Abstract
Evaporation and ablation are fundamental processes which drive laser-material processing performance. In applications where surface shape is important, control of the temperature field and the resulting spatially varying material response must be considered. For that purpose, assist gases are useful in, first, lowering treatment temperatures and, second, in changing interfacial and bulk chemistry to limit capillary-driven flow. Additionally, laser-matter coupling is influenced by pulse length as it determines the heat affected zone. Using infrared imaging of CO2 laser-heated fused silica and surface profile measurements, we derive temperature and time dependent pitting rates along with shapes for a range of gases that include hydrogen, nitrogen, air, and helium. In the range of 1,500–4,500 K, evaporation, flow, and densification are shown to contribute to the pit shape. Analysis reveals a strong and complex dependence of rim formation on heating time and gas chemistry, mostly by lowering treatment temperature. Under dynamic heating, chemicapillarity appears to help in lowering rim height, in spite of the reactants mass transport limitations. Results on this gas-assisted approach suggest the possibility for sub-nanometer “rimless” laser-based machining.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Fused silica is a critical optical component in high-power lasers and will typically damage above a threshold fluence [1]. For stable and long-term high-power laser operation, the surface damage sites are mitigated by laser-based material removal leaving a pit on the order of a few 100 microns, which is precisely shaped to prevent beam modulation and damage re-initiation [2, 3]. Careful control of surface shape and of the laser-induced temperature field remains important to reduce residual stress, redeposit formation, and beam focusing effects from minute surface profile alterations.
Enhancements in laser machining rates and shape control are possible with application of assist gases in place of machining under an ambient atmosphere. Such an approach is well known in the field of laser welding and cutting, for instance laser cutting of metals in which assist gasses are used to physically disperse molten material [4]. Assist gases can also contribute to increased mass transport rates through higher diffusivities and convection, creation of alternative evaporative reaction pathways, and shifting of the equilibrium point for the evaporation products. Previous studies under CW (seconds) laser heating conditions indicated that flow of a reducing gas such as hydrogen (H2) increased evaporation rates manifolds [5] via a reduction reaction SiO2(s) + H2(g) ⇋ SiO(g) + H2O(g) [6]. Even inert gases such as pure nitrogen (N2) produced greater evaporation rates by pushing the reaction equilibrium forward to produce more SiO gas from decomposition of solid SiO2. In contrast, the oxygen (O2) in air, which is a product in the thermal decomposition reaction of silica, tends to inhibit evaporation SiO2(s) ⇋ SiO(g) + ½O2(g) by the reverse silica condensation reaction. Mass transport-limited kinetics in this regime of rapid evaporation near silica boiling point ≈3,000 K (BP) [6]) implies that laser-based evaporation is locally near equilibrium, where both forward and backward reactions can proceed to near completion [5]. Therefore, the temperature dependence of the equilibrium constants and free energy ΔG° (K p = exp(−ΔG°/RT) become important in determining the extent of rate enhancements, and the degree to which a specific gas can be used to reduce treatment temperature, yet still allow sufficient evaporation rates for effective laser machining. Ideally, a gas is selected to produce the most volatile and stable evaporation products at a given temperature, which limits the thermal load on the treated sample.
Along with efficient material removal rates, the final shape of the laser machined pit is also important, particularly when considering laser processing of optical components in high-power laser systems. Indeed, the lowering in laser treatment temperature through use of a dilute reducing gas can also lead to reduced rim formation at the periphery of the heated spots, producing an optically less caustic site [7]. For fluences ~1–10 MW/cm2, evaporation recoil-pressure-induced melt flow can become an important transport mechanism in determining final surface shape from radial melt flow [8–10]. However, with increasing laser fluence, a transition from normal vaporization to phase explosion occurs, so that molten material breaks down into vapor and droplets as a result of explosive boiling [11]. At lower fluences and temperatures well below the BP of fused silica, rim formation is mostly driven by capillarity-related effects which can induce motion of the molten material at the free surface depending on the temperature dependence of the surface tension σ and viscosity μ. A negative dependence, dσ/dT < 0, whether from compositional, thermal gradients, or interaction with a gas [12, 13], induces an outward flow of material from the hot to the cold regions of the laser-heated area, while a positive dependence reverses the flow direction [14, 15]. The flow is kinetically limited by viscosity often represented with an Arrhenius relationship, μ(T) ∝ exp(E a /RT) [16]. The dimensionless Marangoni number represents the balance of these forces for a thermal gradient ΔT over the size of the flowing melt pool, M a = dσ/dT × (R 0ΔT/μα), where R 0 is a characteristic length and α is the thermal diffusivity of silica, with buoyancy forces being negligible over the length scales considered here. Thus, a reduction in temperature should increase μ minimizing or, even, eliminating rim formation if the surface relaxation occurs over time scales much larger than the laser heating and thermal diffusion times.
Left unexplored in previous studies was the question of whether solid–gas phase reaction chemistry remains significant for non-equilibrium situations, when shorter pulses are used, and how short exposures affect rim formation. We present here measurements of pulse length dependent CO2 laser evaporation studies in H2 and in air and related rim height information; and contrast these results with CW steady laser heating for dilute H2, air, N2, and Helium (He) gases. Taken together, these gases represent reducing, oxidizing, neutral, and high diffusivity conditions, respectively. We extend our measurements to lower temperatures by ≈1,000 K from previous studies performed in the 2,500–3,000 K range [5]. For this lower heating regime around 1,500 K, silica densification (≈2–3 %) from structural relaxation is dominant [17–19], but material flow is negligible due to the high viscosity of fused silica at those temperatures [16, 20]. In contrast, with short and intense microsecond pulse exposures, temperatures up to 4,500 K are reached, a regime where evaporation and melt flow dominates final surface shape. Differences in dynamic rim height formation from H2 and air treatments are discussed, along with results of rim formation scaling with gas-assisted laser etch rate and temperature.
2 Experimental
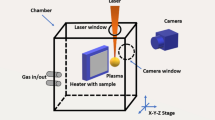
A schematic of the laser setup used to locally heat and apply gas to the surface of the fused silica sample is shown in Fig. 1a. Figure 1b illustrates the heating, resulting surface pit, and mass transport processes for the experimental configuration. Temperature measurements were obtained from our infrared imaging system of the blackbody radiation emitted during heating, which was validated and described as reported elsewhere [21]. The amount of evaporated silica was estimated from the surface shape profiles obtained by interferometry measurements following laser treatment of the surface as described previously [5]. Finally, gas flow rate levels were adjusted with a mass flow controller (400–2,000 sccm), directing a normal incidence gas jet toward the sample surface through a custom nozzle equipped with a laser window for simultaneous co-incident laser heating. In general, the evaporation rate showed a weak, but systematic dependence on gas flow rates in the range used in this study, varying by less than 5 % for all gases used. Additional details of the measurements and setup are provided below.
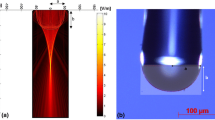
a Schematic of the experimental setup used for evaporation of fused silica with CO2 laser heating. A nozzle with a 3 mm opening and a mass flow controller (MFC) are used to bring an impinging gas coincidently with the laser beam at a controlled flow rate of the treatment gas from 400 to 2,000 sccm. Lenses for beam focusing and magnification are indicated as f1, f2, cf1, cf2. b The laser-based evaporation produces a pit shape measured using white light interferometry ex situ. A surface depth profile is shown by the dashed line drawn through the center of the pit, along with the corresponding surface temperature profile, T(K), captured in situ with the infrared camera imaging system. The reaction zone where species concentration varies is of size δ depending on fluid properties (ex. gas diffusivity), flow rate, flow configuration, and the reactions within the zone and at the surface. Exchange with the bulk flow occurs at a rate q∝D/δ which determines the kinetics of the laser driven evaporation
2.1 Heating of silica surface
For near steady, long exposures, localized heating, and evaporation of the silica surface were achieved with a focused CW (λ L = 10.6 μm) CO2 laser beam from a Synrad Firestar V20 (Synrad, Mukilteo, WA, USA), with a maximum output power of 20 W and power stability of ~1 % over the duration of the exposure. The laser beam profile was nearly a perfect Gaussian with a 1/e2 diameter of ~0.4 mm, as characterized by the knife-edge method. The laser power delivered to the sample was set between 1.5 and 4.0 W (2.4–6.4 kW/cm2)for the steady heating experiments with fixed exposures of 5 s. To study the effects of shorter pulses, another laser system with higher power and microsecond power control was utilized. It consists of a Synrad Firestar T100 RF excited CO2 laser operating at 10 kHz and 50 % duty cycle with a peak power of 110 W and an average power of 55 W and beam diameter of 100 μm (1/e2) as characterized by a Photon Inc. Nanoscan beam profiler. The laser passes through an acousto-optic modulator to modulate the power reaching the silica surface. The modulator was driven with a square pulse with prescribed lengths ranging from 10 μs to 5 s and amplitudes ranging from 0.5 to 55 W average power (12.7–1,400 kW/cm2). The ratio of the average power to pulse length was predetermined to yield an approximately 3 μm deep ablation pit. For pulse lengths above 100 μs, which is the period of the laser output, the silica was exposed to multiple cycles of the inherent power variation of the laser. In this regime, the exposure is quasi-CW, and the average power becomes relevant. For 10 and 50 μs pulse lengths, the output was centered on the peak of one cycle of the power variation. In this regime, the peak power is more relevant. For 10 μs pulse length, the maximum power was not enough to create a 3 μm deep pit in one shot, so 6 successive shots were used.
2.2 Gas treatment of the silica surface
The samples used in this study were Corning 7980 (type III) fused silica (50 mm diameter, 10 mm thick disks). They were fixed vertically on a stage in ambient air and treated by injecting reactive gases using a nozzle with a 3 mm opening in front. Sample surface spacing was kept fixed ~5 mm. The gas jet impinged normal to the surface plane and submerged the treated area well beyond the boundaries of the heated site by displacing the ambient air at the reacting surface before onset of laser heating. The laser beam passes through a transparent ZnSe window mounted on the backside of the nozzle and on to the surface through the nozzle front side opening. Compressed gas cylinders were used as the source for the following four dry gases (<1 ppmv H2O), with trace amounts of impurities (<3 ppmv):(1) dry air (78 % nitrogen, 21 % oxygen, 1 % trace gases), (2) 100 % nitrogen, (3) 5 % hydrogen, 95 % nitrogen, and (4) 5 % hydrogen, 95 % helium. A calibrated OMEGA gas flow controller (FMA 3400) allowed setting the volumetric flow rate to levels around 1 sccm/min. The flow was started at least a minute before laser exposure to insure that all the dead volume was removed from the lines and that surface gas submersion was at steady state.
2.3 Depth profile measurements and evaporation rates
As discussed elsewhere in details [5], the total depth of a pit created by laser heating at a given temperature and exposure length is a contribution from the amount of silica evaporated, the amount of melted silica displaced by flow, and the reduction in silica volume through densification. At temperatures approaching silica boiling point (≈3,000 K), pit depth is dominated by evaporation, while below the softening point of fused silica (1,853 K for Corning 7980), the depth is dominated by densification. To derive pitting and evaporation rates, the depths of the laser-induced pits were measured with a white light interferometer (Zygo Corp., Middlefield, CT, USA) to obtain a three-dimensional image of the silica surface. The maximum depth was measured from the profile with ≈1 nm accuracy and lateral resolution of ≈0.5 μm. Experimental depth ranged from 10 nm up to 40 μm depending on laser power, exposure time, and assist gas used for a particular site. Pitting rate was then calculated by taking the axial depth and dividing by the exposure time for the axial temperature measured.
2.4 Temperature measurement of the silica surface
During laser exposure, the sample surface temperature was derived from in situ measurements of the blackbody emission of the heated spot using a calibrated LN-cooled HgCdTe camera operating at 33 fps and equipped with a λ p = 8.9 μm narrow bandpass cold filter [21]. Although the optical resolution of the camera was ~40 μm (sampled at 40 μm spacing), it can be shown that the calculated temperature profile established by a 0.4 mm beam varies slowly over the length scales studied here. Thermal imaging in a narrow band of the far infrared (λ p ) spectrum is crucial because it probes the surface emission emanating exclusively from within ~1 μm of the outer surface of silica, which results in an accurate temperature measurement of the interface rather than a bulk average over a gradient [22]. For further details, the 2D surface temperature measurement method used in this study was validated and described elsewhere [21–23]. Under the CW conditions used in this study, near steady state temperatures were attained within ~0.2 s of laser exposure [21]. For short laser exposure experiments (t exp < 0.1 s), the IR camera frame rate was too slow, therefore, the peak temperature was roughly estimated using the pit depth, d, and the known laser-based fused silica evaporation rates measured recently and correlated with the following equation, A exp[−E evap/RT] [5, 24], such that T = (−E vap/R)/ln[(ρd/At exp)], where ρ is silica density, A = 6.25 × 103 μg/μm2/s, and E evap = 120.1 kcal/mol. Therefore, the temperatures reported for these short exposure experiments represent a temperature averaged over the course of the temperature rise during the pulse. However, these temperatures will tend to be more heavily weighted toward the highest temperature reached because of the exponential dependence of the evaporation rate and pit depth (d). For these pulse lengths <100 ms, the peak temperatures for both H2 and air gases were nearly identical to within ≈5 K, ranging from 3,250 up to 4,525 K for the shortest pulse of 10 μs.
3 Results and discussion
3.1 Modeling of hydrogen assisted silica evaporation
Since our measurements of silica evaporation rates discussed later were limited to 5 % H2 reactant feed concentration, we use a semi-empirical near-equilibrium model [5] to estimate an upper bound for the efficacy of H2 gas-etching under 100 % H2 atmosphere for steady heating conditions >1 s. We then extrapolate to the experimental rates for short exposure times where dynamic heating conditions prevail and where the system is likely far from equilibrium. Our previous modeling efforts of silica laser-based evaporation kinetics used an empirically derived correlation function to describe the mass transfer coefficient, h m , in the equation relating evaporation rates, R, with the equilibrium evaporation product concentration of silica [SiO]eq [5],
The mass transport coefficient from the equation below was obtained by fitting Eq. (1) to rate data for the parameters C = 2.49 × 10−3.07, m = 0.047, n = −4.02, as reported elsewhere using the same experimental model system and gases in this study [5].
Where Sh represents the Sherwood number, Sc is the Schmidt number (Sc = μ G /ρD), Re is the Reynolds number (Re = ρVL/μ G ), V is the nozzle volumetric flow rate, L is a characteristic length, D is SiO gas diffusivity in N2, ρ is the gas density from the ideal gas law, and μ G is the gas viscosity. Finally, the temperature dependent [SiO]eq(T) was derived from evaporation rate measurements with knowledge of the evaporation chemistry of SiO2 and equilibrium constants in air and in a H2/N2 gas mixture as described elsewhere [5]. Comparison of these evaporation rates calculations with experimental results is presented below.
3.2 Evaporation kinetics for transient laser heating
The gas–solid reaction dynamics was assessed by measurement of the relative evaporation rates of silica in air and in 5 % H2/N2 (\( R_{{{\text{H}}_{2} }} \)/R AIR) for laser exposures from 10 μs up to 5 s as shown in Fig. 2a. H2 reaction with silica is not apparent until the ≈100 ms exposure time is reached, at which point the relative rate rose continuously reaching a threefold increase for 5 s exposures due to the added evaporation reaction of H2 with SiO2. For these experiments, laser power levels were adjusted for each exposure such that approximately a fixed final depth was reached for each evaporation pit (d = 2.75 ± 0.25 μm), hence the trend of lowered temperatures for longer exposure times in Fig. 2b. In this manner, the amounts of material removed by evaporation were similar, and rim height differences more easily interpreted. The relative rate measurements are compared to model-based predictions described in Sect. 3.1, showing first that relative rates increase with pulse length and, second, that much greater silica evaporation rates are possible with 100 % H2 (for example, ≈tenfold increase at 3,000 K). Furthermore, the onset of the effect of H2 is predicted to shift to shorter exposure times ≈10 ms.
a Relative evaporation rates (\( R_{{{\text{H}}_{ 2} }} \)/R AIR) dependence on pulse length in 5 % H2/N2 gas and in air. Laser conditions were adjusted to produce fixed evaporation pit depths (2.75 ± 0.25 μm). The data (black curve) are compared to a model prediction of evaporation rates with 100 % H2 gas (red dashed curve). b Estimated axial temperatures are provided for the conditions used in (a) and (c). Also included in (b) is a comparison of the experimental silica evaporation fluxes with H2 reactant fluxes. The H2 fluxes were roughly calculated using Fick’s diffusion (\( J_{{{\text{H}}_{ 2} }} \) ≈ \( D_{{{\text{H}}_{ 2} }} \) \( C_{{{\text{BH}}_{ 2} }} \)/R 0) and bulk convection from volumetric flow rate, feed concentration of H2 (\( C_{{{\text{BH}}_{ 2} }} \)), and nozzle opening area. c The corresponding rim height measurements are shown for the rims that form at the periphery of the laser-induced evaporation pits for the same conditions as in (a) and (b). The red and black dashed curves in (c) are guides to the eye. d Corresponding experimental laser power used
The lack of H2 effect for short pulses is not surprising, considering that the process is known to be mass transport limited as discussed earlier. A quick estimate of the H2 feed flux based on bulk gas flow rate, H2 concentration, and nozzle size gives a bulk transport rate of ≈1.9 × 10−4 μg/μm2 s. The diffusive flux over the beam heated area R 0 = 0.4 mm diameter and 5 % molar bulk concentration, \( C_{{{\text{BH}}_{2} }} \), can be estimated from Fickian diffusion as \( D_{{{\text{H}}_{2} }} C_{{{\text{BH}}_{2} }} \)/R 0 = 1.0 × 10−6 μg/μm2 s (2.0 × 10−5 μg/μm2s for \( C_{{{\text{BH}}_{2} }} \) = 100 %), with \( D_{{{\text{H}}_{2} }} \) ≈ 10−4 m2/s. These limiting values can be compared to the typical experimental evaporation rates (Fig. 2b) based on pit depths of ≈ 6.0 × 10−5 μg/μm2 s for 100 ms exposure and ≈ 6.0 × 10−1 μg/μm2 s for a 10 μs pulse, which clearly indicates that the thermal decomposition removal rate of silica from laser heating is 1–5 order of magnitude larger than the H2 transport rate, hence the lack of apparent H2 effect in Fig. 1a. As these transport rates become comparable for longer exposures (≈4.4 × 10−6 μg/μm2 s for 5 s exposure), the relative removal rates increase accordingly and should also increase with \( C_{{{\text{BH}}_{2} }} \) concentration as predicted by the model. In addition, the increase in relative rates with pulse length is expected to plateau at longer pulse times >5 s (or lower temperatures), as the H2 reactant transport to the reaction zone becomes less and less limiting relative to the evaporation rates.
Although the gas-etch dynamics described in Fig. 2a are specific to the laser spot size, temperatures, and gas concentration in this study, they reveal that the spontaneous decomposition of silica is much faster than the reactant transport for short pulse, high temperature conditions, and will thus remain unaffected by gas chemistry even for highly reactive species until near steady heating low temperature conditions are reached.
3.3 Rim formation for transient laser heating
The question of whether shorter pulses could be used to reduce rim size, and whether solid–gas reactions can still play a role on these time scales was addressed next. Measurements of the rim height dependence on pulse length are shown in Fig. 2c. The data reveal a complex, but similar dependence for H2 and air gases, although H2 treated sites systematically produced smaller rims than those treated in air except for the lowest and highest exposures around 10 μs and 1 s, respectively. Notably, although H2 has no apparent effect on evaporation rates in the range between 0.1 and 100 ms, it maintains a significant effect on rim height over that same range, indicating that some solid–gas reactions and interactions do take place at the surface, sufficient to produce smaller rims. Since the temperature profiles were nearly identical in both cases given the similarities in depths and laser parameters used, the differences suggest that chemicapillary driven flow must also play a role by opposing the outward thermocapillary force, such that the net result is a smaller driving force for the rim formation in H2, \( |{\text{d}}\sigma /{\text{d}}T\left| {_{{{\text{H}}_{ 2} }} < } \right|{\text{d}}\sigma /{\text{d}}T|_{\text{AIR}}.\)Reaction of hydrogen with silica is known to produce a hydroxylated surface by a so-called tarnishing process [25], involving diffusion–reaction of H2 (SiO2(s) + H2(g)Φ-SiOH(s) + –SiH(s)). The surface tension is expected to be lowered by this tarnishing process for surfaces terminated with silanol (–OH) [26]. However, the OH spatial distribution during laser heating, interaction with the vapor phase, magnitude, and sign of \( {\text{d}}\sigma /{\text{d}}T_{{{\text{H}}_{ 2} }} \) remains uncertain [26]. Thus, interpretation of the rim height differences is not straightforward. Furthermore, one must also assume that most of the flowing melt has not reacted with H2 since OH in the silica network reduces viscosity by an order of magnitude over a few 100 ppm level changes [27], which would tend to facilitate flow and rim formation contrary to what was observed experimentally. In the case of H2 gas application, a lack of OH within the bulk is likely for the shortest pulse of 10 μs for which the expected penetration depth of H2, √4t exp \( D_{{{\text{SH}}_{ 2} }} \) = 200 nm with H2 bulk silica diffusivity, \( D_{{{\text{SH}}_{ 2} }} \) ≈ 10−5 cm2/s [28]. However, for a 1 s exposure, the expected depth is 63 μm, reaching well beyond the melted zone and maximum pit depth in this study, and the assumption of OH-free melt cannot hold.
Focusing now on the possible effects of recoil pressure-driven melt flow, we note that they can become significant for temperatures where the silica vapor pressure becomes comparable to the surface tension pressure, which is mostly invariant with temperature [12]. In this case, little differences in rim height are expected since the evaporated rates in 5 % H2 and air are nearly the same (Fig. 2a) up until the point where evaporation rate differences start to emerge for longer exposure times. However, these recoil-induced effects are expected to produce the opposite effect than what is observed experimentally. Indeed, larger rims should result with 5 % H2 compared to air since greater recoil pressure should accompany the larger evaporation rates produced with H2. The net effect with H2 should be to drive more melted material to the edge of the pit to form a rim, which is the opposite of what was observed. Thus, the results with H2 are counter intuitive, and more experimental work is needed to determine the fundamental properties of H2 treated surfaces under these intense laser heating conditions.
Also, the intricate pulse length behavior in Fig. 2c is still not clear and is currently being addressed for future publication, along with the possible gas chemistry effects noted above. Briefly, however, the dependence is likely related to the complex interplay between viscosity, heating rates and times, interfacial energies, and the peak temperatures applied. As the pulses get shorter, the laser power and temperature needed to produce the fixed target pit depth also increase from ≈3,000 up to 4,500 K (Fig. 2b). Viscosity and resistance to flow decrease exponentially as a result, yet the effective time allowed for flow is also reduced countering the effects of the lowered viscosity. The reversal in rim heights in Fig. 2c could reflect such antagonist tendencies. Likewise, for short pulses, the depth of the heat affected zone, which scales with thermal diffusion and exposure time, would reduce the melted pool available to form a rim and the size of the rim, which is consistent with the lowering of the rim trend observed for the shortest pulses and the highest laser power used (Fig. 2d).
3.4 Evaporation kinetics and rim formation for steady laser heating
After focusing on dynamic pulse length effects, measurements of rim heights, pit profiles, and temperature were made for near steady heating conditions using a fixed exposure of 5 s. For the beam parameter and experimental setup used, the surface temperature reaches a steady state after ≈1 s of CW exposure [5, 21, 22]. In Fig. 3a, a comparison of rim size dependence on pitting rates for 5 % H2/N2, 5 % H2/He, air, and 100 % N2 is shown. Laser heating in air produced the largest rims, followed by 100 % N2, 5 % H2/N2, and 5 % H2/He, in that order. This result is not surprising considering that greater evaporation rates are possible—at a given temperature—with reactive gases, such as H2, or for gases that shift the evaporation equilibrium forward, such as 100 % N2. Helium buffer gas mixture with H2 appears to have the added benefit of greater diffusivity, which increases the transport and evaporation rates. Thus, for equal etching rate and exposure time, gases that promote silica evaporation will have done so at a lower temperature, producing smaller rims. The lower temperatures increase silica viscosity and reduce its ability to flow by capillarity, especially since the temperature dependence of the surface tension is much weaker than that of the viscosity. This is illustrated in Fig. 3b showing a ≈200 K reduction in treatment temperature with 5 % H2 to obtain the same pit depth as that in air, yet with a much lower rim size from the corresponding spatial profiles. Selection of a more active etching gas therefore minimizes rim formation producing a more planar and less optically caustic surface.
a Comparison of measured rim heights produced for the given pitting rates in the presence of the gases indicated. Rims and pitting rates shown are all for steady laser heating conditions (5 s exposures). b The rim spatial profiles illustrate the effect of H2 treatment on rim structures compared to air. The pit profiles shown have the same depths, but different axial temperatures indicated by the vertical black arrows
The observation that differences in rim height are thermally driven for steady heating is further demonstrated in Fig. 4. The temperature dependence of the pit volumes formed (Fig. 4a) for a fixed exposure of 5 s is shown with the corresponding rim height data (Fig. 4b). For the temperature regime where evaporation dominates pit shape, the amount of material removed is strongly dependent on the gas used and increases with application of air, 100 % N2, 5 % H2/N2, and 5 % H2/He in that order for reasons already noted in Sect. 3.4 and consistent with previous measurements using a larger beam size of ≈1 mm [5]. However, in contrast with the apparent gas-related differences in Fig. 3a when the rim size data were given as a function of pitting rate, the same data points collapsed on a single line when plotted over 1/T for all gases, thus demonstrating that rim formation in the steady heating regime is a thermally activated process mostly independent of any solid–gas phase chemistry. The fitted activation energy (402 kJ/mol) closely matches that measured for the Corning 7980 glass (432 kJ/mol) used in this study, which can thus be attributed to the viscous flow activation energy of silica [27]. This result is consistent with the fact that rim formation occurs by viscous flow of the melt.
a Experimental laser-induced pit volumes and b rim height dependence on temperature and gas. Regions for the three surface shaping processes (evaporation, melting/flow, and densification) are delineated with the segmented arrows. The Arrhenius least square fit of the rim height data is shown (black line), with the value for viscous flow activation energy included (E a = 402 kJ/mol). c Spatial profile measurements illustrating the three surface shaping processes using the following conditions. Black curve, “evaporation” T = 2,110 K, with pit depth of 1.85 μm and the rim produced by melting and flow. Red curve, combined evaporation and densification T = 1,940 K, with pit depth of 0.242 μm. Orange curve “densification” T = 1,770 K, with pit depth of 0.053 μm. All laser exposures were fixed at 5 s for the data shown in (a), (b), and (c), with fixed beam diameter of 400 μm (1/e2)
At lower temperatures, evaporation, melting, and densification, all play a role in determining final surface. Pit volume differences persist, but with a weaker temperature dependence than for the evaporation dominated regime, which mostly reflects the temperature dependence of the equilibrium constant and reaction-free energy [5]. At still lower temperatures, below melting from 1,800 down to 1,450 K, the shape of the pit is dominated by silica densification since both evaporation and flow become negligible at these temperatures and over the 1 s exposure time scales. Under these low temperature conditions, gas chemistry seems to have a limited effect on pit volume since most of the pitting would occur through structural relaxation rather than chemical reactivity. The three processes affecting pit shape are illustrated in the Fig. 4c where the spatial profiles clearly show the combined effects of densification and evaporation at the intermediate temperature (red curve), the less complex shape of the densified region with a depth of 0.05 μm at the lowest temperature (orange curve), and the evaporation pit (depth = 1.85 μm) at the highest temperature with the rim protruding from melting and flow (black curve). The rimless pits mostly occur for heating conditions where both evaporation and flow are minimal. However, a more reactive gas should enable practical etching rates while avoiding rim formation, in particular for a material like fused silica with a very high softening temperature (T g = 1,853 K for Corning 7980). The transition points for the creation of rimless pits occur around T g as expected in the 1,820–2,000 K temperature range and appear to be gas dependent, with air being the first in the series to reach the rimless regime as the temperature is increased, followed closely by 5 % H2/He, 100 % N2, and 5 % H2/N2 (Fig. 4b). The reason for this apparent gas-specific shift in T g is not clear, but for the purpose of producing rimless laser-etched features at the sub-nanometer scale, air and 5 % H2/He are most effective, with 5 % H2/He having the advantage of higher etch rates. It is also expected that larger beam sizes would tend to produce smaller rims since from non-dimensional analysis of the governing equations, rim height scales as ~1/L 2, with L representing a characteristic spot size on the treated surface [29]. This particular approach has yet to be addressed experimentally, depending on whether larger treated areas are tolerable for the particular laser machining process.
4 Conclusions
Pitting rates and surface shape of CO2 laser-heated fused silica were measured for oxidizing, reducing, and neutral gas flow conditions over a range of dynamic and steady laser heating times from 10 μs up to 5 s. A reducing atmosphere produced the greatest etch rates, while minimizing rim size, followed by neutral and oxidizing atmospheres. The temperature and etch rate dependence of rim formation reveal that the surface tension driven viscous flow process is mostly thermally activated for steady, low temperature heating, with some possible added effect of gas reactivity-chemicapillarity apparent in the H2 exposure for the dynamic (short pulse) heating experiments. Over the range of laser exposure and power used, the rim height exhibited a complex fluctuating behavior, although H2 treatment produced systematically lower rims, even for <100 ms pulse length where H2 had no apparent effect on etch rates. The interplay of the temperature dependent material properties coupled to the reactive surface environment still has to be determined for a quantitative description of the differences in gas-induced rim formation, especially under dynamic thermal loading. This study suggests, however, that with highly reactive gas conditions, rimless etch pits or machined features can be produced under steady and transient heating. The only limitation being the mass transport rate of the reactants relative to that of the decomposition reaction for practical laser-based etching. The resulting more planar surface should reduce beam modulation and damage in optical materials used for high-power laser application. In general, the reactive gas approach would benefit applications where sub-nanometer “rimless” laser-induced features are required.
References
T.A. Laurence, J.D. Bude, S. Ly et al., Opt. Express 20, 11561 (2012)
I.L. Bass, V.G. Draggoo, G.M. Guss et al., High-Power Laser Ablation VI 6261, A2612 (2006)
I.L. Bass, G.M. Guss, M.J. Nostrand et al., Laser-Induced Damage in Optical Materials, Boulder, CO, USA, 2010
A.F.H. Kaplan, J. Appl. Phys. 79, 2198 (1996)
S. Elhadj, M.J. Matthews, S.T. Yang et al., Opt. Express 20, 1575 (2012)
H.L. Schick, Chem. Rev. 60, 331 (1960)
M.J. Matthews, I.L. Bass, G.M. Guss et al., Presented at the Laser-Induced Damage in Optical Materials Boulder, CO, USA, 2007 (unpublished)
C. Korner, R. Mayerhofer, M. Hartmann et al., Appl. Phys. A Matter. 63, 123 (1996)
V. Semak, A. Matsunawa, J. Phys. D Appl. Phys. 30, 2541 (1997)
V.V. Semak, B. Damkroger, S. Kempka, J. Phys. D Appl. Phys. 32, 1819 (1999)
N.M. Bulgakov, A.V. Bulgakova, Appl. Phys. A Matter. 73, 199 (2001)
W.D. Kingery, J. Am. Ceram. Soc. 42, 6 (1959)
N.M. Parikh, J. Am. Ceram. Soc. 41, 18 (1958)
S.C. Chen, D.G. Cahill, C.P. Grigoropoulos, J. Heat Trans. T ASME 122, 107 (2000)
T.J. Mcneil, R. Cole, R.S. Subramanian, J. Am. Ceram. Soc. 68, 254 (1985)
N. Shen, M.J. Matthews, J.E. Fair et al., Appl. Surf. Sci. 256, 4031 (2010)
R. Bruckner, J. Non-Cryst Solids 5, 123 (1970)
R. Brückner, J. Non-Cryst Solids 5, 123 (1970)
J.E. Shelby, J. Non-Cryst Solids 349, 331 (2004)
R.H. Doremus, J. Appl. Phys. 92, 7619 (2002)
S.T. Yang, M.J. Matthews, S. Elhadj et al., J. Appl. Phys. 106, 103106 (2009)
S. Elhadj, M.J. Matthews, S.T. Yang et al., Laser-Induced Damage in Optical Materials: Proceedings, Boulder, CO, 2009
S. Elhadj, M.J. Matthews, S.T. Yang et al., Appl. Phys. Lett. 96, 071110 (2010)
S. Elhadj, S.R. Qiu, A.M. Monterrosa et al., J. Appl. Phys. 111, 093113 (2012)
J.E. Shelby, J. Appl. Phys. 51, 2589 (1980)
G.A. Parks, J. Geophys. Res. 89, 3997 (1984)
V. Zandian, J.S. Florry, D. Taylor, Br. Ceram. Trans. J. 90, 59 (1991)
V. Lou, R. Sato, M. Tomozawa, J. Non-Cryst Solids 315, 13 (2003)
A. Ben-Yakar, A. Harkin, J. Ashmore et al., J. Phys. D Appl. Phys. 40, 1447 (2007)
Acknowledgments
This work performed under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under Contract No. DE-AC52-07NA27344.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elhadj, S., Matthews, M.J., Guss, G.M. et al. Laser-based dynamic evaporation and surface shaping of fused silica with assist gases: a path to rimless laser machining. Appl. Phys. B 113, 307–315 (2013). https://doi.org/10.1007/s00340-013-5481-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-013-5481-7