Abstract
In this work, we developed a SERS platform for quantitative detection of carcinoembryonic antigen (CEA) in serum of patients with colorectal cancers. Anti-CEA-functionalized 4-mercaptobenzoic acid–labeled Au/Ag core-shell bimetallic nanoparticles were prepared first and then used to analyze CEA antigen solutions of different concentrations. A calibration curve was established in the range from 5 × 10−3 to 5 × 105 ng/mL. Finally, this new SERS probe was applied for quantitative detection of CEA in serum obtained from 26 colorectal cancer patients according to the calibration curve. The results were in good agreement with that obtained by electrochemical luminescence method, suggesting that SERS immunoassay has high sensitivity and specificity for CEA detection in serum. A detection limit of 5 pg/ml was achieved. This study demonstrated the feasibility and great potential for developing this new technology into a clinical tool for analysis of tumor markers in the blood.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Colorectal cancer is the third largest malignant neoplasm in the world. It is the most common malignant tumor of the digestive tract [1, 2]. The annual incidence is approximately 1,000,000 cases, and the annual mortality is more than 500,000. Its incidence and mortality rates are on the rise in recent years [3]. With the development of surgical technology and update of chemotherapy, the survival rate is greatly improved. However, in current clinical practice, the rate of early diagnosis of colorectal cancer is low. Thus, the postoperative recurrence and metastasis is common for this disease. Therefore, a good screening tool is urgently needed, and ideally it should be able to catch early lesions, yield excellent prognosis, and has low cost [4, 5].
Serum carcinoembryonic antigen (CEA) discovered in 1965 is expressed in most colorectal cancer patients, but it is undetectable in normal colonic epithelial cells. Furthermore, it is found that CEA is also expressed in several other malignant neoplasms such as gastric cancer, liver cancer, and lung cancer. Recently, CEA is frequently used for screening colorectal cancer, assessing treatment effect, evaluating prognosis, and monitoring recurrence and metastasis [6]. Currently, it is the only recommended marker for routine screening of patients with colorectal cancer. At present, the clinical methods of detecting serum CEA are mainly ELISA and electrochemical luminescence. And electrochemical luminescence is recognized as a technique with high sensitivity.
SERS spectroscopy is one of the most powerful analytical techniques for the identification of molecular species [7–11]. Surface-enhanced Raman scattering immunoassay (SERIA) combining SERS with biological analysis technology and nanotechnology has arisen as a new labeled immunoassay technique based on antigen antibody–specific interactions [13, 13]. Recently, SERIA has become a hot topic in the field of biomedicine due to its high sensitivity, simple operation, and multiplex labeling capability [14]. It has been implemented for nucleic acid detection, [15–19] protein expression analysis in blood and tissues [20–26], circulating tumor cell detection, and tumor targeting detection in vivo [27–30]. However, there are few reports about SERS-based immunoassay for CEA detection in the serum from patients with colorectal cancer [31].
In this work, we focused on exploring the feasibility of using SERS probes to detect serum CEA in patients with colorectal cancer. Firstly, anti-CEA-functionalized 4-MBA-labeled Au/Ag core-shell bimetallic nanoparticles were prepared and applied to analyze CEA antigen solutions of different concentrations, established a calibration curve, and demonstrated the new SERS probe’s high sensitivity and specificity. Then, the developed anti-CEA SERS probe was used for quantitative detection of CEA in serum from colorectal cancer patients. To the best of our knowledge, this is the first report on SERS probes for quantitative detection of tumor markers in serum of colorectal cancer patients. It has the potential to be developed into a new clinical tool for microarray analysis of tumor markers in the blood.
2 Materials and methods
2.1 Reagents and apparatus
Mouse anti-CEA monoclonal antibody (MAb) (2.0 mg/mL), mouse anti-CEA polyclonal antibody (PAb) (2.0 mg/mL), and CEA protein were purchased from Zhongshan Jinqiao Biotechnology Co. Ltd, Fuzhou, China. The electrochemical luminescence kit was purchased from Roche Company, USA. The bovine serum albumin (BSA) was purchased from Genview, Germany. 4-Mercaptobenzoic Acid (4-MBA), trisodium citrate, glutaraldehyde (GA), HAuCl4·4H2O, and silver nitrate (AgNO3) were obtained from Beijing Dingguo Changsheng Biotechnology Co. Ltd. All the chemicals used were of analytical grade. Borate buffer solution (BBS 2 mM, pH 9.0), citrate buffer (0.01 M, PH6.0), and phosphate buffer solution (PBS 0.01 M, pH 7.2-7.4) were purchased from BioTeke Company, China. All required solution in experiments was prepared by using deionized water with a conductivity of 18 MΩ/cm at 25 °C.
Absorption spectra of Au/Ag core-shell nanoparticles were recorded using PerkinElmer Lambda 950 Spectrophotometer (Waltham, MA, USA). The transmission electron microscopy (TEM) observation of Au/Ag core-shell nanoparticles was performed on a Hitachi model H-800 transmission electron microscope with an accelerating voltage of 200 kV. A Renishaw Raman microscope (InVia system, Great Britain) was used for the collection of SERS spectra. Roche Modular E170 Analytics assay (Switzerland) was used to quantitatively detect CEA in serum from patients with colorectal cancer.
2.2 Tissue sample collection and processing
In this study, blood serum samples were collected from 26 colorectal cancer patients with clinical and confirmed pathological diagnoses. All patients were from Fujian Provincial Tumor Hospital. All the study subjects provided written consent, and ethics permission was obtained for the study.
Tissue samples were fixed in 10 % neutral buffered formalin and embedded in paraffin blocks. For this study, 4-μm-thick sections were cut from each block using a microtome for SERS probe staining. All the tissue sections were baked at 60 °C for 2 h and then were de-paraffinized with xylene, rehydrated through graded ethanol for further experiments.
2.3 Preparation of anti-CEA PAb-conjugated SERS nanoparticles and anti-CEA MAb-conjugated slides
Briefly, Au/Ag core-shell bimetallic nanoparticles were prepared by the seed growth method according to references [22, 32]. Then, CEA-conjugated SERS probe preparation was conducted using the same procedure as our published method in 2012 [33]. The CEA SERS probes are stable for several months when stored at 4 °C.
The pretreatment of slides and conjugation of anti-CEA MAb were processed according to Chrisey et al. [34]. Briefly, ordinary glass slides were first cleaned by immersing in a mixture solution of concentrated hydrochloric acid and methanol (1:1 v/v), and then silanized with fresh 1 % diethylenetriamine (DETA) in 1.0 mM acetic acid solution. After being modified with amino groups, the glass slides were further cross-linked with 2.5 % glutaraldehyde in aqueous solution. Finally, anti-CEA MAb was dropped onto the glass slides and reacted with the aldehyde groups. It is then rinsed with PBS containing 1 % BSA to remove the residual CEA antibodies and finally dried under nitrogen gas.
2.4 Establishing the Raman intensity versus CEA concentration calibration curve
Firstly, antibody-modified glass slides were incubated with different concentrations of CEA proteins for 3 h and then washed with PBS containing 0.1 M NaCl and 3 % BSA to remove the residual antigens as well as to block the non-specific absorption sites. Secondly, the substrates prepared above were incubated with 10 μL of anti-CEA SERS probes in a humidity chamber for 3 h at room temperature. Then, they were washed with PBS containing 1 % BSA and 0.1 M NaCl (pH = 7.0), as well as ultrapure water in sequence, and finally dried under nitrogen gas.
A 785-nm near-IR diode laser was used as the excitation source. The SERS spectra were collected using a Leica 20 × objective; the detection of Raman signal was carried out with a Peltier cooled CCD camera. Raman spectra in the range of 300–1,700/cm were acquired with an integration time of 1 s using the software package WIRE 2.0 (Renishaw). An automated algorithm for autofluorescence background removal was applied to the measured raw data to extract pure Raman spectra [35]. Thirty spectra at different locations of each sample were collected and then averaged. Finally, the SERS peak intensity at 1,077/cm (originated from 4-MBA) for each sample was calculated to establish the Raman intensity versus CEA concentration calibration curve.
2.5 Quantification of the CEA concentration in serum from patients with colorectal cancer via SERS immunoassay
A total of 10 μL of serum samples was pipetted onto antibody-modified glass slides and then incubated for 3 h at room temperature. After rinsed with PBS containing 0.1 M NaCl and 3 % BSA, anti-CEA SERS probes were dropped to the substrates prepared above and incubated for 3 h at room temperature. Then, the substrates were rinsed with PBS containing 0.1 M NaCl (pH = 7) and 1 % BSA, as well as with water in sequence. Finally, the glass slides were dried under nitrogen gas before SERS measurements. Testing conditions and data processing were the same as above.
2.6 Quantification of the CEA concentration in serum from patients with colorectal cancer via electrochemical luminescence
Quantitative detection of the CEA concentration in serum from patients with colorectal cancer was performed using an electrochemical luminescence method–based CEA detection Kit (Roche, USA) according to the manufacturer’s protocol.
3 Results and discussion
3.1 Establishment of the calibration curve of Raman intensity versus CEA concentration
The relationship between the SERS response and the CEA concentration was investigated under optimized experimental conditions mentioned above. Firstly, the CEA antigen solutions with different concentrations of 5 × 105, 5 × 104, 5 × 103, 5 × 102, 5 × 101, 5 × 100, 5 × 10−1, 5 × 10−2, 5 × 10−3 ng/ml were prepared, and then SERS measurements were taken. Figure 1 shows SERS spectra of different concentrations of CEA solutions. When PBS substituted for CEA protein as a control, no reliable Raman signals were obtained in the control group. It is obvious that the higher the concentration of CEA is, the stronger the Raman signals observed are, suggesting reliable concentration–dependent SERS spectral changes. It is easier to see the tendency from the bar charts of the intensity of SERS signals versus concentration of CEA (as shown in Fig. 2).
Taking into account the minor variations of the SERS signals detected from different locations of the slide, thirty spectra of each sample were collected and averaged. Because the intensity of the peak at 1,077/cm was strongest and most stable at different locations over the slide at a given CEA concentration, the peak intensity at 1,077/cm was selected to establish the Raman intensity versus concentration calibration curve for CEA detection. The calibration curve constructed by mean spectra of each sample in the CEA concentration range of 500 μg/ml–5 pg/ml is shown in Fig. 3. The linear regression equation was I = 1069logC + 3,611. Here, I represents the Raman intensity and C is the concentration of CEA. The correlation coefficient (R 2) was 0.9955. To test the reproducibility of this method, we repeated the experiment 4 times under the same condition. We found that the relative standard deviation (R.S.D.) is 15.6 %. Furthermore, we examined the R.S.D. of the signals of the same concentration at thirty different locations on the slide is 5.8 %. Using this strategy, concentration of CEA was detected in the range from 500 μg/ml to 5 pg/ml.
3.2 Quantification of the concentration of CEA in serum from patients with colorectal cancer
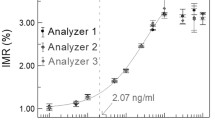
CEA concentrations in serum samples collected from 26 colorectal cancer patients were analyzed with both SERS immunoassay and electrochemical luminescence. In SERS immunoassay, thirty readings at different locations of each sample were obtained and averaged, and then the Raman intensities at 1,077/cm of the mean spectra were put into the equation of the calibration curve to derive the concentration of CEA. Table 1 lists the values of CEA in serum from 26 colorectal cancer patients by electrochemical luminescence and SERS immunoassay, respectively. The values of CEA concentration measured by the two methods were consistent with each other except a few data points (black points as shown in Fig. 4). Using electrochemical luminescence, concentration of CEA was detected in the range from 1,000 to 0.2 ng/ml, while the detection limit of CEA in this immunoassay system was as low as 5 pg/ml. This suggests that the new SERS immunoassay method has potential for developing into a clinical tool for detecting tumor markers in the serum.
4 Conclusion
In summary, we have developed a highly specific and sensitive methodology for CEA detection in serum using 4-MBA-labeled Au/Ag core-shell nanoparticle-enabled SERS. To the best of our knowledge, this is the first report on rapid detection of CEA concentrations in serum obtained from colorectal cancer patients using SERS immunoassay. The reliability and applicability of the new method was further validated by comparison with electrochemical luminescence method. The results from SERS immunoassay were well consistent with those of electrochemical luminescence for CEA concentration quantification. Our study demonstrated the feasibility and great potential for developing this new technology into a clinical tool for sensitive analysis of tumor markers in blood.
References
A. Jemal, F. Bray, M. Center, J. Ferlay, E. Ward, D. Forman, Global cancer statistics. CA Cancer J. Clin. 61, 69–90 (2011)
R. Siegel, E. Ward, O. Brawley, A. Jemal, Cancer statistics, 2011. CA Cancer J. Clin. 61, 212–236 (2011)
M. Center, A. Jemal, E. Ward, International trends in colorectal cancer incidence rates. Cancer Epidemiol. Biomark. Prev. 18, 1688–1694 (2009)
R.M. McLoughlin, C.A. O’Morain, Colorectal cancer screening. World J. Gastroenterol. 12, 6747–6750 (2006)
R. Labianca, G. Beretta, S. Mosconi, L. Milesi, M. Pessi, Colorectal cancer: screening. An. Oncol. 16, 127–132 (2005)
M.J. Duffy, Carcinoembryonic antigen as a marker for colorectal cancer: is it clinically useful? Clin. Chem. 47, 624–630 (2001)
R.A. Alvarez-Puebla, L.M. Liz-Marza, SERS-Based diagnosis and biodetection. Small 6, 604–610 (2010)
J. Kneipp, H. Kneipp, K. Kneipp, SERS—a single-molecule and nanoscale tool for bioanalytics. Chem. Soc. Rev. 37, 1052–1060 (2008)
Y.S. Huh, A.J. Chung, D. Erickson, Surface enhanced Raman spectroscopy and its application to molecular and cellular analysis. Microfluid. Nanofluid. 6, 285–297 (2009)
S. Hudson, G. Chumanov, Bioanalytical applications of SERS (surface-enhanced Raman spectroscopy). Anal. Bioanal. Chem. 394, 679–686 (2009)
D. Graham, R. Goodacre, Chemical and bioanalytical applications of surface enhanced Raman scattering spectroscopy. Chem. Soc. Rev. 37, 883–884 (2008)
K.C. Bantz, A.F. Meyer, N.J. Wittenberg, H. Im, Kurtulu, S.H. Lee, N.C. Lindquist, S.H. Oh, C.L. Haynes, Recent progress in SERS biosensing. Phys. Chem. Chem. Phys 13, 11551–11567 (2011)
S. Xu, X. Ji, W. Xu, B. Zhao, X. Dou, Y. Bai, Y. Ozaki, Surface-enhanced Raman scattering studies on immunoassay. J Biomed Opt 10, 031112 (2005)
B. Lutz, C. Dentinger, L. Nguyen, L. Sun, J. Zhang, A. Allen, S. Chan, B. Knudsen, Spectral analysis of multiplex Raman probe signatures. ACS Nano 2, 2306–2314 (2008)
Y. Cao, R. Jin, C. Mirkin, Nanoparticles with Raman spectroscopic fingerprints for DNA and RNA detection. Science 297, 1536 (2002)
M. Culha, D. Stokes, L.R. Allain, T. Vo-Dinh, Surface-enhanced Raman scattering substrate based on a self-assembled monolayer for use in gene diagnostics. Anal. Chem. 75, 6196–6201 (2003)
R.J. Stokes, A. Macaskill, J.A. Dougan, P.G. Hargreaves, H.M. Stanford, W.E. Smith, K. Faulds, D. Graham, Highly sensitive detection of dye-labelled DNA using nanostructured gold surfaces. Chem. Commun. 27, 2811–2813 (2007)
J.J. Storhoff, A.D. Lucas, V. Garimella, Y.P. Bao, U.R. Müller, Homogeneous detection of unamplified genomic DNA sequences based on colorimetric scatter of gold nanoparticle probes. Nat. Biotechnol. 22, 883 (2004)
T. Vo-Dinh, L.R. Allain, D.L. Stokes, Cancer gene detection using surface-enhanced Raman scattering (SERS). J. Raman Spectrosc. 33, 511–516 (2002)
J.W. Chen, Y. Lei, X.J. Liu, J.H. Jiang, G.L. Shen, R.Q. Yu, Immunoassay using surface-enhanced Raman scattering based on aggregation of reporter-labeled immunogold nanoparticles. Anal. Bioanal. Chem. 392, 187–193 (2008)
B. Du, Z. Li, Y. Cheng, Homogeneous immunoassay based on aggregation of antibody-functionalized gold nanoparticles coupled with light scattering detection. Talanta 75, 959–964 (2008)
X. Ji, S. Xu, L. Wang, M. Liu, K. Pan, H. Yuan, L. Ma, W. Xu, J. Li, Y. Bai, Immunoassay using the probe-labeled Au/Ag core-shell nanoparticles based on surface-enhanced Raman scattering. Colloids Surf. A Physicochem. Eng. Asp. 257, 171–175 (2005)
C. Lin, Y. Yang, Y. Chen, T. Yang, H. Chang, A new protein A assay based on Raman reporter labeled immunogold nanoparticles. Biosens. Bioelectron. 24, 178–183 (2008)
B. Lutz, C. Dentinger, L. Sun, L. Nguyen, J. Zhang, A. Chmura, A. Allen, S. Chan, B. Knudsen, Raman nanoparticle probes for antibody-based protein detection in tissues. J. Histochem. Cytochem. 56, 371–379 (2008)
S. Schlücker, B. Küstner, A. Punge, R. Bonfig, A. Marx, P. Ströbel, Immuno-Raman microspectroscopy: in situ detection of antigens in tissue specimens by surface-enhanced Raman scattering. J. Raman Spectrosc. 37, 719–721 (2006)
L. Sun, K. Sung, C. Dentinger, B. Lutz, L. Nguyen, J. Zhang, H. Qin, M. Yamakawa, M. Cao, Y. Lu, Composite organic-inorganic nanoparticles as Raman labels for tissue analysis. Nano Lett. 7, 351–356 (2007)
K.K. Maiti, U. Dinish, C.Y. Fu, J.J. Lee, K.S. Soh, S.W. Yun, R. Bhuvanaeswari, M. Olivo, Y.T. Chang, Development of biocompatible SERS nanotag with increased stability by chemisorption of reporter molecule for in vivo cancer detection. Biosens. Bioelectron. 26, 398–403 (2010)
A. Pallaoro, G. Braun, N. Reich, M. Moskovits, Mapping local pH in live cells using encapsulated fluorescent SERS nanotags. Small 6, 618–622 (2010)
X. Qian, X.H. Peng, D.O. Ansari, Q. Yin-Goen, G.Z. Chen, D.M. Shin, L. Yang, A.N. Young, M.D. Wang, S. Nie, In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat. Biotechnol. 26, 83–90 (2007)
M.Y. Sha, H. Xu, M.J. Natan, R. Cromer, Surface-enhanced Raman scattering tags for rapid and homogeneous detection of circulating tumor cells in the presence of human whole blood. J. Am. Chem. Soc. 130, 17214–17215 (2008)
D. Lin, S. Feng, J. Pan, Y. Chen, J. Lin, G. Chen, S. Xie, H. Zeng, R. Chen, Colorectal cancer detection by gold nanoparticle based surface-enhanced Raman spectroscopy of blood serum and statistical analysis. Opt. Express 19, 13565–13577 (2011)
Y. Yang, J. Shi, G. Kawamura, M. Nogami, Preparation of Au-Ag, Ag-Au core-shell bimetallic nanoparticles for surface-enhanced Raman scattering. Scr. Mater. 58, 862–865 (2008)
Y. Chen, X. Zheng, G. Chen, C. He, W. Zhu, S. Feng, G. Xi, R. Chen, F. Lan, H. Zeng, Immunoassay for LMP1 in nasopharyngeal tissue based on surface-enhanced Raman scattering. Int. J. Nanomed. 7, 73 (2012)
L.A. Chrisey, G.U. Lee, C.E. O’Ferrall, Covalent attachment of synthetic DNA to self-assembled monolayer films. Nucleic Acids Res. 24, 3031–3039 (1996)
J. Zhao, H. Lui, D. McLean, H. Zeng, Automated autofluorescence background subtraction algorithm for biomedical Raman spectroscopy. Appl. Spectrosc. 61, 1225–1232 (2007)
Acknowledgments
We thank Jing Wang and Gangqin Xi for excellent technical assistance. This work was supported by the National Natural Science Foundation of China (No. 61210016, 61178090, 81101110), the Canadian Institutes of Health Research International Scientific Exchange Program, the Natural Science Foundation of Fujian Province (No.2012J01326), and the Medical Innovation Program of Fujian Province (No.2012-CX-7).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, G., Chen, Y., Zheng, X. et al. Surface-enhanced Raman scattering study of carcinoembryonic antigen in serum from patients with colorectal cancers. Appl. Phys. B 113, 597–602 (2013). https://doi.org/10.1007/s00340-013-5515-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-013-5515-1