, S.A. Rangwala, Measurement of collisions between rubidium atoms and optically dark rubidium ions in trapped mixtures. Phys. Rev. A 87, 052701 (2013)
M.H. Shah, H.A. Camp, M.L. Trachy, G. Veshapidze, M.A. Gearba, B.D. DePaola, Model-independent measurement of the excited fraction in a magneto-optical trap. Phys. Rev. A 75(5), 053418 (2007)
R.W. Schmieder, A. Lurio, W. Happer, Quadratic stark effect in the \(^{2}p_{\frac{3}{2}}\) states of the alkali atoms. Phys. Rev. A 3, 1209–1217 (1971)
Acknowledgments
W.S. acknowledges support for the experiments from the National Science Foundation (NSF) under Grant No. PHY0855570. The work of S.B. was supported in part by the U.S. Department of Energy, Office of Basic Energy Sciences, and the work of R.C. by NSF Grant No. PHY-1101254.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smith, W.W., Goodman, D.S., Sivarajah, I. et al. Experiments with an ion-neutral hybrid trap: cold charge-exchange collisions. Appl. Phys. B 114, 75–80 (2014). https://doi.org/10.1007/s00340-013-5672-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-013-5672-2