Abstract
A portable instrument based on two-color laser-induced incandescence (LII) technique has been designed and developed for the detection of carbonaceous particles for environmental applications. The instrument has been calibrated by performing LII measurements at the exhaust of a home-made soot generator. The incandescence signal from particles sampled into the instrument has been compared with in situ, calibrated, LII measurements to correlate the incandescence signal by the instrument with particles concentration. Measurements of particulate with the LII instrument were then conducted in different environmental conditions, covering a wide range of concentration (from ambient air to cars’ exhaust). The detection limit of the LII instrument has been estimated to be in the range of 200 ng/m3. These measurements have been also compared with results obtained with a commercial aethalometer. The results show a linear relationship between the two sets of measurements, also in the case where significant variation of the carbon particles concentration has been observed over time. These observations allow us to infer that the two instruments are responding in the same way to different carbon particles load, size and nature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The study of combustion-generated particulate matter has received particular attention by the research community for different aspects such as the global climate change [1, 2], environmental pollution and the effects on human health [3]. Carbonaceous particles emitted from engines up to power generation facilities are responsible for a loss of useful energy and for air pollution. Freshly emitted particles are composed of fractal hydrophobic aggregates of carbonaceous primary particles with diameter in the range of ~20–40 nm [4]. Fractal cores are often coated with aliphatic organic compounds as well as polycyclic aromatic hydrocarbons [5, 6]. In polluted urban air with increasing residence time on a time scale of ~12 h, carbonaceous particulates acquire additional coating through deposition of semi-volatile atmospheric species or coagulation with pre-existing particles. Several definitions of atmospheric particles, such as soot carbon, black carbon (BC), elemental carbon (EC), brown carbon and light absorbing carbon (LAC), are reported in the literature [7]. These classes of particles are not universally recognized and the distinction between them is not clear.
The need to measure, characterize and monitor these particles emission triggered the interest for the development of advanced diagnostic techniques based either on their thermal or optical properties. These two classes of techniques do not necessarily yield the same results. Several studies have been performed in the recent years for comparing the results of different instruments [8, 9].
Common techniques for measuring aerosols through their optical properties mainly include: cavity ring-down technique [10], nephelometry for aerosol scattering [11] and real-time filter-based methods such as the aethalometer [12] and the multi-angle absorption photometer (MAAP) [13].
Laser-Induced incandescence (LII) technique is also a powerful technique capable of measuring concentration and size of carbonaceous particles. A Single Particle Soot Photometer (SP2) (Droplet Measurement Technology, Inc., Boulder, CO, USA) has been developed for high sensitivity BC mass concentration measurements in the environment [14–16]. The principle of the SP2 instrument consists in measuring the time-dependent scattering and two-color incandescence signals from individual aerosol particles passing through a continuous-wave, intra-cavity Nd:YAG laser beam. The minimum BC detection limit is around 10 ng/m3 with individual particles in the range of 70–500 nm mass-equivalent diameter and a counting rate of 0–8,000 particles/cm3. Being based on an optical technique, the response of the instrument depends, in part, on the refractive index and shape of the particles and needs a careful calibration to accurately determine the BC mass [17, 18]. The instrument is able to measure the BC content inside individual particles without interference from internally mixed semi-volatile materials or mineral dust particles.
Pulsed LII is a widely used technique for soot measurements in harsh environments such as flames and combustion systems [19, 20]. Many papers can be found in the literature on the development and application of this technique, but an exhaustive analysis of the potential of this diagnostic is far from being reached [21]. Differently, from the SP2 instrument in which single soot particles are heated on a μsec time scale flowing through a cw laser beam, pulsed LII technique implies a rapid heating of particles during a single pulse of the laser of about 10 ns. Many studies have been carried out to better understand and to model the processes of laser heating, possible vaporization and cooling, especially for soot particle size determination [22–25]. Experimentally, a two-color LII technique together with an absolute optical calibration of the detection system is used for absolute soot volume fraction measurements without the need of scaling with other techniques [26, 27]. In contrast to the SP2 instrument, the pulsed LII technique is able to obtain in situ absolute soot measurements in high soot concentration environments without the need of using a probe and a dilution system.
Instruments have been also developed and used in different experimental situations [28–30]. In particular, the LII300 (Artium Technologies Inc., CA, USA) is able to measure soot concentration from 2 μg/m3 up to 20 g/m3 with primary particle size in the range of 10–100 nm. Therefore, the pulsed LII technique exhibits a high dynamic range and can be used for the evaluation of carbonaceous particles content both at the exhaust of engines (2–200 mg/m3, depending on the engine operating conditions [31]) and in the environment (less than 50 μg/m3).
Aim of this work is to further investigate the capability of the pulsed LII technique to be applied for environmental applications such as ambient air quality and source emission monitoring. Based on previous work [32] and on the use of an integrating sphere, we have recently developed an experimental arrangement for high sensitivity carbonaceous particles measurements (Italian Patent ITRM20090617). Pulsed LII measurements have been performed in different experimental conditions and compared with the ones derived using a commercial aethalometer. The results confirm the possibility of using pulsed LII technique for environmental measurements.
2 Experimental
2.1 Laser-induced incandescence apparatus
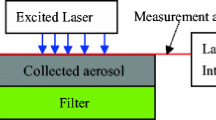
In this section, the main features of the implemented instrument are presented and the apparatus is shown in Fig. 1. Soot particles are sampled using a stainless steel sucking probe, driven by a Dynamic Dilution Sampler (DDS, TCR Tecora, Milan, Italy) and sent in a test cell, which is essentially a Pyrex tubelet (I.D. = 6.7 mm, O.D. = 9 mm, 10 cm long) with two quartz windows at either ends. The IR beam of a Nd:YAG laser (Quantel, Big-Sky, CFR 400, 10 Hz) is properly aligned within the tubelet. The beam intensity distribution at the center of the tubelet (36 cm from the laser exit, near field) is checked to be a top-hat using a Gentec Beamage Focus I. Image of the beam intensity is reported in the inset in Fig. 1. Similar light distributions were obtained by moving the detector ± 2.5 cm from the center of the tubelet. The laser fluence is set to about 350 mJ/cm2 in the saturation regime [27]. At this fluence, sublimation is mostly present in the first part of the laser pulse affecting the decay rate but not the peak intensity. To avoid scattering from the tubelet walls, a diaphragm (4 mm diameter) is used to limit the laser beam diameter. The test cell is placed inside an integrating sphere (Sphere Optics Hoffman LLC, Contoocook, NH, USA). The integrating sphere has the aim of collecting the largest part of the signal generated inside the tubelet and emitted by the particles in every direction. Besides the two circular apertures used for allocating the tubelet, the sphere is provided with a further circular aperture placed perpendicular to the tubelet axis. This aperture is used to allocate the receiving optics for LII signal collection.
The overall LII signal is detected through a system of optical blocks (Hamamatsu) containing a short pass filter (CVI, λ < 850 nm), a dichroic mirror (660 nm) and two arms. Each arm consists of a band-pass filter and a PMT module (Hamamatsu H5783-20) powered by a power supply (Hamamatsu C7169). The two band-pass filters are centered at 530 nm (40 nm FWHM) and at 700 nm (60 nm FWHM). The choice of the filter widths (FWHM) is a compromise between a good sensitivity and a negligible effect of the effective central wavelength [26] on the temperature evaluation.
A fast digital oscilloscope (Agilent, 1 GHz, 4Gs/s), triggered by the laser Q-switch pulse is used for data acquisition and storage. The two-color LII time-resolved curves are obtained with an average of about 500 acquisitions and processed using a MATHCAD program. In order to evaluate the “prompt” signal value, the average over a 4-ns interval (1 ns before and 3 ns after) around the maximum of the LII curve is performed [27, 33].
As it will be extensively discussed in the results section, measurements are carried out using a sampling flow rate of 1 Nl/min and a repetition rate of 5 Hz.
2.2 LII calibration procedure
In order to calculate the soot concentration, a calibration procedure is required. Due to the difficult access to the probe volume inside the cylindrical test cell, the standard calibration procedure with a calibrated lamp cannot be performed. Therefore, a different procedure is implemented. The calibration is carried out at the exhaust of a home-made soot generator by comparing LII measurements of soot sampled into the instruments with in situ LII measurements, which are properly calibrated by means of a calibrated tungsten ribbon lamp. The home-made soot generator consists of a quenched diffusion flame, fed with ethylene or methane depending on the soot load needed. Around the flame at a certain height, an annular duct with small holes is positioned, so that, a nitrogen flow is able to quench the flame. In order to change the soot load, other than using different fuel, the position of the N2 quenching flow with respect to the flame or, alternatively, the fuel flow rate can be changed. The soot produced is then confined in a cylindrical chimney.
Concerning the LII instrument, the sampling probe has been positioned immediately above the probe volume of the in situ measurements. In order to condense the water vapor coming from the combustion gases, an ice trap is placed upstream of the sampling line.
As for in situ LII, the IR beam of a Nd:YAG laser (Quanta System, SYL 202) is used and properly aligned by imaging a uniformly illuminated small aperture (ϕ = 4 mm) on the probe volume. To obtain particle concentration, one-color incandescence signal is sufficient, once the incandescence temperature has been verified to be at the sublimation value in the plateau laser fluence regime [27]. In this work, the temperature has been checked to be 4,000 ± 50 K, as obtained using the E(m) values of [34] in consistency with our previous measurements [32]. The influence of the choice of E(m) is discussed later in the paper. The incandescence signal is collected with an achromatic lens on a 1.8 mm diameter diaphragm positioned just before a photosensor (Hamamatsu H5783-03) coupled with an interference filter (400 nm wavelength, 70 nm FWHM). The time-resolved LII signal is acquired and stored with a fast digital oscilloscope (Tektronix, 1 GHz, 5 Gs/sec) triggered by the laser. The laser is running at 6 Hz and the LII curve integrated over 500 acquisitions. This allows us to perform in situ measurements simultaneously with the LII instrument and over the same integration time.
Replacing the soot generator with the calibrated lamp, the emission radiation is detected in the same experimental conditions. The processing calibration procedure to derive soot concentration is then carried out as widely described in the literature [27]. Plotting the soot concentration measured with the in situ LII versus the corresponding prompt incandescence signal collected with the LII instrument at 530 nm wavelength, the instrument calibration curve can be derived.
In Fig. 2, the calibration curve is shown together with the corresponding linear fitting. A linear curve passing through the origin of the axes is obtained and the relative standard error of the slope is 2.5 %.
2.3 Micro-aethalometer
In order to validate LII measurements, real-time concentration measurements of optically absorbing aerosol particles have been also carried out using a commercial aethalometer (microAeth AE51, MAGEE Scientific Co.; Berkeley, USA). The basic principle of this instrument is the continuous measurement of the attenuation of a LED source light at 880 nm wavelength through a particle-loaded Teflon-coated glass fiber filter [35]. The filter is blackened over time with the aerosol picked up inside the instrument at a controlled flow. Each measurement results from an average over a time interval ranging from 1 s to 5 min. At a constant air flow rate Q, the deposition rate of particulate on the filter is proportional to its mass concentration rate and the corresponding rate of increase of the optical attenuation ATN is given by
where I o is the transmittance of a blank filter and I the transmittance of a particle-loaded filter. The factor of 100 is introduced for numerical convenience. The black carbon mass loading (ng/m3) can be obtained according to the following relationship:
where A is the sample spot area (3 mm diameter), ΔATN indicates the ATN variation during the time period Δt and σ ATN is the apparent mass attenuation cross-section for the black carbon collected on the glass fiber filter. This parameter is essentially related to physical properties of the particles such as density, radius and size distribution as well as the optical function of the complex refractive index [36]. The sigma value depends on the wavelength of the light source used. In our case, the value of σ ATN is provided by the manufacturer to be 12.5 m2/g.
In our case, the air sample flow rate is kept at 0.1 Nl/min. In order to keep the loading of the filter within the limits specified by the manufacturer, as soon as the ATN reaches a certain value, a manual change of the filter is performed. In this way, a kind of saturation effect in the detection of the filter blackening, with a consequent underestimation of the concentration, is avoided.
The aethalometer is mainly sensitive to solid (non-volatile, or “black”) carbon, even if the measurement of the concentration can be affected by the presence in the sample of other materials which may also prevent light from reaching the sensor. In fact, light attenuation could depends on interactions between particle morphology, nature of the deposit on the filter, and scattering and absorption of light by non carbonaceous materials, which can be responsible in this case for an overestimation of the concentration. Consequently, care has to be taken in the interpretation of the concentration data provided by the instrument.
Another important aspect to take into account in these measurements is the limitation obtained in the case of high time resolution and, at the same time, low particles load. In fact, at short time interval ΔATN can be sufficiently small to be significantly influenced by the measurement noise. To overcome this aspect, an algorithm has been developed by [37] and applied in our measurements where needed.
3 Results and discussion
A preliminary investigation concerning the choice of the sampling flow rate at different laser frequencies is carried out for the LII instrument. This analysis is dictated by the particular geometry of the sampling test cell, in agreement with the work reported in [32]. In fact, changing the sampling flow rate changes the corresponding average residence time of the particles in the cell as well, resulting in a different number of pulses hitting the probe volume. In the case under study, the 10-cm-long test cell is positioned inside the integrating sphere having a 5 cm diameter. Therefore, the integral incandescence signal comes from all the particles receiving different number of laser pulses depending on the position reached inside the sphere.
In order to investigate the effect of multiple laser pulses hitting soot on the incandescence signal, two-color incandescence measurements are carried out in different condition of flow rate and laser beam frequency. In Fig. 3, the LII signal detected at 530 nm is reported versus the sampling flow rate at 2, 5 and 10 Hz laser frequency, respectively. Similar curves (not reported here) are obtained for LII signal detected at 700 nm.
As for the measurements at 2 Hz, a slight decrease of the LII signal is observed moving toward low value of the sampling flow rate. This result is consistent with the fact that decreasing the flow rate soot particles in the probe volume is hit by more than one laser pulse. Such condition can account, especially in the laser fluence regime used, for particles sublimation or modification, which in turn results in an incandescence signal reduction. This effect is more significant at higher values of the laser frequency, and particularly at 10 Hz. For this reason, in this work the measurements are carried out at 5 Hz and 1 Nl/min sampling flow rate.
The LII instrument has been tested in different environmental conditions and in conjunction with measurements performed simultaneously using the aethalometer. Different ambient air (office, laboratory and parking lot) as well as the exhaust of the home-made soot generator and of gasoline- and diesel-fuelled cars have been monitored, collected and measured to cover a range of carbonaceous particles load as wide as possible. Both measurements were carried out with almost the same integration time, which was fixed at 1 min for the aethalometer and at 51 s for the LII instruments. In this last case, in fact, the integration time results from an average over 256 acquisitions, with the laser beam running at 5 Hz.
The sampling probes of the two instruments have been placed in an adjacent position. An exception has been made for the measurements at the exhaust of the soot generator. Due to the presence of a high temperature of the combustion gas flow, and consequently the need to introduce an ice trap, the sampling procedure has been performed as follows. The gas flow after the ice trap is sucked in a measuring cell and then sampled by the two instrument probes. It is important to stress that being the aethalometer an instrument for air monitoring, concentration on the order of μg/m3 has to be produced to avoid the aethalometer filter saturation. Consequently, in this work, the soot generator has been fed with methane and the fuel flow rate has been properly varied.
In Fig. 4, measurements performed in different environmental conditions, such as an office (a), a parking lot (b), the exhaust of gasoline- (c) and diesel-fuelled (d) cars are reported versus time. The prompt of the incandescence signal detected at 530 nm is presented on the left y-axis, while the right y-axis refers to the concentration measurements performed with the aethalometer and properly scaled. The aethalometer measurements have been collected every minute while the incandescence signal intensity has been detected every 3 min. Concerning Fig. 4a (office), an almost constant behavior over time is observed, while for the other cases a significant change in the concentration allows us to verify the linear correlation of the two sets of measurements.
The significant decrease of the concentration over time observed in the case of the measurements at the exhaust of the gasoline-fuelled car (Euro3) is due to the heat-up transient during the start-up regime. The agreement in the trend of the two sets of measurements is emphasized in Fig. 4d (diesel-fuelled car, Euro 4, without diesel particulate filter DPF) where a strong fluctuation of the concentration is registered.
It is interesting to observe that in Fig. 4b (parking lot), the peak detected by the aethalometer is due to cigarette smoke and is not registered by the LII instruments. Cigarette smoke is essentially a liquid-like aerosol of a low molecular weight and high hydrogen content. Being almost gas phase it is not detected by the incandescence. Such observation allows to stress that the concentration measurements obtained with the aethalometer, which are derived from extinction measurements, can be overestimated due to the contribution of the scattering signal.
In order to evaluate the accuracy of the incandescence measurements, the set of data shown in Fig. 4a with an almost constant concentration is considered. The concentration relative standard error results to be 1.6 %.
In Fig. 5, the ratio of the incandescence signals at 700 and 530 nm is shown versus time for different environments. This is an important parameter as the value of the ratio of the incandescence signal at two wavelengths is strictly related to the incandescence temperature and consequently to the optical absorption properties of the particles under investigation. As it is evident from the figure, the results obtained in the case of air monitoring present almost the same value. On the contrary, a slight decrease (about 3.9 %) is observed for measurements performed at the exhaust of the diesel-fuelled car and the soot generator. The relative standard error of the incandescence signals ratio is about 2 %, as obtained applying the error propagation theory. Then, the slight difference, being higher than the error bars, can be considered related to some difference in the nature of the particles investigated [14]. More work is needed to better understand this aspect.
Taking into account the calibration, in Fig. 6 the particles concentration measured with the LII instrument is reported versus the values obtained from the aethalometer for all the environments investigated. As a wide concentration range is considered, a logarithmic scale is used for both axes. It is interesting to observe that the data referring to the air monitoring are in the same range of values, while a wide spread is registered for measurements collected at the exhaust of the gasoline-fuelled car compared to the diesel-fuelled one (without DPF). The relative standard error of about 7 % is estimated for the concentration measured with the LII instrument, starting from the relative standard error of the LII signal and of the signal ratio [27]. In the figure, the slope and the R 2 values are reported. From the statistical analysis, the relative standard error of the slope is 0.39 %. A good linear correlation is obtained for the two sets of measurements.
Directly comparing the particulate concentrations measured with the two instruments, the values obtained with LII instrument are about 25 % higher than the aethalometer ones for every environmental condition tested. Taking into account that the two techniques are based on different phenomena, such difference can be considered acceptable.
In fact, as already observed in the Sect. 2.3, the aethalometer calibration process depends upon the knowledge of the specific optical attenuation cross-section (sigma value). Since the sigma value is a function of the aerosol type, the use of the same sigma value to calculate the BC concentration in all the environments under study could introduce error in the estimation of the real concentration value. For example, considering a sigma of 12.5 m2/g (used in this work) or 16.6 m2/g, as widely used by other authors [38, 39], a 30 % difference in the particles concentration can be estimated. Nevertheless, the good agreement between the two sets of data allows us to infer that the two instruments are sensitive to the same class of carbonaceous particles.
On the other hand, as noticed before the absolute LII signal is influenced by the choice of E(m) and the calibration curve (Fig. 2). Concerning E(m), the values used in this work are consistent with our previous papers and fall in the average of the values reported in the literature [33, 40]. Recently, Bond et al. [41], Snelling et al. [22] and Cleon et al. [42] suggest that a value of E(m) = 0.39 for the visible spectral range should be used. With this value, the absolute volume fraction decreases about 28 %, approaching the values obtained with the aethalometer. However, it is not the aim of this work to evaluate the soot optical properties and consequently to determine exactly the absolute soot concentration in the environment. More work is needed to assess this topic.
Starting from the intensity of the LII signals, the limit of the detection of the developed LII instrument can be estimated to be in the range of 200 ng/m3, which is sufficient for most of the environmental applications. Unfortunately, we were not able to test the instrument in this range due to the lack of such a “clean” environment. By increasing the band pass of the filters and enlarging the laser beam diameter, even higher sensitivity can be reached.
The time decay of the LII signal, giving information on the size of the primary particle forming an aggregate [21], has not been analyzed, since probably not fundamental for environmental application. Nevertheless, it would be interesting to couple the two-color LII technique with elastic light scattering measurements, as already implemented in the SP2 instrument and by Snelling et al. [43], to derive the soot aggregate size.
4 Conclusions
In this work, a new instrument for carbonaceous particle detection and concentration measurement is presented. Details of the implemented apparatus and the calibration procedure performed are described. The instrument, which is based on the two-color LII technique, is able to detect particle concentration with a considerable wide dynamic range, from 200 ng/m3, measured in the case of air monitoring up to heavy concentration detected at the exhaust of combustion systems. In order to validate the LII instrument, comparison with concentration measurements carried out using a commercial aethalometer is performed. Different environmental conditions, such as the office, the parking lot, the exhaust of the soot generator and of gasoline and diesel-fuelled cars, have been monitored to have a range of carbonaceous particles load as wide as possible. A good agreement in both the fluctuations over time and the absolute value of the concentration is obtained, which confirms the applicability and reliability of the LII instrument in a wide range of applications. Moreover, the monitoring of the LII intensities at the two wavelengths could be implemented to discriminate between carbonaceous particles coming from different sources.
References
S.E. Schwartz, P.R. Buseck, Science 288, 989 (2000)
V. Ramanathan, G. Carmichael, Nature GeoSci. 1, 221 (2008)
J.S. Lighty, J.M. Veranth, A.F. Sarofim, J. Air Waste Manag. Assoc. 50, 1565 (2000)
R.K. Chakrabarty, H. Moosmuller, M.A. Garro, G.X. Tian, J.G. Slowik, E.S. Cross, J.-H. Han, P. Davidovits, T.B. Onasch, D.R. Worsnop, Phys. Rev. Lett. 102, 1 (2009)
H. Sakurai, H.J. Tobias, K. Park, D. Zarling, S. Docherty, D.B. Kittelson, P.H. McMurry, P.J. Ziemann, Atmos. Environ. 37, 1199 (2003)
L.C. Marr, L.A. Grogan, H. Wohrnschimmel, L.T. Molina, M.J. Molina, T.J. Smith, E. Garshick, Environ. Sci. Technol. 38, 2584 (2004)
T.C. Bond, R.W. Bergstrom, Aerosol Sci. Technol. 40, 27 (2006)
J.G. Slowik, E.S. Cross, J.-H. Han, P. Davidovits, T.B. Onasch, J.T. Jayne, L.R. Williams, M.R. Canagaratna, D.R. Worsnop, R.K. Chakrabarty, H. Moosmuller, W.P. Arnott, J.P. Schwarz, R.-S. Gao, D.W. Fahey, G.L. Kok, A. Petzold, Aerosol Sci. Technol. 41, 295 (2007)
Y. Kondo, L. Sahu, N. Moteki, F. Khan, N. Takegawa, X. Liu, M. Koike, T. Miyakawa, Aerosol Sci. Technol. 45, 295 (2011)
J.D. Smith, D.B. Atkinson, Analyst 126, 1216 (2001)
J. Heintzenberg, R.J. Charlson, J. Atmos. Ocean. Technol. 13, 987 (1996)
A.D.A. Hansen, H. Rosen, T. Novakov, Sci. Total. Environ. 36, 191 (1984)
A. Petzold, M. Schonlinner, J. Aerosol Sci. 35, 421 (2004)
M. Stephens, N. Turner, J. Sandberg, Appl. Opt. 42, 3726 (2003)
N. Moteki, Y. Kondo, Aerosol Sci. Technol. 41, 398 (2007)
N. Moteki, Y. Kondo, Aerosol Sci. 39, 348 (2008)
N. Moteki, Y. Kindo, Aerosol Sci. Technol. 44, 663 (2010)
G.R. McMeeking, N. Good, M.D. Petters, G. McFiggans, H. Coe, Atmos. Chem. Phys. 11, 5099 (2011)
M. Kohler, K.P. Geigle, W. Meier, B.M. Crosland, K.A. Thomson, G.J. Smallwood, Appl. Phys. B 104, 409 (2011)
D. Herter, R. Stirn, S. Arndt, R. Grzeszik, A. Dreizler, Appl. Phys. B 104, 399 (2011)
C. Schulz, B.F. Kock, M. Hoffmann, M. Michelsen, S. Will, B. Bougie, R. Suntz, G. Smallwood, Appl. Phys. B 83, 333 (2006)
D.R. Snelling, F. Liu, G.J. Smallwood, O.L. Gulder, Comb. Flame 136, 180 (2004)
H.A. Michelsen, F. Liu, B.F. Kock, H. Bladh, A. Boiarciuc, M. Charwath, T. Dreier, R. Hadef, M. Hofmann, J. Reimann, S. Will, P.-E. Bengtsson, H. Bockhorn, F. Foucher, K.-P. Geigle, C. Mounaim-Rouselle, C. Schulz, R. Stirn, B. Tribalet, R. Suntz, Appl. Phys. B 87, 503 (2007)
H.A. Michelsen, M.A. Linne, B.F. Kock, M. Hofmann, B. Tribalet, C. Schulz, Appl. Phys. B 93, 645 (2008)
F. Liu, G.J. Smallwood, J. Quant. Spectrosc. Radiat. Transf. 111, 302 (2010)
D.R. Snelling, G.J. Smallwood, F. Liu, O.L. Gulder, W.D. Bachalo, Appl. Opt. 44, 6773 (2005)
S. De Iuliis, F. Cignoli, G. Zizak, Appl. Opt. 44, 7414 (2005)
S. Dankers, A. Leipertz, S. Will, J. Arndt, K. Vogel, S. Schraml, A. Hemm, Chem. Eng. Technol. 26, 966 (2003)
P.O. Witze, G.A. Payne, W.D. Bachalo, G.J. Smallwood, SAE International, 04ANNUAL-174 (2004)
J. Liggio, M. Gordon, G. Smallwood, S.-M. Li, C. Stroud, R. Staebler, G. Lu, P. Lee, B. Taylor, J.R. Brook, Environ. Sci. Technol. 46, 4819 (2012)
A. Neer, U.O. Koylu, Combust. Flame 146, 142 (2006)
S. De Iuliis, F. Cignoli, S. Maffi, G. Zizak, Appl. Phys. B 104, 321 (2011)
S. De Iuliis, F. Migliorini, F. Cignoli, G. Zizak, Appl. Phys. B 83, 397 (2006)
H. Chang, T.T. Charalampopoulos, Proc. R. Soc. Lond. Ser. A. 430, 577 (1990)
L. Ferrero, G. Mocnik, B.S. Ferrini, M.G. Perrone, G. Sangiorgi, E. Bolzacchini, Sci. Total. Environ. 409, 2824 (2011)
C. Liousse, H. Cachier, S.G. Jennings, Atmos. Environ. 27A, 1203 (1993)
G.S.W. Hagler, T.L.B. Yelverton, R. Vedantham, A.D.A. Hansen, J.R. Turner, Aerosol Air Qual. Res. 11, 539 (2011)
I. P. Ng, H. Ma, D. Kittelson, A. Miller, SAE technical paper 2007-01-0334 (2007)
K. Park, J.C. Chow, J.G. Watson, D.L. Trimble, P. Doraiswamy, K. Park, W.P. Arnott, K.R. Stroud, K. Bowers, R. Bode, A. Petzold, A.D.A. Hansen, J. Air Waste Manag. Assoc. 56, 474 (2006)
K.C. Smyth, C.R. Shaddix, Combust. Flame 107, 314 (1996)
T.C. Bond, R.W. Bergstrom, Aerosol Sci. Tech. 40, 27 (2006)
G. Cleon, T. Amodeo, A. Faccinetto, P. Desgroux, Appl. Phys. B 140, 297 (2011)
D.R. Snelling, O. Link, K.A. Thomson, G.J. Smallwood, Appl. Phys. B 104, 385 (2011)
Acknowledgments
The authors would like to acknowledge the financial support for this project from the Ministero dello Sviluppo Economico (MSE) and the technical assistance of Mr. Enio Fantin.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Migliorini, F., De Iuliis, S., Maffi, S. et al. Environmental application of pulsed laser-induced incandescence. Appl. Phys. B 112, 433–440 (2013). https://doi.org/10.1007/s00340-013-5385-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-013-5385-6