Abstract
The multi-photon ultraviolet upconversion emission properties and synergistic effect are investigated in BaSr2Y6O12:Er3+ phosphor. The deep-ultraviolet emissions centered at 274, 297 and 324-nm are observed under the 1,540-nm excitation, which results from a seven-, six- and six-photon upconversion process, respectively. A synergistic effect is found, which shows that the red emission intensity under 351- and 1,540-nm dual excitation is 4.7 % time stronger than the sum of red emission intensities under the 351 and 1,540-nm single excitation. This phenomenon is attributed to the 4I13/2 and 4I11/2 levels of Er3+ from non-radiative transition process under the 351-nm excitation are excited again to 4F9/2 level by absorbing 1,540-nm photon in the 351- and 1,540-nm dual-excitation process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent years, the upconversion luminescent properties research has been dominated by the conversion of infrared to visible region [1–3]. While the ultraviolet upconversion emission under 1,540-nm excitation is still greatly restricted, populating the high-energy states needs a multi-photon upconversion process. In the previous reports, the deep-ultraviolet upconversion emissions of Gd3+ and Nd3+ were detected under the excitation of near-infrared or visible laser [4–7], and the ultraviolet upconversion emission in the region of 270–330 nm from Er3+ ions which was sensitized by Yb3+/Gd3+ in fluoride material hosts under 1,560-nm excitation has also been reported. These high-lying excited levels were populated through excited state absorption (ESA) and complex energy transition (ET) processes [8, 9].
Among the rare-earth ions, trivalent erbium ions (Er3+) provide several long-lived intermediate levels as well as metastable high energy levels, and the energy gap between the ground level 4I15/2 and the first excited level 4I13/2 is 6,400 cm−1, which matches well with the absorption of 1,540-nm photon [8, 9]. Also, the wavelengths around 1,540 nm have a relative strong peak in the air-mass 1.5 global (AM1.5G) spectrum [10]; therefore, investigating the upconversion emission properties under the excitation of 1,540 nm may have a potential application value to reduce the solar cells’ sub-bandgap losses and optical data storage, color display, infrared sensor and so on [11–17].
In this paper, the single-phase BaSr2Y6O12:Er3+ powders are prepared by solid-state reaction method for the first time, and the deep-ultraviolet upconversion emissions are detected under 1,540-nm excitation. Particularly, red emission combines downshifting with upconversion under 351- and 1,540-nm dual-wavelength excitation is also investigated. The properties of material combined downshifting with upconversion effect have been studied in the previous reports [18–20]. We discovered the synergistic effect between ultraviolet downconversion and infrared upconversion by employing the dual-wavelength (369 and 980 nm) excitation source. Further, in our work, the synergistic effect is also explored in BaSr2Y6O12:Er3+ powders using the 1,540-nm infrared and 351-nm ultraviolet dual wavelength as excitation source.
2 Experimental
Powder samples of BaSr2Y6O12:Er3+ are synthesized via conventional high-temperature solid-state reaction in air. Analytical reagents BaO and SrO, high-purity Y2O3 (99.99 %) and Er2O3 (99.99 %) are used as raw materials. Each sample is weighed according to the nominal compositions BaSr2Y6(1−x)O12:6xEr3+(x = 0.02, 0.04, 0.06, 0.08, 0.10, 0.15, 0.20) and then mixed thoroughly. The samples are filled in alumina crucibles and kept at 1,350 °C for 7 h in air atmosphere before decreasing temperature to ambient temperature.
The crystal structures of obtained samples are investigated by X-ray diffraction (XRD, Cu tube with Kα radiation at 1.54056 Å for 2θ = 10−80°). The Raman spectrum is measured by a laser micro-Raman spectrometer system. The excitation spectra and emission spectra of samples are measured by an Omni-λ3007 spectrophotometer with a photomultiplier tube with a CW Xe lamp (150 W) and a 1,540-nm variable power laser (0–150 mW) as the excitation light source. Among them, the 351-nm light source origin from a Xe lamp equipped with an optical grating monochromator. All the measurements are taken at room temperature.
3 Results and discussion
3.1 Sample synthesis
In our experiment, the BaSr2Y6O12 powders with different Er3+ concentrations are obtained by simple solid-state method. Figure 1a shows the XRD patterns of BaSr2Y6(1−x)O12:6xEr3+ as a function of Er3+ concentration. The XRD patterns of BaSr2Y6(1−x)O12:6xEr3+ match well with the standard data in JCPDS Card file of NO. 72-0023, and the doping of Er3+ ions does not cause any significant change in the BaSr2Y6O12 host structure. The Raman spectrum is excited by a 488-nm argon laser of BaSr2Y6O12, which is shown in Fig. 1b. It is found that the spectrum shows several strong peaks corresponding to phonon modes with energy 217, 324, 379, 410 and 485 cm−1. It is known that the host material with low phonon energies would have a high upconversion efficiency. The dependence of unit cell parameters a(Å), c(Å) and v(Å3) on Er3+ concentration in BaSr2Y6(1−x)O12:6xEr3+ is shown in Fig. 2. The unit cell parameters a, c and v decrease linearly with the increasing Er3+ concentration, which accords with the Vegard’s rule [21]. The linear decreasing trend of unit cell parameters is due to the substitution of Y3+ ions by small Er3+ ions in the host lattice. All these results indicate that the hexagonal phase BaSr2Y6O12 is fully developed and a small amount of Er3+ ions successfully doped into the host.
3.2 Photoluminescent properties
Figure 3 shows the upconversion emission spectra of BaSr2Y6(1−x)O12:6xEr3+ (x = 0.02) under 1,540-nm excitation. The ultraviolet and visible upconversion emissions are obtained. In particular, the ultraviolet emissions in the range of 260–350 nm are detected in these samples, as depicted in Fig. 3a. These emission peaks centered at 274, 297 and 324 nm come from the transitions of 4G9/2, 2K13/2 and 2P3/2 → 4I15/2 of Er3+ ions, respectively. All these ultraviolet emission peaks of Er3+ ions are rarely detected under the 1,540-nm excitation in the previous reports. In addition, it should also be noted that peaks centered at 382 and 408 nm are assigned to the 4G11/2, 2H9/2 → 4I15/2 transition, as shown in Fig. 3b. Figure 3c depicts the upconversion emission spectrum in the range of 500–900 nm; these peaks are assigned to the following transitions: Near-infrared emission centered at 808 nm, red emission centered at 659 nm and green emission centered at 550 and 560 nm are assigned to the 4I9/2 → 4I15/2, 4F9/2 → 4I15/2 and 2H11/2, 4S3/2 → 4I15/2 transitions of the Er3+ ions, respectively. Figure 3d gives the upconversion emission in the infrared region of 900–1,100 nm, the emission peak centered at 980 nm corresponding to the 4I11/2 → 4I15/2 of Er3+. It should be pointed out that ultraviolet and blue emissions are measured with a higher voltage value of photomultiplier tube due to the weak emission (the same blow).
To investigate the contribution of Er3+ ions toward the photoluminescent (PL) properties of BaSr2Y6O12:Er3+ powders, the emission intensities under 1,540-nm excitation are recorded for samples with different Er3+ ion concentrations, as shown in Fig. 4. The emission intensity is highly dependent on the Er3+ concentration. With the increasing Er3+ ion concentration, the intensity of all the emission regions increases firstly and reaches a maximum and then decreases remarkably when Er3+ concentration is further increased. This phenomenon is due to the Er3+ ions at high concentration that leads to the decrease in energy transfer effect, which is known as concentration quenching effect [22]. It is noted that the maximum emission intensity from all the regions of spectrum is observed for a critical concentration of 8 mol % Er3+.
To better understand the physical mechanism responsible for the PL properties, the emission intensities are measured as a function of pumping power under 1,540-nm excitation. The upconverted emission intensity (Iem) depends on the pumping laser power density (P) according to the following equation [23]:
where I em is the fluorescence intensity, P is the pumping power density of the 1,540-nm laser, the power density is calculated from the ratio between the laser power and the area of light spot, and n is the number of pumping photons absorbed per upconverted photon emitted. Figure 5 shows the logarithmic plot of the integrated emission intensities of the upconverted emission bands in the region of ultraviolet light, violet, green and red as a function of the pumping laser power density. The output slopes (n) for ultraviolet and violet upconversion emissions centered at 274, 297, 324, 382 and 408 nm are 6.87, 5.84, 5.87, 4.96 and 3.94, respectively, indicating that seven-, six-, six-, five- and four-photon processes are responsible for the populating of 2P3/2, 4G11/2, 2K13/2, 4G9/2 and 2H9/2, states of Er3+ ions. When the power density increases to about 90 mW/mm2, the output slope (n) of log-log graph is out of shape, and the corresponding slopes of linear fittings are reduced to 3.01, 2.28, 2.94, 2.53 and 2.26 for 274, 297, 324, 382 and 408 nm, respectively. The numbers of photons decrease rapidly when the excitation power exceeds digital, owing to the competition between the linear decay and the upconversion processes for the depletion of the intermediate excited states [24]. In addition, the n values for red and green emission bands are all around 3, indicating that populating the 2H11/2, 4S3/2 and 4F9/2 levels needs three 1,540-nm photons and is a three-photon process. For the emission peaked at 808 and 980 nm, the n value is 2.22 and 2.11, respectively, indicating two-photon process.
The photoluminescence excitation spectrum (PLE) of Er3+: 4F9/2 → 4I15/2 emission (659 nm) is shown in Fig. 6a, in which an excitation peak in UV region centered at 351 nm is observed, which corresponds to the 4I15/2 → 2G7/2 transition of Er3+ ion. Figure 6b shows the downshifting emission spectrum of sample under 351-nm excitation, the red region emission centered at 659 nm is assigned to the transition of 4F9/2 → 4I15/2 of Er3+ ion, and the infrared characteristic emissions of Er3+ from 4I11/2 (980 nm) and 4I13/2 (1,540 nm) levels are presented here clearly. The insert of Fig. 6 shows the dependence of red emission intensities on Er3+ doping concentration under the excitation of 351 nm. It is noticed that the emission intensity of red emission increases rapidly with the increasing Er3+ doping concentration; however, a decreasing trend is observed clearly when concentration is set to more than 8 mol % due to concentration quenching.
a The PLE spectra of Er3+: 4F9/2 → 4I15/2 emission (659 nm) in BaSr2Y6O12:2 %Er3+. b The visible and infrared photoluminescent spectrum of sample under 351-nm excitation. The inset shows the dependence of red emission intensity on Er3+ ions doping concentration under 351-nm excitation in BaSr2Y6(1−x)O12:6xEr3+ (x = 0.02, 0.04, 0.06, 0.08, 0.10, 0.15, 0.20)
As described above, the emission band of Er3+ ions centered at 659 nm is observed under 1,540- and 351-nm single excitation, respectively. However, under the 1,540- and 351-nm dual excitation, a synergistic effect is witnessed in these samples, which shows that the red emission intensity under the 1,540-nm and 351-nm dual excitation is stronger than the sum of red emission intensity under the 351- and 1,540-nm single excitation. To quantitatively describe the synergistic effect, the absolute enhancement rate (ψ) of red emission can be defined as follows [20]:
where I 1, I 2 and I 3 are the integrated intensity of red emission bands under 1,540-nm excitation, under 351-nm excitation and under 1,540- and 351-nm dual excitation, respectively. The red emission bands, the dependence of red emission absolute enhancement rate on 1,540-nm power density and Er3+ doping concentration are shown in Fig. 7a, b and inset, respectively. When the maximum value (ψ = 4.7 %) is witnessed, the excitation power density of 1,540-nm semiconductor laser is adjusted to 115 mW/mm2 and the excitation power density of 351 nm is fixed at 0.8 mW/mm2 in 8 mol %Er3+-doped BaSr2Y6O12 sample.
a The red emission spectra of BaSr2Y6O12:Er3+ under 351-nm excitation, under 1,540-nm excitation and under 1,540- and 351-nm dual excitation. b The dependence of absolute enhancement rate (ψ) of red emission on Er3+ concentration in BaSr2Y6(1−x)O12:6xEr3+ (x = 0.02, 0.04, 0.06, 0.08, 0.10, 0.15, 0.20), on 1,540-nm laser power density when 351-nm power density fixed at 0.8 mW/mm2 (the inset)
3.3 Upconversion process and synergistic effect mechanism
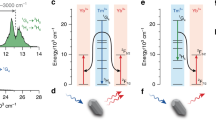
It is no doubt that ET, excited/ground-state absorption (ESA/GSA) and non-radiative relaxation (NR) processes can be accounted for the upconversion emission mechanisms in BaSr2Y6O12:Er3+ under the excitation of 1,540-nm semiconductor laser [25, 26]. The possible upconversion procedure is schematically illustrated in Fig. 8. As to the ultraviolet emission, for populating 2K13/2 and 4G9/2 levels as examples, a possible upconversion process is provided as follows:
Populating 2K13/2 and 4G9/2 are assigned to six- and seven-photon upconversion processes, respectively, as the above process, which accords with the calculated n as shown in Fig. 5a.
The mechanism resulting in upconversion emission occurs via two distinct processes acting concomitantly: ESA and ET processes. It is well known that both processes are dependent upon doping concentration. While the ET rate is stronger depending on the distance between the ions involved than the ESA process, and once over a critical distance, the efficiency of energy transfer mechanism will be decreased rapidly [27]. The different slopes of intensity in different regions of emission are observed with the increasing concentration as shown in Fig. 4; the slope of red emission is greater than the others; we deduce that ET process is the dominant mechanism for populating the low energy levels, while the ESA process becomes the dominant mechanism, which is responsible for populating the high energy level of Er3+.
In addition, the possible mechanism of synergistic effect is also proposed, as shown in Fig. 8. Under the 1,540-nm excitation, the Er3+ ions are excited to the 4I9/2 level through successively absorbing two 1,540-nm photons, some of Er3+ ions at 4I9/2 level into the 4I11/2 level through a non-radiative relaxation process. Then, the cross-relaxation process from Er3+(4I13/2) to Er3+(4I11/2) and ESA process occur simultaneously: Er3+(4I13/2 → 4I15/2), Er3+(4I11/2 → 4F9/2), which leads to the red upconversion emission (4F9/2 → 4I15/2). Under the 351-nm excitation, 2G7/2 level of Er3+ ion is populated through GSA process, and then the red luminescent centered at 659 nm due to a NR process from 2G7/2 to 4F9/2 [28, 29]. Besides, the 4I11/2 and 4I13/2 levels are also populated through non-radiative relaxation process in downshifting process. In general, some of the energy in the 4I11/2 and 4I13/2 levels of Er3+ ions is dissipated via thermal energy; however, under the 351- and 1,540-nm dual excitation, the Er3+ ions in the 4I11/2 and 4I13/2 levels from NR process can be excited again (EA) to the 4F9/2 level by absorbing 1,540-nm photons as follows:
Therefore, the energy of non-radiative relaxation is excited again, resulting in the synergistic enhancement effect of red emission (4F9/2 → 4I15/2) under the dual excitation.
4 Conclusion
In summary, the preparation and photoluminescence properties of single-phase BaSr2Y6O12:Er3+ are investigated systemically for the first time. Power-dependent studies reveal that ultraviolet emissions of Er3+ under 1,540-nm excitation result from a seven-, six- and five-photon process, and the ultraviolet upconversion mechanism is discussed based on complex ESA and GSA/ESA processes. Additionally, a synergistic effect combined ultraviolet downshifting with infrared upconversion, which shows that the red emission intensity under the 1,540- and 351-nm dual excitation is stronger than the sum of red emission intensities under the 351- and 1,540-nm single excitation, and the maximum absolute enhancement rate is 4.7 %. It is believed that the 4I13/2 and 4I11/2 levels of Er3+ from non-radiative relaxation process under 351-nm excitation are excited again to 4F9/2 level by absorbing 1,540-nm infrared photon in the 351- and 1,540-nm dual-excitation process, which is responsible for the synergistic effect of red emission.
References
X.S. Wang, J. Song, H.Y. Sun, Z.Z. Xu, J.R. Qiu, Opt. Express 15, 1384 (2007)
X.F. Wang, X.H. Yan, C.X. Kan, J. Lumin. 131, 2325 (2011)
Q.J. Chen, W.J. Zhang, X.Y. Huang, G.P. Dong, M.Y. Peng, J. Alloy Compd. 513, 1814 (2012)
Y.Y. Zhang, L.W. Yang, C.F. Xu, J.X. Zhong, C.Q. Sun, Appl. Phys. B 98, 243 (2010)
D.Q. Chen, Y.S. Wang, Y.L. Yu, J. Rare Earth. 26, 428 (2008)
C.Y. Cao, W.P. Qin, J.S. Zhang, Opt. Lett. 33, 857 (2008)
F. Qin, Y.D. Zheng, Y. Yu, C.B. Zheng, P.S. Tayebi, Opt. Commun. 284, 3114 (2011)
K.Z. Zheng, D. Zhao, D.S. Zhang, N. Liu, W.P. Qin, Opt. Lett. 35, 2442 (2010)
K.Z. Zheng, Z.Y. Liu, D. Zhao, D.S. Zhang, G.S. Qin, W.P. Qin, Opt. Mater. 33, 783 (2011)
S.K.W. MacDougall, I. Aruna, J. Marques-Hueso, K.W. Krämer, B.S. Richards, Opt. Express 15, A879 (2012)
M. Yan, V.D. Ende, L. Aarts, A. Meijerink, Phys. Chem. 11, 11081 (2009)
V.K. Tikhomirov, V.D. Rodriguez, J. Méndez-Ramos, J. Castillo, D. Krilenko, G. Van Tendeloo, V.V. Moshchalkov, Sol. Energ. Mat. Sol. C 100, 209 (2012)
A. Shalav, B.S. Richards, M.A. Green, Sol. Energ. Mat. Sol. C 91, 829 (2007)
T. Trupke, A. Shalav, B.S. Richards, P. Würfel, M.A. Green, Sol. Energ. Mat. Sol. C. 90, 3327 (2006)
K. Deng, T. Gong, L.X. Hu, X.T. Wei, Y.H. Chen, Opt. Express 19, 1749 (2011)
D. Mihailovic, D. Dvorsek, V.V. Kabanov, J. Demsar, L. Forro, H. Berger, Appl. Phys. Lett. 80, 871 (2002)
E. Downing, L. Hesselink, J. Ralston, R. Macfarlane, Science 273, 1185 (1996)
H. Lin, J. Marqués-Hueso, D.Q. Chen, Y.S. Wang, B.S. Richards, Mater. Res. Bull. 47, 4433 (2012)
Q.J. Chen, W.J. Zhang, X.Y. Huang, G.P. Dong, M.Y. Peng, Q.Y. Zhang, J. Alloy. Compd. 513, 139 (2012)
X.F. Wang, X.H. Yan, Opt. Lett. 36, 4353 (2011)
L. Vegard, Z. Angew. Phys. 5, 17 (1921)
G. Blasse, Phys. Lett. A. 28, 444 (1968)
M. Pollnau, D.R. Gamelin, S.R. Lüthi, H.U. Güdel, Phys. Rev. B. 61, 3337 (2000)
Y.Q. Lei, H.W. Song, L.M. Yang, L.X. Yu, Z.X. Liu, G.H. Pan, X. Bai, L.B. Fan, J. Chem. Phys. 123, 174710 (2005)
P. Ghosh, S. Sadhu, T. Sen, A. Patra, Bull. Mater. Sci. 31, 461 (2008)
H. Guo, M. Yin, W.P. Zhang, J. Rare Earth. 24, 740 (2006)
J.A. Capobianco, F. Vetrone, J.C. Boyer, J. Phys. Chem. B 106, 1181 (2002)
L. Aarts, B.M. van der Ende, A. Meijerink, J. Appl. Phys. 106, 23522 (2009)
R. Mupparapu, K. Vynck, I. Malfanti, S. Vignolini, M. Burresi, P. Scudo, R. Fusco, D.S. Wiersma, Opt. Lett. 37, 368 (2012)
Acknowledgments
This work was supported financially by National Natural Science Foundation of China (No. NSFC51032002), the key Project of the National High Technology Research and Development Program (“863” Program) of China (No. 2011AA050526) and the Science and Technology Support Plan of Jiangsu Province (BE2011191), Natural Science Youth Foundation of Jiangsu Province (BK20130865).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zheng, J., Wang, X.F., He, W.Y. et al. Seven-photon ultraviolet upconversion emission of Er3+ induced by 1,540-nm laser excitation. Appl. Phys. B 115, 443–449 (2014). https://doi.org/10.1007/s00340-013-5623-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-013-5623-y