Abstract
The stand-off detection and analysis of environmental land samples have been demonstrated using laser-induced breakdown spectrometry. The samples of interest have included soils and vegetation powder. Elements Hg, As, Pb, Zn, Cd and Cr have been spectrally analysed with a focus on Hg as a trace contaminant in the samples. It is found that element Fe, usually contained in land samples, is a main source of spectral interference for Hg detection due to its ever present iron emission line at 253.68 nm that is closely adjacent to the strongest Hg emission line at 253.65 nm, and hence, a high resolution of spectral detection is necessary. The strong spectral signals from Bremsstrahlung emission in laser-induced plasma and atomic emission of Fe of high concentration caused a significant reduction in detection resolution in the use of image intensifier of an ICCD. The limit of detection at ~8 ppm for Hg detection in soil samples with iron as a minor constituent has been achieved, using an optical chopper and a CCD detector for laser-induced breakdown spectroscopy (LIBS) signal detection. Such detection method in LIBS system has shown a great advantage in determining trace elements from interfering elemental constituents in land sample matrixes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Laser-induced breakdown spectroscopy (LIBS) as a viable analytical tool has been applied to elemental detection and analysis in a wide range of analytical fields in the last decade [1–10], and it has attracted increasing attention from both the scientific and commercial communities. The laser used in this technique as an atomisation and ionisation tool offers some major advantages over the conventional atomisation or ionisation devices in atomic spectroscopy. In particular, it allows in situ, real-time and stand-off detection/monitoring without the need for any sample preparation protocol. The major emphasis in the development of LIBS system has so far been focused on its use with solid samples, culminating in commercial systems for remote and in situ elemental analysis for hazardous and inaccessible targets [11–15]. One of the most important applications of LIBS is the determination of trace elements in contaminated land for environmental monitoring and waste management, particularly when some locations are inaccessible or sample targets are in hostile environments.
The pollution of land through soil and plants is a big threat to the environment. Among those pollutants, heavy metals are often present as a consequence of anthropogenic activities (Intensified industrialisation and human activities) and are also the most concerned polluting elements due to their toxicity and their susceptibility to bioaccumulation. There have been numerous investigations and application demonstrations reported by world-wide researchers and technologists using LIBS technique for soil and plant analysis [16–22]. In the present state of this development, LIBS cannot compete with the established analysis methods, such as inductively coupled plasma-atomic emission spectroscopy (ICP-AES), gas chromatography mass spectroscopy (GCMS) etc., in terms of limits of detection and reproducibility. However, these methods need the use of trained personnel and long time for sample preparation and presentation for extracting elements from solid samples and making the sample solution prior to measurements. Therefore, LIBS has its own great role to play in rapid elemental analysis of solid samples.
The experimental study for LIBS in soils and plants has been carried out in our laboratory and showed that for the monitoring of some priority elements such as Hg, As, Pb etc. in soils, the detection resolution and sensitivity need to be further improved. Spectral interferences from the elements contained in the sample matrixes have also caused main difficulties for identifying trace contaminants from the minor or major elemental constituents. Iron, an element that is naturally and mostly present as a minor constituent in land environmental samples, produces a great spectral interference in trace mercury detection due to Fe emission line at 253.68 nm being close to that of Hg at 253.65 nm. This paper presents the experimental results and analysis on the trace detection of heavy metals, e.g. mercury, etc. in soil and plant samples. The detection limit for mercury has been clearly improved when a high-resolution Echelle grating spectrograph coupled with CCD and an optical chopper as a gating device was used, indicative of the prospect of its commercial viability. These results were compared with those using a LIBS system with ICCD which may have a significant effect on spectral detection resolution, restricting the utilisation of the high resolution of a spectrograph.
2 Materials and methods
2.1 Samples
A selection of environmental samples of soil and plant were used for this experimental study. The soil materials were clay soil (LGCQC3004, LGC Promochem, UK), and the plant material was dry powder of tomato leaves (locally grown) in which As and Hg were added at a trace concentration (50 ppm) for detection purpose. An archived soil material (Cranfield Natural Resources, UK) was also doped with trace mercury at a set of various concentrations and used for generating Hg concentration curve. The contents of the elements of interest for detection in the clay soil and plant samples are listed in Table 1. All samples were pressed into ~10 mm diameter disc of 2 mm thickness under a pressing pressure of ~15 tons on each dry sample. Sample discs were placed on the sample translational device allowing data collection from a fresh surface for each laser pulse, thereby reducing data averaging error.
2.2 Experimental apparatus
A laboratory-based experimental apparatus was set up to carry out initial laser-induced breakdown spectrometry. As shown schematically in Fig. 1, it consisted of a laser source, a sample translation stage, a double-grating monochromator (SPEX 1404) and a detection system which is an intensified diode array detector connected to an optical multichannel analyser (EG&G 1420/OMA III). The spectral dispersion is achieved by the monochromator with a maximum resolution of 10 cm−1, as quoted by the manufacturer, which allows a spectral resolution of <0.063 nm at 250 nm. The excitation source was an Nd-YAG laser (Litron 200-UK), operating at 1,064 nm wavelength (λ) and delivering a maximum energy of 200 mJ over pulse duration of ~6 ns (FWHM) at a maximum pulse repetition rate of 20 Hz. The beam diameter (D) at the exit was 5 mm, and the beam divergence was <2 mrad. A 100 mm focal length (f) lens was used to focus the beam, being delivered through a mirror and a prism, on to a sample pellet whose surface was placed in the focal plane of the lens. The radium (r) of the laser focus hitting the sample surface was approximately estimated as 26 μm using diffraction-limited imaging, i.e. r = 1.22 λf/D. The laser pulse energy and pulse repetition rate were maintained at ~100 mJ and 20 Hz, respectively, for the present experiments. The spectral coverage extended from UV to near IR wavelengths depending upon the grating type being used. The plasma source induced with the exciting laser on the sample surface was imaged on to the entrance slit of the monochromator by both F/2 and F/8 lenses of 50 mm diameters, giving an f# nearly matching that (f/7.8) of the monochromator. The gating on the detector intensifier was controlled by the external trigging pulses in synchronisation with laser output. The system parameters have been optimised for maximum signal detection with reasonable spectral resolutions and noise filtering by gating. A one-dimension translation sample stage was integrated into the system, which allows sampling fresh sample points for spatial averaging of spectral signals.
3 Results and analysis
3.1 LIBS of tomato leaves
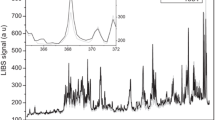
The spectral measurements and analysis were carried out to evaluate their limits of detection based on the intensities of their spectral peaks and background noises. In all spectral recording and measurements, gate delay and width were optimally set as 2 and 5 μs, respectively. Each spectrum was acquired with 200 shots signal averaging at the pulse repetition rate of 20 Hz to achieve good signal-to-noise ratio (S/N). The region of interests for LIBS spectral detection was focused on those of prominent emission lines of elements contained in the samples. Using the experimental apparatus of Fig. 1, LIBS spectra of these elements in the sample of tomato leaves were recorded and examples of these are shown in Fig. 2. The trace elements Zn (62 ppm), Cd (3 ppm), As (50 ppm) Pb (6.3 ppm) and Cr (4.5 ppm) were clearly detected at their strongest emission lines as shown in the spectra. However, the emission line of Hg (50 ppm) at 253.65 nm appeared to be interfered with those of Fe at 253.68 nm and P at 253.56 nm. The lines of Hg and Fe were not resolved although their overlapped signal was detected. These spectral interferences have given rise to an uncertainty for mercury identification and made Hg undetectable in this measurement.
For the spectra in Fig. 2, the signal-to-noise ratios (S/N) were measured at the chosen emission line of each element, and the limits of detection (LOD) were estimated as 3C/S/N, where C is elemental concentration in the sample. This estimation was based on the assumptions of a linear concentration calibration and no spectral interference. Such LOD estimation may not be evidently biased in the cases of Zn, Pb, Cr, Cd and As where the spectral interferences were weak and the signals (S) were measured from the local background at non-interference side. The limits of detection for these elements are listed in Table 2 when the LIBS system in Fig. 1 was used. Such detection sensitivities for most of these elements can reach the concentration levels of sub-ppm or ppm which may meet the requirement of minimum permissible limits (MPL) for disposal. This has demonstrated that the LIBS system is a viable tool for the detection of these elements. However, not all of the elements in the sample of tomato leaves can be detected with the same level of sensitivities. For example, arsenic and mercury was found to be not amenable to detection with the sensitivity meeting the MPL with the existing LIBS system.
3.2 LIBS of clay soil
To observe and analyse the effect of sample matrix containing minor concentrations of constituent, iron, on the detection of mercury and arsenic, LIBS spectra of the certificated clay soil sample (LGCQC3004, LGC) with high concentrations of Hg at 670 ppm, As at 83 ppm and minor iron at 4 % were recorded at the regions of emission lines 234.98 nm for As and 253.65 nm for Hg, as shown in Fig. 3. Element As was evidently detected on the shoulder of spectral signal of iron centred at 234.83 nm although this emission line of iron produced serve interference. However, the Hg line could not be resolved from the Fe interfering lines at 253.68 nm, due to insufficient spectral detection resolution. This could be due to insufficient resolution of the spectrograph and also the overexposure of strong Bremsstrahlung emission of continuous spectrum on the photocathode of the image intensifier for the detector. The light overexposure produces a strong photo-electron noise, and thus, the photocathode could not sufficiently recover before the intensifying gate is opened. Therefore, it is evident that the existence of iron at a relatively high concentration in environmental sample matrix has made it difficult particularly to detect mercury and demands a more dedicated system of high-measurement resolution.
3.3 High-resolution LIBS detection of Hg
For the detection of mercury in a sample matrix containing minor constituent of iron, two factors were considered in the LIBS system for this study: use of a higher resolution spectrograph and blocking of Bremsstrahlung emission. Therefore, two same Echelle grating spectrographs (ARYELLE Butterfly, Lasertechnik Berlin GmbH) were used. One of these was coupled with ICCD as a gateable detector, and the other coupled with CCD as a detector and a mechanical chopper as a physical gating device in front of its entrance slit. The spectrograph is of spectral resolving power of ~30,000 (as quoted by the manufacturer), giving a spectral resolution ∆λ = λ/30,000 ≈ 0.008 nm at the wavelength (λ) of 250 nm. Using these two different detection techniques with same LIBS parameter settings, the LIBS spectra of pressed samples of clay soil and tomato leaf powder were recorded over 100 shots averaging and their regions of interest at 253.65 nm are shown in Figs. 4 and 5, respectively. The gating delay was set as 2 μs for both the ICCD and the optical chopper with respect to the laser pulse.
It is shown that spectral signals of the emission line at 253.56 nm of phosphorus, as a minor constituent in both of the samples, are clearly away from those around 253.65 nm, and thus, the existence of phosphor may not affect the detection of mercury when a high-resolution spectrograph is used. However, using ICCD detector, the spectral lines of Hg at 253.65 nm and Fe at 253.68 nm were not resolved, as shown in Figs. 4a and 5a, in spite of the apparently sufficient high resolution (~0.008 nm) of the used spectrographs. Mercury was not detected in this case due to the interfering line of iron contained in the samples. When CCD detector and a chopper were used, LIBS spectra in both Figs. 4b and 5b evidently show that the Hg line from both of the samples has been identified and resolved from an interfering spectral line of iron at 253.68 nm. For tomato leaf sample, the Fe line was better resolved because of its lower concentration. In the clay soil sample, iron concentration was much higher (4 %) than that (0.07 %) in the sample of tomato leaves, and thus, its emission line at 253.68 nm caused worse interference to its nearby Hg line of 253.65 nm. From the signal-to-noise ratio measurements of these two spectral signals acquired with CCD and the chopper, the limits of detection for mercury were estimated as ~50 ppm in clay soil and ~18 ppm in tomato leaves.
Strong Bremsstrahlung emission of continuous spectrum in laser-induced breakdown spectrometry takes place prior to atomic emission of elements in their samples. In the use of ICCD, such strong light directly strikes onto the photocathode of its image intensifier and generates massive photon electrons locally as background noise. The photocathode may not recover before receiving the following spectral signal of atomic emission in a few microseconds. Thus, the remaining shot noise is also intensified together with desired spectral signal during gated detection. Moreover, in the case of high concentration of iron contained in the samples, the strong spectral signal of Fe emission may cause overexposure of its amplified electronic signal on to the phosphor screen of image intensifier and consequently reduce resolving capability of the detector. In use of a CCD and a chopper for gating, this effect is avoided, and the strong bremsstrahlung emission is physically blocked from entering the detector to greatly reduce or eliminate the shot noise. This allows the CCD still be able to detect the weak spectral signal from trace mercury with good signal-to-noise ratio, without the use of an image intensifier which compromises the utilisation of an ultra-high-resolution spectrograph. Therefore, the detection of trace mercury in soil and plant sample matrixes containing minor iron can be achieved with this detection technique.
3.4 Calibration curve for Hg detection
As a result from the above analyses, an ultra-high-resolution spectrograph connected with a CCD detector and an optical chopper as a gating device is able to undertake LIBS measurement of trace mercury in its contaminated land samples, where one of the minor constituents, iron usually poses strong spectral interference. To further evaluate the application using this system, concentration calibration for Hg contained in soil was carried out. The soil (provided by Cranfield Natural Resources, UK) was used as a sample matrix containing ~0.08 ppm of mercury and ~1 % of iron. Mercury of different concentrations in aqueous solutions was added to the matrix samples, which were then dried in a vacuum oven at a temperature of 50 °C and pressed into pellets. The mercury concentrations of these samples were 0.08, 25, 50, 100, 200 and 400 ppm. Their LIBS spectra were acquired over 1,000 shots averaging at 20 Hz pulse repetition rate of exciting laser. Figure 6 shows the spectra around 253.65 nm Hg emission line for Hg concentrations of 0.08, 50 and 200 ppm. All the spectra are normalised to signal intensity of Fe line at 253.68 nm, and their peak intensities were measured as their peak values subtracting the local background and marked in Fig. 7. A linear calibration curve of lower (<100 ppm) Hg concentrations (the first four data points) was also plotted in this figure, with its extension (dot line) to show a strong deviation of the data points at higher Hg concentrations (>100 ppm). The deviation may indicate that at higher concentrations of Hg, the laser-induced plasma is of higher electron density and the plasma absorption (self-absorption) of emission signal increases proportionately, leading to a non-linear effect. Therefore, a linear calibration curve only applies to the case of lower concentration. Based on the slope (ρ) of this curve, 0.0029, and measured standard deviation (σ) of noise, ~0.0053, the limit of detection (LOD = 3σ/ρ) was estimated to be ~6 ppm. This also shows a great improvement with more shots averaging on spectral acquisition for weak spectral signal of trace elements, such as mercury, using CCD detector.
4 Conclusions
Laser-induced breakdown spectroscopy (LIBS) is a feasible technique for stand-off detection and analysis of trace elements in contaminated land samples, e.g. soils and tomato leaves in this study. The elemental constituents of sample matrixes may pose strong spectral interference within a spectral range of the resolution limit. The results clearly indicated that the LOD for the detection of Hg at its emission line of 253.65 nm was governed by the interference effect of the ever present Fe line at 253.68 nm. The high concentration of Fe usually contained in land samples makes mercury detection a very challenging task. Therefore, an ultra-high resolution in spectral detection of a LIBS system is required to take this challenge. The use of image intensifier in ICCD significantly reduced the overall performance in spectral resolution, due to the exposure to strong Bremsstrahlung emission and Fe emission signals. This problem can most effectively be addressed by uses of a CCD and an optical chopper to physically block the strong light from Bremsstrahlung emission and also to avoid over-multiplication of emission signal from interfering elements. The detection and analysis of trace elements including mercury in an iron-reach land environmental sample can be achieved at ppm concentration levels using this detection technique with an ultra-high-resolution spectrograph. For practical applications, further work is being considered to determine the lower limit of laser pulse energy when using the CCD as compared to the ICCD and also to understand how well the signal is modulated by the optical chopper.
References
F. Anabitarte, A. Cobo, J.M. Lopez-Higuera, ISRN Spectrosc. 285240, 1 (2012)
P.K. Diwakar, K.H. Loper, A. Matiaske, D.W. Hahn, J. Anal. At. Spectrom. 27, 1110 (2012)
L. Huang, M. Yao, Y. Xu, M. Liu, Appl. Phys. B Lasers Opt. 111, 45 (2013)
V.K. Unnikrishnan, R. Nayak, K. Aithal, V.B. Kartha, C. Santhosh, G.P. Gupta, B.M. Suri, Anal. Methods 5, 1294 (2013)
M.A. Khater, Spectrochimica Acta Part B Atom Spectrosc. 81, 1 (2013)
M.Z. Martin, S. Allman, D.J. Brice, R.C. Martin, N.O. Andre, Spectrochimica Acta Part B Atom. Spectrosc. 74–75, 177 (2012)
A.F.M.Y. Haider, R.S. Lubna, K.M. Abedin, Appl. Spectrosc. 66, 420 (2012)
X. Fang, S.R. Ahmad, M. Mayo, S. Iqbal, Lasers Med. Sci. 20, 132 (2005)
A. Giacomo, M. Dell’Aglio, A. Casavola, G. Colonna, O. Pascale, M. Capitelli, Anal. Bioanal. Chem. 385, 303 (2006)
X. Fang, S.R. Ahmad, Environ. Technol. 26, 1271 (2005)
F.J. Fortes, J.J. Laserna, Spectrochimica Acta Part B Atom. Spectrosc. 65B, 975 (2010)
F.C. De Lucia Jr., A.C. Samuels, R.S. Harmon, R.A. Walters, K.L. McNesby, A. LaPointe, R.J. Winkel Jr., A.W. Miziolek, IEEE Sens. J. 5, 681 (2005)
M.A. Gondal, T. Hussain, Z.H. Yamani, M.A. Baig, J. Hazard. Mater. 163, 1265 (2009)
L. Peng, D. Sun, M. Su, J. Han, C. Dong, Opt. Laser Technol. 44, 2469 (2012)
M.A. Gondal, M.A. Dastageer, A.A. Naqi, A.A. Isab, Y.W. Maganda, Appl. Opt. 51, 7395 (2012)
J. El Haddad, M. Villot-Kadri, A. Ismael, G. Gallou, K. Michel, D. Bruyere, V. Laperche, L. Canioni, B. Bousquet, Spectrochimica Acta Part B Atom. Spectrosc. 79–80, 51 (2013)
J. Kwak, K. Kim, M. Park, J. Kim, K. Park, Environ. Technol. 33, 2177 (2012)
D. Santos Jr., L. C. Nunes, G.G.A. de Carvalho, M. da S. Gomes, P.F. de Souza, F. de O. Leme, L.G.C. dos Santos, F.J. Krug, Spectrochimica Acta Part B Atom. Spectrosc., 71–72, 3 (2012)
Q. Peng, M. Liu, M. Yao, T. Chen: Proceedings of SPIE, 8205, 82050G/1 (2011)
O.T. Butler, W. Cairns, J.M. Cook, C.M. Davidson, J. Anal. At. Spectrom. 26, 250 (2011)
A.K. Pathak, R. Kumar, V.K. Singh, R. Agrawal, S. Rai, A.K. Rai, Appl. Spectrosc. Rev. 47, 14 (2012)
G.G.A. de Carvalho, D. Santos Jr., L.C. Nunes, M. da S. Gomes, F. de O. Leme, F.J. Krug, Spectrochimica Acta Part B Atom. Spectrosc., 74–75, 162 (2012)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fang, X., Ahmad, S.R. Elemental analysis in environmental land samples by stand-off laser-induced breakdown spectroscopy. Appl. Phys. B 115, 497–503 (2014). https://doi.org/10.1007/s00340-013-5630-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-013-5630-z