Abstract
The capabilities of time-resolved laser-induced incandescence (TiRe-LII), a combustion diagnostic used almost exclusively to measure soot primary particles, could potentially be extended to size aerosolized metal nanoparticles. In order to do this, however, it is necessary to characterize the thermal accommodation coefficient, α, which specifies the heat conduction rate between the laser-energized nanoparticles and the surrounding gas. This paper extends a molecular dynamics (MD) methodology to calculate α for Fe/He, Fe/Ar, Mo/He, and Mo/Ar systems. A comparative analysis of the results shows that α is most strongly influenced by the potential well between the gas molecule and nanoparticle surface. Finally, the MD-derived value for α is used to recover the nanoparticle size distribution for TiRe-LII measurements made on molybdenum nanoparticles in argon.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The distinctive attributes of metal nanoparticles place them prominently in the frontiers of materials science. For example, their unique electromagnetic properties, arising from plasmon/photon resonance, can be exploited to improve the performance of photovoltaic materials [1] and form the basis of novel biomedical imaging and treatments [2]. Also, the high surface-to-volume ratio of metal nanoparticles makes them potent catalysts for many chemical processes, including gas-phase synthesis of carbon nanotubes [3]. The functionality of metal nanoparticles in virtually every application strongly depends on their size, presenting a need for an optical diagnostic capable of making in situ, temporally and spatially resolved sizing of aerosolized metal nanoparticles to fully realize their commercial potential. This diagnostic is also needed to assess and mitigate the unintended impacts of metal nanoparticles on human health and the environment, which recent research has shown to be strongly size-dependent [4, 5].
Time-resolved laser-induced incandescence (TiRe-LII) appears to be a promising candidate to fulfill this need. Since it was initially proposed by Melton [6], TiRe-LII has become a standard combustion diagnostic and is widely used for sizing the primary particles of soot in flames [7], combustion chambers [8], and exhaust streams [9]. This diagnostic uses a laser pulse to energize the nanoparticles within a sample of aerosol, and the resulting incandescence is measured as the nanoparticles re-equilibrate with the ambient gas. Since larger nanoparticles cool more slowly than smaller nanoparticles, the nanoparticle size distribution can be inferred by regressing simulated incandescence data, generated using a model of the nanoparticle temperature decay, to the measured values. A key parameter in this model is the thermal accommodation coefficient, α, which specifies the average energy transfer when a gas molecule scatters from the nanoparticle surface.
While TiRe-LII is an established combustion diagnostic for sizing soot, there have been surprisingly few attempts to extend this tool to non-carbonaceous aerosols. Vander Wal et al. [10] were the first to explore the feasibility of using TiRe-LII on metal aerosols, specifically iron, titanium, molybdenum, and tungsten nanoparticles in helium; while they did not actually infer particle sizes, the spectral emission decay they observed at longer cooling times was consistent with laser-induced incandescence, suggesting that TiRe-LII sizing of metal particles should be possible. In the same year, Filippov et al. [11] inferred the distribution of silver nanoparticles in argon, albeit by erroneously assuming α = 1.
Subsequent TiRe-LII experiments have been done on metallic aerosols of iron [12–15], nickel [16], and molybdenum [17] nanoparticles. While the majority of these studies assess the feasibility of using TiRe-LII to characterize metal nanoparticles, very few actually recover nanoparticle sizes from TiRe-LII data because α is generally unknown for the aerosols in question. Most published thermal accommodation coefficients [18] are not directly useful since they are obtained under vastly different conditions from LII experiments; instead, thermal accommodation coefficients for TiRe-LII particle sizing must be found using TiRe-LII data, a somewhat circular process. This is most often done by making TiRe-LII measurements on aerosols containing nanoparticles of known sizes, which are usually found through transmission electron microscopy (TEM) of extracted nanoparticles. Following this procedure, Starke et al. [12] obtained α = 0.33 for iron nanoparticles in argon, while Eremin et al. [13] found α = 0.1, 0.2, and 0.01 for iron nanoparticles in argon, carbon monoxide, and helium, respectively. These values were also used to infer the size of iron nanoparticles using TiRe-LII in a subsequent study [14]. Kock et al. [15] inferred α without prior knowledge of the particle size distribution; instead, they assumed that particle sizes obeyed a lognormal distribution having a width typical of mature aerosols in which coalescence and fragmentation mechanisms have equilibrated. They then recovered α and the geometric mean particle diameter simultaneously from two-wavelength LII data. Using this procedure, they estimated α = 0.13 for iron nanoparticles in both argon and nitrogen. This technique only works for nanoparticles in which evaporation is important to heat transfer, however, since α is not independent of d p,g in conduction-dominated cooling.
It is not always possible to complement TiRe-LII measurements with a detailed TEM analysis of extracted nanoparticles. While this procedure is notoriously difficult and time-consuming for in-flame soot, it is even more challenging for metal aerosols since they are often at near-ambient temperatures, which complicates thermophoretic extraction, and have lower nanoparticle loading compared to soot-laden aerosols. For example, Reimann et al. [16] carried out TiRe-LII measurements on nickel nanoparticles formed by inert gas condensation in an argon atmosphere; while they performed a limited TEM study on extracted nanoparticles, the number of images was insufficient to derive a nanoparticle size distribution. Murakami et al. [17] carried out TiRe-LII measurements on molybdenum nanoparticles formed by laser photolysis of Mo(CO)6 in He, Ar, N2, and CO2. They were unable to extract any nanoparticles for TEM imaging, and a subsequent statistical analysis of the TiRe-LII data [19] found that the high melting temperature of molybdenum disallows simultaneous inference of α and d p following the methodology of Kock et al. [15]. Accordingly, in these scenarios, one must have a priori knowledge of α in order to infer nanoparticle sizes from TiRe-LII measurements.
As an alternative to experimental characterization, Daun et al. [20, 21] pioneered the use of molecular dynamics (MD) to estimate α between graphite (as a surrogate for soot) and various gases. In this procedure, pairwise potentials are defined between the constituent surface and gas atoms in the system, which are then differentiated with respect to displacement to obtain forces. Newton’s equations of motion are then integrated with respect to time to obtain atomic trajectories through an individual gas/surface scattering event. The MD simulation becomes the kernel of a Monte Carlo integration over the incident trajectories of the gas molecule. This methodology was first used to model thermal accommodation coefficients between soot in monatomic [20] and polyatomic gases [21]; MD-derived accommodation coefficients matched ones found by experiment [22]. More recently, Daun et al. [23] applied MD to find α for the Ni/Ar system, to complement the experimental study of Reimann et al. [16]. The main challenge in this technique lies in accurately modeling the gas/surface potential; in the case of soot, Daun et al. [20, 21] fit a pairwise Lennard-Jones 6–12 potential to desorption energies and equilibrium distances between graphite and various gases, while for the Ni/Ar system, a pairwise Morse potential was fit to ab initio-derived ground state energies of an argon atom at various heights above a nickel surface, obtained using density functional theory (DFT) [23].
The present paper extends this technique to other metal aerosols containing monatomic gases, specifically Fe/Ar, Fe/He, Mo/Ar, and Mo/He systems. MD-derived accommodation coefficients for Fe/Ar and Fe/He are consistent with experimentally derived values. A comparative analysis of these results, along with the MD-derived accommodation coefficients from previous studies, reveals that the potential well between the gas atom and the metal nanoparticles strongly influences α. This is confirmed through a sensitivity study on α for the Fe/Ar system. Finally, for the first time, a MD-derived accommodation coefficient will be used to recover particle sizes from previously un-interpreted TiRe-LII measurements made by Murakami et al. [17] on molybdenum nanoparticles formed in argon.
2 Time-resolved laser-induced incandescence
In time-resolved laser-induced incandescence (TiRe-LII), a short laser pulse energizes the nanoparticles contained in a sample volume of aerosol, and the resulting spectral incandescence is recorded, usually at multiple wavelengths, as the nanoparticles re-equilibrate with the surrounding gas. The instantaneous spectral incandescence measured by the detector is due to emission from all nanoparticle sizes,
where T p is the temperature of nanoparticles of diameter d p at time t after the laser pulse, I λ is the corresponding blackbody intensity, P(d p) is the probability density of the nanoparticle size distribution, Q abs,λ is the absorption efficiency, and C λ is a constant that depends on the experimental apparatus and nanoparticle loading. If the nanoparticle diameters are much smaller than the excitation laser and detection wavelengths, the nanoparticles emit radiation in the Rayleigh regime, in which case
where m λ is the complex index of refraction. Because the absorption cross-section C abs,λ = πd 2p /4Q abs,λ/4 is proportional to d 3p , and due to the short duration of the laser pulse, all particles reach the approximately same initial peak temperature provided that the excitation beam fluence has a spatially uniform (i.e., “top-hat”) profile. After the pulse, the nanoparticles cool at different rates depending on their size, so the spectral incandescence decay contains information about P(d p).
While it is possible to recover P(d p) by deconvolving Eq. (1) directly (e.g. [11]), more often the spectral incandescence is measured at multiple wavelengths and then reduced to a pyrometric temperature T eff(t), defined in such a way that it would correspond to the true temperature of the nanoparticles where the aerosol monodisperse. (If the nanoparticle sizes were identical, they would cool at the same rate, and consequently, all the nanoparticles would be at the same temperature at any instant.) In this scenario, a distribution type (most often lognormal) is assumed for P(d p), and the unknown distribution parameters are found by regressing simulated effective temperatures, found by substituting modeled values of T p(t, d p) into Eq. (1), to experimental data. Figure 1 shows a sample fit between measured and model effective temperatures for molybdenum nanoparticles in argon [17, 19].
Model nanoparticle temperatures can be found by solving
starting from the peak nanoparticle temperature, which, again, is similar for all nanoparticle sizes provided the excitation laser has a spatially uniform fluence. If d p is smaller than the mean free molecular path in the gas (i.e., Kn = λ MFP/d p > 1), heat conduction occurs within the free molecular regime,
where N g″ is the incident gas molecular number flux, n g = P g/(k B T g) and c g = [8k B T g/(πm g)]1/2 are the number density and mean thermal speed in the equilibrium gas, and <E o−E i> is the average energy transferred to a gas molecule that scatters from the laser-energized nanoparticle. The latter term is defined using the thermal accommodation coefficient, α ≡ <E o−E i>/<E o−E i> max, where the denominator is the maximum average energy transfer allowed by the 2nd Law of Thermodynamics. For monatomic gases, <E o−E i> max = 2k B(T p−T g), and thus,
In the free molecular regime, heat transfer by evaporation is given by [24]
where Δh v is the enthalpy of vaporization per atom, N v″ is the evaporating molecular number flux, n v = P v/(k B T p) and c v = [8k B T p/(πm v)]1/2 are the number density of evaporated metal atoms and their mean thermal speed, respectively, and ζ is a coefficient between zero and unity that accounts for the fraction of evaporated atoms that do not have sufficient kinetic energy to overcome the potential well and thus re-condense on the nanoparticle. (This coefficient is either chosen heuristically [24] or simply set equal to unity, e.g. [15], as is done in this paper.) The main challenge in calculating q evap lies in finding P v, the vapor pressure of the metal atoms at the gas/surface interface. This is usually done by assuming quasi-equilibrium between the phases and then applying the Clausius–Clapeyron relationship (e.g. [15]), although the accuracy of this approach may be questionable given the very short timescales involved in nanoparticle cooling.
Finally, the thermal radiation from the nanoparticles is given by
Figure 2 shows the relative magnitudes of conduction, evaporation, and radiation for 15 nm nanoparticles composed of iron, molybdenum, and nickel in argon at 0.5 atm and 300 K, representative of conditions in most gas-phase nanoparticle reactors. For the purposes of comparison, α is assumed to be 0.15 based on the range of accommodation coefficients for Fe/Ar reported in the literature [13, 15], although this value is reassessed later in the paper. For the molybdenum system, P v, was solved by equating the Gibbs free energy of the vapor and solid phases [25], while for nickel and iron, P v is found through the enthalpy of vaporization via the Clausius–Clapeyron relationship, assuming Δh v obeys a square root dependence on temperature [26]. In all three cases, conduction accounts for a considerable amount of the heat transfer as the nanoparticles re-equilibrate with the surrounding gas. Accordingly, in order to infer accurate nanoparticle sizes from TiRe-LII data, it is essential to know the thermal accommodation coefficient with a high degree of certainty.
As noted in the introduction, in principle, the thermal accommodation coefficient can be derived experimentally by carrying out TiRe-LII measurements on well-characterized aerosols, most often through TEM analysis of extracted nanoparticles. While TEM analysis is time-consuming at the best of times, in many situations it is simply impossible to extract nanoparticles from the aerosol. Moreover, while Kock et al. [15] were able to derive α for iron nanoparticles in argon and nitrogen due to the dominance of evaporation in nanoparticle cooling, the accuracy of thermal accommodation coefficients derived by this technique must be examined critically in light of the uncertainties in the nanoparticle evaporation model. Accordingly, in these cases, a priori knowledge of α is essential in order to interpret TiRe-LII measurements.
3 Molecular dynamics modeling
Molecular dynamics (MD) simulations present an alternative approach for deriving the thermal accommodation coefficient. In contrast to the techniques described above, it does not require TEM characterization of extracted nanoparticles, nor is it susceptible to uncertainties in the heat transfer model. The first step is to define the potential energies between the constituent atoms of the gas molecule and nanoparticle surface. Interatomic potentials of metals are often expressed in the form
where U tot is the total potential, r ij is the distance between the ith and jth atoms, V(r ij) is the pairwise (repulsive) potential between the ith atom and the jth atom, and ρ i is the electron cloud density of the ith atom,
The functional form of V(r ij) and ϕ(r ij) depends on the type of metal.
The Finnis–Sinclair potential [27, 28] is often used for body-centered cubic (BCC) metals like iron and molybdenum and has successfully predicted the melting behavior of these metals [29]. In this potential, V(r ij) is defined as
and ϕ(r ij) is given by
where the coefficients for iron and molybdenum are summarized in Table 1.
For face-centered cubic (FCC) metals, including nickel, the Sutton–Chen potential is often used [30, 31]. In this model, the pairwise repulsive potential is given by
where r c is the cutoff distance, while ϕ(r ij) is given by
This potential was later re-parameterized by Kimura et al. [32, 33] to correct for quantum effects; these parameters are summarized for nickel in Table 2. The cutoff distance is set to twice the lattice parameter, r c = 2a.
The gas/surface pairwise potential is more problematic since, in contrast to homogeneous systems, rigorously derived interatomic potentials between gas molecules and surface atoms are not generally available in the literature. Instead, many studies employ the Lorentz–Berthelot combining rules [34] to define the gas/surface potential. In this approach, the interaction between dissimilar atoms is represented with a pairwise Lennard-Jones 6–12 potential
where the parameters for the heterogeneous system are the arithmetic and harmonic averages of those from the corresponding homogeneous systems, σ gs = (σ ss + σ gg)/2 and ε gs = (ε ss ε gg)1/2. Many studies, among them [35–37], have used this technique to model gas molecules scattering from metal surfaces. When Daun et al. [23] used this potential to model α for laser-energized nickel nanoparticles in argon, they obtained α ≈ 1, due to the exceptionally large potential well shown in Fig. 3. Other studies have also shown that the Lorentz–Berthelot combining rules produce non-physical results in MD simulations [38–41]. For example, Chase et al. [41] were only able to reproduce experimentally observed results from low incident energy scattering of argon from liquid indium using a potential that was approximately an order of magnitude less than that predicted by the Lorentz–Berthelot rules. The inadequacy of the Lorentz–Berthelot combining rules should not be surprising, since the interatomic potentials between metal atoms, dominated by electron sharing, are completely unlike metal/gas potentials, in which charge transfer is likely.
In this paper, we instead fit a pairwise Morse potential, having the general form
to ab initio-derived ground state energies of a gas molecule at various heights above a 2 × 2 × 2 metal FCC supercell containing 32 atoms and periodic boundary conditions along the lateral surfaces, following Daun et al. [23]. The ground state energies are calculated using the WIEN2k [42] density functional theory program with a generalized gradient approximation (DFT-GGA) and the parameterization of Perdew et al. [43] for the exchange and correlation potentials. In this approach, the unit cell is divided into muffin-tin spheres that are centered on atoms and the interstitial region. The calculation then uses a linear combination of atomic orbitals for the muffin-tin spheres and plane waves in the interstitial region. Figure 3 shows the ground state energy for various argon molecule heights above the surface, along with the best fit obtained by summing Morse potentials over the gas/surface pairs. This methodology has also been followed in other MD studies, e.g. [44]. The resultant Morse potential parameters used in the classical molecular dynamics simulations are given in Table 3.
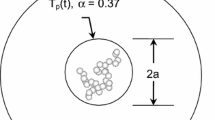
The pairwise potentials are differentiated with respect to atomic displacement to obtain forces. Newton’s equations of motion are then integrated in time using a velocity-Verlet algorithm [45, 46] having a time step of 1 fs, to obtain atomic trajectories. Iron and molybdenum nanoparticles are represented by 637 atoms, initially arranged in a BCC lattice, while the nickel nanoparticle is represented by 600 atoms in an FCC lattice. Periodic boundary conditions are applied on the lateral surfaces. The metal is then heated to 2,500 K, representative of peak temperatures obtained during TiRe-LII experiments done on metal nanoparticles, using a Lowe–Andersen thermostat [47–49]. The thermostat is operated for 20 ps (20,000 timesteps) after which the simulation continues for 1 ps (1,000 time steps) without the thermostat to confirm that the ensemble has reached a steady state. The Finnis–Sinclair and Quantum Sutton–Chen codes were verified by comparing the outputs of independent code implementations and validated by checking the MD-derived density at 2,500 K with experimentally derived values [50–52]. Figure 4 shows how the temperature and density of molybdenum, normalized by the target temperature and experimentally determined density, are very close to the specified values after the warm-up procedure [51]. At the conclusion of the warm-up procedure, the location and velocities of the metal atoms are stored in a restart file.
The thermal accommodation coefficient is then obtained by performing a Monte Carlo integration over the incident gas molecular trajectories. Each trial is initiated with gas molecular velocity components sampled from Maxwell–Boltzmann distributions corresponding to an equilibrium gas at 300 K, as described in [20], while the positions and velocities of the metal atoms are defined by the restart file. The gas molecule starts at a height of approximately 10 Å above the surface, beyond the range of the potential well. The trajectories of the gas molecule and surface atoms are then tracked through the scattering process until the gas molecule reemerges from the surface with a constant escape velocity. Figure 5 shows a sample trajectory of an argon atom scattering from a nickel surface. The atom accelerates as it enters the potential well and then “hops” along the potential surface until it receives enough translational energy from the vibrating surface atoms to overcome the potential well and then scatters from the surface.
Trajectory of an argon molecule scattering from a nickel surface where w g and z g are vertical speed of the argon atom and its height above the surface, respectively. The argon molecule initially accelerates toward the surface in the presence of the potential well. Energy is transferred to the argon molecule from the vibrating potential surface. When the argon molecule obtains enough energy to overcome the potential well, it scatters to the equilibrium gas with a constant exit velocity
The thermal accommodation coefficient is estimated by
where v g,o and v g,i are the incident and exit velocities of the gas molecule, respectively, and <·> denotes the average of all trials. Normal and tangential components of the accommodation coefficient are also found by
and
respectively, where w g and v g,t are the normal and tangential velocities of the gas molecule.
4 Results and discussion
The resulting MD-derived thermal accommodation coefficients, and their normal and tangential components, are listed in Table 4. Each parameter is found by averaging 500 Monte Carlo trials. Table 4 also includes MD-derived values for graphite found previously by Daun et al. [20]. Error bars correspond to two standard deviations of the mean. As noted in previous studies, α n is consistently larger than α t because the energized surface atoms oscillate primarily in the normal direction. This effect is less pronounced for liquid surfaces (Fe, Ni) compared to solid surfaces (Mo, Gr), due to the increased surface roughness of the liquids.
The majority of pioneering TiRe-LII studies on metal aerosols focused on iron nanoparticles, particularly the Fe/Ar system. These studies each report a different thermal accommodation coefficient: α = 0.33 [12]; α = 0.13 [15]; and α = 0.1 [13], which are generally in line with α = 0.23 ± 0.03 found in this study. Eremin et al. [13] also report α = 0.01 for Fe/He, which is considerably smaller than the value found by MD, α = 0.11 ± 0.03, although the relative magnitudes of the thermal accommodation coefficients reported in Ref. [13] for Fe/He and Fe/Ar follow the general trend of the MD-derived values.
Figure 6 shows the normal and tangential scattering energies across a range of incident energies corresponding to velocities sampled from M–B distributions at corresponding gas temperatures, for the Fe/Ar system. (Horizontal and vertical error bars correspond to two standard deviations of the mean of the Monte Carlo trials.) The results agree with the well-known observations of Janda et al. [53] and others, who noted that <E o> = c 1 <E i> + c 2 <E s>, where <E s> = 2k B T s, in gas/surface scattering experiments in which trapping/adsorption was negligible. Figure 6 also shows that the vibrating surface atoms increase the translational energy of the gas molecule when T g > T s and quench the gas molecule when T g < T s as one would expect from the Second Law of Thermodynamics. The respective slopes of the lines show that energy is preferentially transferred to and from the normal mode, which is also indicated by the relative values of α n and α t in Table 4.
Scattering energy versus incident energy for the Fe/Ar system. Error bars denote two standard deviations of the mean. Surface energy is transferred more effectively into the normal mode over the tangential mode, and surface quenches the incident gas molecule at incident energies greater than the surface temperature
Figure 7 shows α plotted as a function of the potential well depth scaled by k B T s, which represents the energy of the vibrating surface atoms. The thermal accommodation coefficient increases monotonically with potential well depth, but becomes less sensitive at large values of D. This trend is generally consistent with thermal accommodation coefficients for other systems as summarized in [18] and is perhaps due to the fact that increasing D allows the gas molecule to penetrate more deeply into the surface potential, improving energy transfer with the vibrating surface atoms.
In classical gas/surface scattering theory (e.g. [54]), thermal accommodation coefficients are often presented in terms of the reduced mass, μ = m g/m s, where m s is the atomic mass of the surface. Figure 8 shows that α generally increases with respect to μ. It has been suggested in the literature that, when T s ≫ T g, the energy transfer between the surface and the gas molecule depends strongly on matching the vibration phase of the surface atom with that of the incident gas molecule [36], which is an implicit function of the molecular mass ratio. If the collision event is sufficiently slow and the incident gas atom is in phase with the vibrating surface atom, the gas molecule will be continuously repelled as the surface atom moves upwards. In the case of the lighter, faster moving molecules, however, there is less energy transfer since the collision duration is much shorter than the oscillations of the vibrating surface atom.
This does not necessarily mean that μ is the only parameter influencing the collision dynamics, however, since D also tends to scale with μ. The exception to the trend is the Ni/Ar system. While some other gas/surface potentials are mainly due to dispersive effects, the Ni/Ar interaction is dominated by Casimir–Polder forces [55, 56], resulting in a much deeper potential well compared to other gas/metal systems having a similar μ. This result suggests that D has a much larger effect on α compared to μ.
The relative influences of μ and D on α are investigated further through a parametric study on the Fe/Ar system. Figure 9 shows that the accommodation coefficient increases with both μ and D, although α is more sensitive to D; also, however, the sensitivity of α to D drops with increasing D, which is consistent with the overall trend shown in Fig. 6. The normalized sensitivities of α to μ and D are approximated by taking the derivative of a quadratic function fitted to the MD-derived accommodation coefficients, as shown in Fig. 8. The results show that, at the nominal values of μ and D for the Fe/Ar system, μ∂α/∂μ = 0.06, and D∂α/∂D = 0.12.
We also examine the sensitivity of α to D for the Ni/Ar system. As noted above, the Lorentz–Berthelot combining rules severely overestimate the true potential well depth for this system, resulting in a strong trapping/desorption channel and consequently near-perfect thermal accommodation [23]. Our ab initio-derived potential well depth of 83 meV is supported by Kao et al. [57] who inferred D = 84 meV based on the results of a low-energy molecular beam experiment. In contrast to these values, two older experimental studies, [58, 59], reported in [60], cite a shallower potential well of 30 meV for the Ni/Ar system. We investigate this shallower potential by scaling a LJ 6–12 pairwise potential to maintain the same equilibrium distance predicted by the Lorentz–Berthelot combining rules, but a potential well depth of 30 meV; this potential is also plotted in Fig. 3. This scaled potential results in α = 0.40 ± 0.04 versus α = 0.50 ± 0.04 using the DFT-derived value of D = 84 meV. Reducing D by nearly 1/3 causes a small change in α compared to the Fe/Ar sensitivity study because the potential well depth for the Ni/Ar system is in the region where α is relatively insensitive to D as shown in Fig. 7.
The most common and important source of uncertainty in DFT-calculated binding energies comes from the single particle approximation of the Kohn–Sham equations. Often, within the local density approximation (LDA) formulation of the exchange and correlation energy, this approximation tends to underestimate the binding energy. The extent of the underestimation varies, generally ranging from a few percent to 10 % and above. Gradient corrections using the generalized gradient approximation (GGA) lead to overestimation. Our experience on carbonaceous materials [61], III–V semiconductors [62], and noble metals Au and Ag put the error to less than 8 %. Another source of error, related to the method of linearized-augmented plane-wave used here, is related to the incompleteness of basis set. We have checked this by performing calculations with various values of the RKmax parameter (the product of the smallest muffin-tin radius in the system and the maximum plane-wave vector) and the total number of k-points in the irreducible Brillouin zone. The error resulting from this is below 0.5 %. For the former source of error, attempts have been made to improve upon this. Among the methods used are many-body effects included through the GW approximation [63] and empirical van der Waals correction (e.g. [64]). While this latter correction is straightforward, the former is extremely computationally expensive and is feasible only on systems made of few atoms. The empirical approach tends to strongly overestimate the binding energy of metal-noble gas pair as we have found for the case of Ni–Ar.
An additional source of uncertainty is the finite number of metal atoms used to represent the surface. To remain computationally tractable, MD simulations must use far fewer atoms than would be contained in a moderately sized metal nanoparticle; for example, a 30 nm nickel nanoparticle is composed of approximately 106 nickel atoms. This could lead to two potential errors: underestimation of the potential well between the gas molecule and the surface, and an incorrect sampling of the dynamics of the laser-energized metal atoms due to the finite simulation domain. Periodic boundary conditions applied to the lateral surfaces of the metal contribute to an accurate representation of the potential well and ensure that the motion of the metal atoms in the MD simulation is representative of those in the nanoparticle.
5 Sizing molybdenum nanoparticles using an MD-derived thermal accommodation coefficient
As noted above, in many experiments involving metal nanoparticles, it is difficult or impossible to extract nanoparticles for TEM analysis, which is a necessary step toward experimental characterization of the thermal accommodation coefficient. In the case of Murakami et al’s experiments on molybdenum in He, Ar, N2, and CO2 [17], for example, until now there was no way to infer particle size from the TiRe-LII data. Recently, Sipkens et al. [19] reanalyzed this data using an improved heat transfer model, assuming the nanoparticle sizes obeyed a lognormal distribution
While Kock et al. [15] were able to infer α and d p,g from TiRe-LII measurements of iron nanoparticles by assuming σ g = 1.5, a typical value for a mature aerosol in which particle growth and fragmentation processes have equilibrated, this was only possible because of the prominence of evaporation in the heat transfer model. In the case of molybdenum, however, Sipkens et al. [19] showed that nanoparticle cooling is dominated by conduction, and consequently, Eqs. (3) and (5) show that α and d p cannot be separated. Instead, these authors carried out a statistically robust analysis of the TiRe-LII data and reported values for d p,g/α and σ g. (Details of Murakami et al.’s experiment and the heat transfer model and the statistical analysis are provided in Refs. [17] and [19], respectively.)
Using the MD value of α = 0.15 derived for Mo/Ar results in the posterior probability shown in Fig. 10. Posterior marginal probability densities are generated using wild bootstrapping [65]. The first step of this procedure is to identify the most probable distribution parameters, \( \left( {d_{\text{p,g}}^{*} ,\sigma_{\text{g}}^{*} } \right) \) through nonlinear least-squares regression of modeled effective temperatures to measured values, as shown in Fig. 1, and the residual vector, \( \left[ {T_{\text{eff}} \left( {d_{\text{p,g}}^{*} ,\sigma_{\text{g}}^{*} } \right) - T_{\text{eff}} } \right]^{T} \), is calculated. Next, a set of 250 artificial measurement datasets are generated by contaminating the best-fit data with random error vectors formed by multiplying the elements of the residual vector with normal random deviates. Each point in Fig. 10 corresponds to the solution found by nonlinear least-squares regression with an artificial dataset. Finally, kernel density estimation [66] is performed on the marginalized histograms to obtain marginal probability densities, P(d p,g) and P(σ g). The resulting distribution parameters and 68 % credible regions are shown graphically in Fig. 10 and are summarized in Table 5. (These credible regions do not account for model parameter uncertainty, which is discussed in greater detail in [19].) The medians of the marginal distributions, d p,g = 35.4 nm and σ g = 1.69, are generally consistent with the size distribution reported in Kock et al.’s experiment on Fe nanoparticles formed in a hot wall reactor containing argon [15]. In this study, a TEM analysis of extracted nanoparticles showed the primary particle diameters to obey a lognormal distribution with σ g = 1.34 and d p,g = 26.9 nm, and they inferred d p,g = 30 nm from their TiRe-LII measurements by assuming that primary particle diameters obeyed a lognormal distribution with σ g = 1.5. On the other hand, Eremin et al. [13] reported considerably smaller nanoparticles formed through UV photolysis of argon doped with Fe(CO)5. These authors found particles sizes to obey a lognormal distribution with d p,g = 10.5 nm and σ g = 1.39 in a 100 kPa argon atmosphere through a TEM survey of extracted nanoparticles, which agreed with nanoparticle sizes inferred through TiRe-LII. A subsequent investigation that used TiRe-LII to measure iron nanoparticles formed through photolysis found similar particle sizes [14]. Further research, in particular experimental validation of the MD-derived accommodation coefficients, is needed to resolve this discrepancy.
6 Conclusions
Scientists and engineers need a diagnostic that can measure the size distribution of aerosolized metal nanoparticles. Time-resolved laser-induced incandescence appears to be a promising candidate to fulfill this need provided the thermal accommodation coefficient is known for these systems. Unfortunately, it is often difficult or impossible to characterize α experimentally for metal aerosols.
An alternative approach is to derive α using molecular dynamics (MD). This paper presents MD-derived thermal accommodation coefficients for Fe/Ar, Fe/He, Mo/Ar, and Mo/He. In this procedure, it is critical to accurately model the gas/surface potential, which was done by fitting Morse pairwise potentials to ab initio-derived ground state energies for gas molecules at various heights above the metal surface. A comparative analysis of these results, along with previous calculations for Ni/Ar and graphite and the noble gases, reveals that α is a strong function of the potential well depth, and a weaker function of the reduced mass, μ = m g/m s. Finally, the MD-derived thermal accommodation coefficient for Mo/Ar was used, for the first time, to analyze TiRe-LII data that previously eluded interpretation due to the fact that, until now, α was unknown for this aerosol.
Although the MD implementation of the potentials used to represent the metal nanoparticles was validated and verified, and the gas/surface potential calculated using DFT was checked against the results of a recent molecular beam experiment, it is still necessary to validate the MD-derived thermal accommodation coefficients against values inferred from TiRe-LII measurements and TEM-derived nanoparticle sizes. The next phase of this research will focus obtaining experimentally derived thermal accommodation coefficients for this purpose.
The MD models derived through this research may also elucidate aspects of nanoparticle synthesis within gas-phase reactors that presently are not fully understood. In particular, Eremin et al. [13] note an intriguing relationship between experimentally derived thermal accommodation coefficients and nanoparticle sizes within gas-phase reactors containing different compositions and pressures of bath gas. In this scenario, experimental values of α will serve to validate the MD models, which in turn will be used to investigate the relationship between bath gas molecular structure and nanoparticle coalescence rates.
Abbreviations
- a 0 :
-
Lattice parameter [Å]
- c :
-
Mean thermal speed [m/s]
- c p(T p):
-
Specific heat [J/(kg K)]
- C λ :
-
Constant in Eq. (1)
- D :
-
Potential well depth [eV]
- d p :
-
Nanoparticle diameter [nm]
- d p,g :
-
Geometric mean nanoparticle diameter [nm]
- E(m λ):
-
Absorption function of the complex refractive index
- I b,λ :
-
Blackbody intensity [W/(m2sr μm)]
- J λ :
-
Spectral incandescence
- Kn:
-
Knudsen number
- k B :
-
Boltzmann’s constant, 1.38 × 10−23 [J/(molecule K)]
- m g :
-
Gas molecular mass [kg/molecule]
- m s :
-
Surface atomic mass [kg/atom]
- m λ :
-
Complex refractive index
- n :
-
Number density [molecules/m3]
- N″:
-
Number flux [molecules/(m2s)]
- P(d p):
-
Probability density of nanoparticle diameter [nm−1]
- P :
-
Pressure [Pa]
- Q abs,λ(d p):
-
Absorption efficiency
- q cond :
-
Conduction heat transfer [W]
- q evap :
-
Evaporation heat transfer [W]
- q rad :
-
Radiation heat transfer [W]
- r :
-
Radial distance [m]
- t :
-
Time [s]
- T :
-
Temperature [K]
- T eff :
-
Effective temperature [K]
- U :
-
Potential [eV]
- v :
-
Velocity [m/s]
- V ij(r ij):
-
Pairwise repulsive potential
- z :
-
Distance from surface [Å]
- α :
-
Thermal accommodation coefficient
- α n :
-
Normal thermal accommodation coefficient
- α t :
-
Tangential thermal accommodation coefficient
- λ :
-
Wavelength
- μ :
-
Reduced mass, m g /m s
- ρ :
-
Density [kg/m3]
- ρ i :
-
Electron cloud density of ith atom
- Δh v :
-
Enthalpy of vaporization [J/atom]
- g :
-
Gas
- p :
-
Particle
- v :
-
Vapor (evaporated surface atoms)
- ζ :
-
Sticking coefficient, Eq. (6)
References
D. Schaadt, B. Feng, E. Yu, Appl. Phys. Lett. 86, 3–063106 (2005)
F.E. Kruis, H. Fissan, A. Peled, J. Aerosol Sci. 29, 511–535 (1998)
T. Saito, S. Ohshima, W.C. Xu, H. Ago, M. Yumura, S. Iijima, J. Phys. Chem. B 109, 10647–10652 (2005)
T. Xia, N. Li, A.E. Nel, Annu. Rev. Public Health 30, 137–150 (2009)
M. Auffan, J. Rose, J.Y. Bottero, G.V. Lowry, J.P. Jolivet, M.R. Wiesner, Nat. Nanotechnol. 4, 634–641 (2009)
L.A. Melton, Appl. Opt. 23, 2201–2208 (1984)
D.R. Snelling, G.J. Smallwood, F. Liu, Ö.L. Gülder, W.D. Bachalo, Appl. Opt. 44, 6773–6785 (2005)
B. Kock, T. Eckhardt, P. Roth, Proc. Combust. Inst. 29, 2775–2782 (2002)
S. Schraml, S. Will, A. Leipertz, SAE Technical Paper 1999-01-0146 (1999)
R. Vander Wal, T. Ticich, J. West, Appl. Opt. 38, 5867–5879 (1999)
A. Filippov, M. Markus, P. Roth, J. Aerosol Sci. 30, 71–87 (1999)
R. Starke, B. Kock, P. Roth, Shock Waves 12, 351–360 (2003)
A. Eremin, E. Gurentsov, C. Schulz, J. Phys. D Appl. Phys. 41, 055203 (2008)
A. Eremin, E. Gurentsov, E. Popova, K. Priemchenko, Appl. Phys. B. 104, 285–295 (2011)
B.F. Kock, C. Kayan, J. Knipping, H.R. Orthner, P. Roth, Proc. Combust. Inst. 30, 1689–1697 (2005)
Reimann J., Oltmann H., Will S., Bassano Carotenuto, E.L. Lösch S., Günther B.H. Laser sintering of nickel aggregates produced from inert gas condensation. Proceedings of the world congress on particle technology 6, Nuremberg, Germany (2010)
Y. Murakami, T. Sugatani, Y. Nosaka, J. Phys. Chem. A 109, 8994–9000 (2005)
S.C. Saxena, R.K. Joshi, Thermal accommodation and adsorption coefficients of gases (McGraw-Hill, New York, 1989)
T.S. Sipkens, K.J. Daun, G. Joshi, Y. Murakami, J. Heat Transfer 135, 052401 (2013)
K. Daun, G. Smallwood, F. Liu, Appl. Phys. B: Lasers Optics 94, 39–49 (2009)
K. Daun, Int. J. Heat Mass Transf. 52, 5081–5089 (2009)
K. Daun, G. Smallwood, F. Liu, J. Heat Transfer 130, 121201 (2008)
K. Daun, J.T. Titantah, M. Karttunen, Appl Phys B 107, 221–228 (2012)
H.A. Michelsen, F. Liu, B.F. Kock, H. Bladh, A. Boïarciuc, M. Charwath et al., Appl. Phys. B 87, 503–521 (2007)
A. Fernández Guillermet, Int. J. Thermophys. 6, 367–393 (1985)
X. Xu, G. Chen, K. Song, Int. J. Heat Mass Transf. 42, 1371–1382 (1999)
M. Finnis, J. Sinclair, Philos. Mag. A 50, 45–55 (1984)
M. Finnis, J. Sinclair, Philos. Mag. A 53, 161 (1986)
Y. Shibuta, T. Suzuki, J. Chem. Phys. 129, 144102 (2008)
A. Sutton, J. Chen, Philos. Mag. Lett. 61, 139–146 (1990)
H. Rafii-Tabar, A. Sulton, Philos. Mag. Lett. 63, 217–224 (1991)
T. Cagin, Y. Kimura, Y. Qi, H. Li, H. Ikeda, W.L. Johnson et al., MRS Symposium series 554, 43–48 (1999)
Y. Qi, T. Çagin, Y. Kimura, W.A. Goddard III, Phys Rev B 59, 3527 (1999)
M.P. Allen, D.J. Tildesley, Computer simulation of liquids (Clarendon Press, Oxford, 1989)
N. Lümmen, T. Kraska, Nanotechnology 15, 525–533 (2004)
V. Chirita, B. Pailthorpe, R. Collins, J. Phys. D Appl. Phys. 26, 133 (1993)
Y. Cheng, C. Lee, Nucl. Instrum. Methods Phys. Res., Sect. B 267, 1428–1431 (2009)
J. Delhommelle, P. Millié, Mol. Phys. 99, 619–625 (2001)
D. Boda, D. Henderson, Mol. Phys. 106, 2367–2370 (2008)
J. Forsman, C.E. Woodward, Langmuir 26, 4555–4558 (2010)
D. Chase, M. Manning, J. Morgan, G. Nathanson, R.B. Gerber, J. Chem. Phys. 113, 9279 (2000)
P. Blaha, K. Schwarz, G. Madsen, D. Kvasnicka, J. Luitz, WIEN2 k, an augmented plane wave plus local orbitals program for calculating crystal properties (Vienna University of Technology, Vienna, 2001)
J.P. Perdew, K. Burke, M. Ernzerhof, Phys. Rev. Lett. 77, 3865–3868 (1996)
B. Qiu, X. Ruan, Phys Rev B 80, 165203 (2009)
D. Frenkel, B. Smit, Understanding molecular simulation: from algorithms to applications (Academic Press, San Diego, 2002)
W.C. Swope, H.C. Andersen, P.H. Berens, K.R. Wilson, J. Chem. Phys. 76, 637–649 (1982)
E. Koopman, C. Lowe, J. Chem. Phys. 124, 204103 (2006)
C. Lowe, Europhys. Lett. 47, 145 (1999)
P. Nikunen, M. Karttunen, I. Vattulainen, Comput. Phys. Commun. 153, 407–423 (2003)
R. Hixson, M. Winkler, M. Hodgdon, Phys. Rev. B 42, 6485 (1990)
P.F. Paradis, T. Ishikawa, S. Yoda, Int. J. Thermophys. 23, 555–569 (2002)
P. Nasch, S. Steinemann, Phys. Chem. Liq. 29, 43–58 (1995)
F.O. Goodman, H.Y. Wachman, Dynamics of gas-surface scattering (Academic Press, New York, 1976)
K.C. Janda, J.E. Hurst, J.P. Cowin, L. Wharton, D.J. Auerbach, Surface Sci. 130, 395 (1983)
H. Casimir, D. Polder, Phys. Rev. 73, 360 (1948)
A.W. Rodriguez, F. Capasso, S.G. Johnson, Nat. Photonics 5, 211–221 (2011)
C.L. Kao, A. Carlsson, R.J. Madix, Surf. Sci. 565, 70–80 (2004)
J.C.P. Mignolet. The charge transfer in physical films on metals. In ed. by W.E.G. Chemisorption. (Butterworths Scientific Publications, London 1955)
P. Gundry, F. Tompkins, Q. Rev. Chem. Soc. 14, 257–291 (1960)
G. Vidali, G. Ihm, H.Y. Kim, M.W. Cole, Surf. Sci. Rep. 12, 135–181 (1991)
J. Titantah, D. Lamoen, E. Neyts, A. Bogaerts, J. Phys.: Condens. Matter 18, 10803 (2006)
J. Titantah, D. Lamoen, M. Schowalter, A. Rosenauer, J. Appl. Phys. 101, 123508 (2007)
L. Hedin, Phys. Rev. 139, A796–A823 (1965)
S. Grimme, J. Comput. Chem. 25, 1463–1473 (2004)
C.F.J. Wu, Ann. Stat. 14, 1261–1295 (1986)
A.W. Bowman, A. Azzalini, Applied smoothing techniques for data analysis: the kernel approach with S-plus illustrations (Oxford University Press, Oxford, 1997)
Acknowledgments
This study was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC). Computational resources were provided by Sharcnet [www.sharcnet.ca].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Daun, K.J., Sipkens, T.A., Titantah, J.T. et al. Thermal accommodation coefficients for laser-induced incandescence sizing of metal nanoparticles in monatomic gases. Appl. Phys. B 112, 409–420 (2013). https://doi.org/10.1007/s00340-013-5508-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-013-5508-0