Abstract
The ejection of molecules from a pressed solid target of lysozyme induced by laser ablation in the UV-regime at a wavelength of 355 nm was investigated. The ablation studies were carried out in vacuum at a laser fluence of 2 J/cm2 for which a significant fraction of proteins remains intact. This was verified by matrix-assisted laser desorption ionization (MALDI) spectrometry of thin films deposited on silicon substrates. The deposition rate of lysozyme was found to decrease with the number of shots and was correlated with increasing thermal damage of the lysozyme. This was monitored by measurements of the optical reflectivity of dry lysozyme. The angular distribution of the mass deposition can be fitted well by Anisimov’s hydrodynamic model. The total deposited yield over the entire hemisphere from direct laser ablation of lysozyme was estimated from this model and found to be three orders of magnitude less than the ablated mass.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Thin films of biomaterials (e.g., polymers, proteins, enzymes) are expected to be extensively used in medical devices and implants due to their versatility and processability. This prospect requires that the biomaterials must demonstrate several critical features for potential applications, including structural integrity, biocompatibility and slow biodegradation. For film production, matrix-assisted pulsed laser evaporation (MAPLE) has become a frequently explored laser-based technique for deposition of biomaterials [1–3], such as lysozyme and myoglobin [4], urease [5], the enzymes ribonuclease [6] and insulin [7]. However, direct laser irradiation of the target in terms of pulsed laser deposition (PLD) has also been employed in some cases, e.g., for urease [8] and papain [9].
In the present work, we have studied the production of thin films of the protein lysozyme by PLD. Lysozyme, also known as muramidase, is a natural thermally stable enzyme found in colostrum, hen egg whites, and human nasal mucus and tears [10]. We have investigated the deposition of lysozyme films by MAPLE with a water ice matrix previously and found that the films contain a significant fraction of intact lysozyme molecules and maintain their functionality after MAPLE transfer [11–13]. Preliminary results have showed that the deposition rate from a pressed target of lysozyme is up to one order of magnitude larger than that from a MAPLE target containing 1 wt % lysozyme in ice [11]. The results were encouraging, and we have subsequently carried out a series of experiments of the direct laser ablation of dry lysozyme.
In this paper, we investigate the total ablation yield of lysozyme molecules irradiated by a UV laser beam at 355 nm. The angular distribution of the mass deposition of lysozyme was determined and compared with the predictions of Anisimov’s model for the adiabatic expansion of the plume, which describes the expansion of a neutral gas in vacuum [14]. Furthermore, the chemical composition of the deposits was investigated.
2 Experimental section
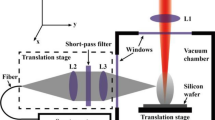
The experimental setup consists of a Nd:YAG laser directed at an angle of 45° degrees with respect to normal onto a target located in a vacuum chamber with a base pressure of 5 × 10−5 mbar. The laser operates at 355 nm with a pulse length of about 6 ns and with a fluence of ~2 J/cm2. The laser beam was focused on the target on an area of 0.017 cm2. The wavelength of the laser is well above the absorption threshold of the lysozyme molecules in an aqueous solution, i.e., 310 nm, but, nevertheless, pressed dry lysozyme does absorb laser light at this wavelength.
We have positioned a double array of 11 + 3 probe holders around the target at a distance of 60 mm and with the angles varying from 0° to 50°, as shown in Fig. 1. To check the symmetry of the system, three holders were positioned at negative angles, 15° apart from each other. Each holder may contain either a quartz crystal microbalance (QCM) or a Si < 100 > substrate of an area of 7 × 7 mm2. The quartz crystal electrode has a sensor area with 6 mm diameter, and the accuracy of the measurements is ~1.29 × 1016 amu/cm2 [15]. Typically, a deposition measurement with the quartz crystal microbalance was carried out for 600 shots.
Schematic of the experimental setup. The laser beam has an angle of incidence of 45° on the target. The arrays of QCMs and Si substrates are placed at ~60 mm away from the target and at various angles with respect to the normal to the surface varying from θ = 0 to 500 with a step of 5°. Three holders were positioned at negative angles (15°, 30° and 45°) to verify the symmetry of system. The black dots denote the positions of the QCMs, while the red dots denote the positions of the Si substrates
The target was a freshly pressed chicken egg white lysozyme from Sigma-Aldrich (molecular weight: 14,307 amu). The target cup has a diameter of 20 mm and a depth of 6 mm. The laser beam was raftered across the target to minimize the drilling of holes, and the target was also rotated.
The reflectivity of dry lysozyme prior and after laser irradiation was monitored using an integrating sphere. The basic principle of operation is described elsewhere [16]. The collimated beam from a deuterium tungsten-halogen light source (DH2000 from Ocean Optics) illuminates the sample at an angle of 8° with respect to normal. The beam goes through multiple reflections and is scattered uniformly around the interior of the sphere. The total flux of light is measured with an optical fiber coupled to a spectrometer (QE 6500 from Ocean Optics). All reflectance spectra were integrated over 2 s and referenced against high diffuse reflectivity standards.
The matrix-assisted laser desorption ionization (MALDI) analysis was performed on a commercial MALDI-TOF–MS system (Bruker Reflex IV MALDI-TOF) equipped with a nitrogen laser (λ = 337 nm, 3 ns pulse length). The sample preparation was performed according to the procedure described in Refs [11, 12]: A matrix solution was prepared by mixing 70 mg sinapinic acid with 2 ml acetonitrile and 1 ml water with 0.1 % trifluoroacetic acid (TFA). A drop of 0.5 μl solution was poured onto the lysozyme film; then, the sample was transferred onto a MALDI plate and left to dry in vacuum prior the analysis. Each MALDI spectrum was averaged over 2000 laser shots. The spectra were recorded with the linear TOF in positive ion mode.
3 Results and discussion
The variation of the mass deposition rate of lysozyme (ng/cm2 per pulse) with respect to the number of pulses is given in Fig. 2. Each point represents an average over the previous 110 shots. The average over 550 shots gives a value of 6.6 ng/cm2 per pulse for the deposition rate of lysozyme at a laser fluence of 2 J/cm2 (dashed line in Fig. 2). The preliminary data for PLD on lysozyme in Refs. [11, 12] was obviously too low. We have compared this value with the mass deposition rate of a MAPLE target containing 1 wt % lysozyme in water, which is 0.98 ng/cm2 per pulse at similar laser fluence [11]. The results indicate that the deposited mass from ablation of dry lysozyme is only about 7 times higher compared to MAPLE deposition.
The QCM results indicate that the deposited mass of pressed lysozyme decreases slowly with the number of pulses. This behavior can partly be attributed to changes in the surface morphology of the solid lysozyme target upon laser irradiation, but also partly to thermal degradation of intact lysozyme molecules to fragments which are less efficient in driving the laser ablation. The changes in optical reflectance of lysozyme were monitored by UV–visible spectroscopy. Optical reflectance spectra of non-irradiated and irradiated lysozyme were measured using the integrating sphere in the wavelength region from 250 to 850 nm are shown in Fig. 3. Each spectrum was taken on a freshly pressed lysozyme target irradiated under the same conditions as those used for deposition rate measurements. The reflectance spectrum of non-irradiated lysozyme shows an absorption edge of around 300 nm (black curve in Fig. 3). A clear decrease in the optical reflectance of lysozyme (dip at around 400 nm) was observed for laser irradiation of dry lysozyme. The total irradiated area after beam rastering on the dry lysozyme target was ~1.6 cm2, meaning that after about 100 pulses, all points on the target have been irradiated. This indicates that one pulse can already change the structure of the protein. A similar change in the absorption spectra of lysozyme has been reported elsewhere, and it has been attributed to partly unfolding of the lysozyme protein triggered by UV illumination [17]. The optical reflectance of lysozyme at 355 nm decreases with number of pulses, as shown in Fig. 2 (right axis). The results indicate that there is a correlation between the change in the optical reflectance of the laser-irradiated lysozyme and the deposition rate of lysozyme. Upon laser beam impact, the ejection of lysozyme from UV-irradiated areas can be partly reduced as discussed above, but also the probability of lysozyme molecules being trapped with increasing etching depth can increase. Both effects will result in a reduction in the deposition rate of dry lysozyme.
The relatively low deposition rate of dry lysozyme might be explained if the majority of the ablated molecules and fragments are ejected with large angles relative to the target normal and not collected at the electrode surface of the quartz crystal. Therefore, we have proceeded to investigate the angular distribution of the ablated material with a QCM facing the target and positioned 60 mm from the ablation spot as shown in Fig. 1. The QCM measurements were taken in steps of 5°, and occasionally, measurements were taken also for negative angles to control the symmetry around the normal incidence at angle θ = 0. The mass deposition per pulse (ng/cm2 per pulse) at different angles with respect to normal is shown in Fig. 4. Each point represents an average over 480–600 shots. The results were compared to the predictions of Anisimov’s model, which is a hydrodynamic model based on an isentropic expansion of an ablation plume. The model was developed for the expansion of a neutral gas cloud, but has been successful also for reproducing the behavior of the expansion of both ions and neutrals for laser-produced plasmas [18–22]. For the ablation of lysozyme, the deposition yield measured with QCM comprises neutral intact molecules, fragments as well as ions.
Mass deposition per pulse (ng/cm2 per pulse) versus angle of incidence for dry lysozyme. The continuous line is a fit to Eq. (1) with k = 1.86 from Anisimov’s model. Fluence, 2 J cm−2, λ = 355 nm
The angular distribution of the plume, F(θ), on a circular collector can be described by the Anisimov’s model using the equation:
As usual, k = Z inf /X inf is the ratio of the limiting value Z inf of the cloud front along the Z-axis directed into vacuum along the normal and the limiting value X inf parallel to the surface. The model describes the expansion of the plume after the laser pulse terminates and typically at times of the order of microsecond. The fitting to Eq. (1) is shown in Fig. 4, and it has been performed with the center F(0) of the distribution as fitting parameter. A high value of the constant k means that the distribution is peaked in forward direction, while a low value of k describes a broad distribution of the plume. A k value of 1.86 (±0.6) was obtained, which is comparable with the value obtained from ablation of silver at low fluence [19]. Once the k value is determined, the total deposited mass Y per pulse can be calculated from Anisimov’s model for the full hemisphere:
where d is the distance from the target to the hemispherical substrate [21]. The total yield of the ablation at laser fluence of 2 J/cm2 is ~507 ng/pulse. We have compared this value with the total mass loss per pulse of the pressed lysozyme target, which is ~155 μg/pulse [21]. The surprisingly low collected mass indicates that only a small fraction of 3.27 × 10−3 of the total ablated material is deposited. This low efficiency is probably caused by thermally induced fragmentation which leads to the release of volatile gases during the laser irradiation [23].
Furthermore, we have measured the chemical spectra of the lysozyme films deposited on Si and under the same ablation conditions as the QCM. At each angle, the number of pulses was varied appropriately to achieve films of lysozyme of similar mass area density of 17 μg/cm2. The MALDI spectra of lysozyme films deposited at normal incidence are shown in Fig. 5. The MALDI spectra show distinctive peaks corresponding to the single charge protonated lysozyme ion [M + H]+ at m/Q = 14308 Th (14308 thomson) as well as protonated doubly charged molecular ion [M + 2H]2+ at m/Q = 7154.5 Th. The MALDI spectrum confirms that a fraction of lysozyme molecules remain intact upon laser transfer from target to substrate. Since there is no clear evidence of fragmentation of lysozyme in the MALDI spectrum, that implies that a significant number of photo-fragments are released in the gas phase.
Zhigilei et al. [24] have recently pointed out that photothermal processes during laser irradiation of lysozyme may lead to ablation. However, as soon as the fluence is sufficiently high to induce a significant ablation, no lysozyme molecules survive the thermal ejection process and only fragments will be deposited on a substrate. This is in a clear contradiction with the present results, and further investigations will be carried out to resolve this issue.
4 Conclusion
We have investigated the ablation of organic molecules of lysozyme during PLD by a UV laser beam at 355 nm at a moderate laser fluence of 2 J/cm2. Our results show that the deposited mass decreases as function of number of pulses. Also, the optical reflectivity of laser-irradiated lysozyme decreases considerably even after 110 pulses as a result of thermal disintegration. The angular distribution of the deposited mass is peaked in forward direction, and we have shown that the plume expansion in vacuum of the organic solid can be well described by Anisimov’s model. The deposit contains a significant number of intact lysozyme molecules which have been detected by MALDI analysis. Mass balance measurements reveal that only a fraction of 3.27 × 10−3 of the total ablated material is deposited. This number is surprisingly low, indicating that a large number of fragments are released as gas products during ablation.
References
D.B. Chrisey, A. Pique, R.A. McGill, J.S. Horwitz, B.R. Ringeisen, D.M. Bubb, P.K. Wu, Chem. Rev. 103, 553 (2003)
A. Caricato, A. Luches, Appl. Phys. A Mater. Sci. Process. 105, 565 (2011)
A. Piqué, Appl. Phys. A Mater. Sci. Process. 105, 519 (2011)
A. Matei, J. Schou, C. Constantinescu, P. Kingshott, M. Dinescu, Appl. Phys. A Mater. Sci. Process. 105, 629 (2011)
E. Gyorgy, A.P. del Pino, G. Sauthier, A. Figueras, J. Appl. Phys. 106, 114702 (2009)
C. Popescu, J. Roqueta, A. Perez del Pino, M. Moussaoui, M.V. Nogues, E. Gyoergy, J. Mater. Res. 26, 815 (2011)
B.R. Ringeisen, J. Callahan, P.K. Wu, A. Piqué, B. Spargo, R.A. McGill, M. Bucaro, H. Kim, D.M. Bubb, D.B. Chrisey, Langmuir 17(11), 3472 (2001)
T. Smausz, G. Megyeri, R. Kékesi, C. Vass, E. György, F. Sima, I.N. Mihailescu, B. Hopp, Thin Solid Films 517, 4299 (2009)
E. Gyorgy, A.P. del Pino, G. Sauthier, A. Figueras, J. Appl. Phys. 106, 114702 (2009)
G. Kim, M. Gurau, J. Kim, P.S. Cremer, Langmuir 18, 2807 (2002)
A. Purice, J. Schou, P. Kingshott, M. Dinescu, Chem. Phys. Lett. 435, 350 (2007)
A. Purice, J. Schou, P. Kingshott, N. Pryds, M. Dinescu, Appl. Surf. Sci. 253, 6451 (2007)
A. Purice, J. Schou, N. Pryds, A. Filipescu, M. Dinescu, Appl. Surf. Sci. 254, 1244 (2007)
S. Anisimov, D. Bauerle, B. Lukyanchuk, Phys. Rev. B. 48, 12076 (1993)
W. Svendsen, J. Schou, T.N. Hansen, O. Ellegaard, Appl. Phys. A Mater. Sci. Process. 66, 493 (1998)
S. Daviðsdóttir, J. Soyama, K. Dirscherl, S. Canulescu, J. Schou, R. Ambat, Surf. Coat. Tech. 216, 35 (2013)
H. Durchschlag, T. Hefferle, P. Zipper, Radiat. Phys. Chem. 67, 479 (2003)
T. Donnelly, J.G. Lunney, S. Amoruso, R. Bruzzese, X. Wang, X. Ni, J. Appl. Phys. 108, 043309 (2010)
B. Toftmann, J. Schou, Appl. Phys. A Mater. Sci. Process. 112, 197 (2013)
B. Thestrup, B. Toftmann, J. Schou, B. Doggett, J. Lunney, Appl. Surf. Sci. 197, 175 (2002)
T.N. Hansen, J. Schou, J. Lunney, Appl. Phys. A Mater. Sci. Process. 69(7), S601 (1999)
B. Toftmann, J. Schou, J. Lunney, Phys. Rev. B. 67, 104101 (2003)
A. Matei, M. Tabetah, C. Constantinescu, J. Schou, L.V. Zhigilei, M. Dinescu, to be published (2013)
L.V. Zhigilei, A. Volkov, E. Leveugle, M. Tabetah, Appl. Phys. A Mater. Sci. Process. 105, 529 (2011)
Acknowledgments
The authors acknowledge the competent technical assistance from Arne Nordskov and Lotte Nielsen. We would also like to acknowledge Dennis D. Corell and Carsten Dam-Hansen for fruitful collaboration and support on reflectance measurements using the integrating sphere.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Constantinescu, C., Matei, A., Schou, J. et al. Pulsed laser deposition of lysozyme: the dependence on shot numbers and the angular distribution. Appl. Phys. B 113, 367–371 (2013). https://doi.org/10.1007/s00340-013-5485-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-013-5485-3