Abstract
The detection of multiple species using a single laser and single detector employing multi-mode absorption spectroscopy (MUMAS) is reported. An in-house constructed, diode-pumped, Er:Yb:glass micro-laser operating at 1,565 nm with 10 modes separated by 18 GHz was used to record MUMAS signals in a gas mixture containing C2H2, N2O and CO. The components of the mixture were detected simultaneously by identifying multiple transitions in each of the species. By using temperature- and pressure-dependent modelled spectral fits to the data, partial pressures of each species in the mixture were determined with an uncertainty of ±2 %.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Optical methods for remote sensing of gases in atmospheric, industrial or combustion environments have been greatly enhanced by tunable diode laser absorption spectroscopy (TDLAS). The narrow linewidth of single-mode diode lasers provides high spectral resolution and thus species selective detection by measurement of the absorption at an isolated molecular resonance. The restricted, mode-hop free, tuning range of these lasers, however, limits the technique to detection of a single species since only a single absorption line can be detected. A major goal of gas sensing is to find a method that combines the high species selectivity of TDLAS with a broad spectral range to detect multiple species and which can be implemented using a simple, robust, compact and inexpensive optical system. Spectroscopic detection of multiple species, or multiple transitions of a single species, requires a device providing both high spectral resolution and spectral coverage wide enough to encompass multiple absorption features.
Extension of single-line absorption methods, such as TDLAS, to multiple species usually involves multiplexing several diode lasers and detectors, one for each transition in each species [1, 2]. It has been shown that, in some cases, a single multi-section diode laser and integrated optical devices based on DFB lasers, originally developed for telecommunications, can be used for multi-species detection [3, 4]. Light sources using frequency chirping of propagating light in optical fibres to provide wavelength agility to cover a wide spectral range have been developed and applied to gas sensing [5, 6]. Another approach is to use light sources with broad spectral bandwidth such as super-luminescent light emitting diodes (SLEDs) [7], super-continuum sources based on self-phase modulation in optical fibres [8] or wide-bandwidth frequency combs produced by mode-locked lasers [9]. These methods suffer from the disadvantages of requiring some kind of wavelength dispersion, which limits the resolution and may also involve large, complex and expensive laser systems making them unsuitable for field use or applications in hostile environments.
An alternative method based on gas correlation spectroscopy has been reported that offers the advantage of simplicity and relatively low cost [10, 11]. The technique employs a single, multi-mode, diode laser in which temporal correlations of fluctuating intensities are used to identify absorptions of specific modes that occur simultaneously in the probed gas and in a reference cell containing the gas being detected. The disadvantage is that a separate reference cell and detector system is needed for each target gas.
Another technique, also using multi-mode lasers, multi-mode absorption spectroscopy (MUMAS) has been introduced that achieves, simultaneously, wide spectral coverage and high spectral resolution [12–14]. By using a single short cavity, multi-mode, laser and scanning each mode over only the short frequency interval separating the modes, a spectral range may be covered that is determined by the overall emission bandwidth of the laser. Absorption that occurs when any of the individual modes comes into resonance with a molecular transition results in a reduction in the total transmitted intensity recorded by the detector. Thus, each segment of the spectral range covered by the laser is probed, at high resolution, by each of the individual modes as it scans across the frequency interval separating the modes. The resulting signal produced by n modes is effectively a super-position of n single-mode spectra and provides, for a given laser, a unique signature for absorption by a particular species or mixture of species. Thus, MUMAS achieves high spectral resolution and coverage of multiple transitions or species using only a single laser and single detector and needs no multiplexing and de-multiplexing, spectral dispersion, complex or expensive laser sources nor reference cells. The principle of the method is similar to a device employed by Svanberg and co-workers who used the transmission modes of a scanning Fabry–Perot interferometer to effectively narrow the spectrum of a pulsed dye laser for high resolution spectroscopy of the hyperfine structure of rubidium and ytterbium [15]. The interferometer in this case was external to the pulsed dye laser and was scanned over several free spectral ranges to aid in identifying the spectral features and to provide a frequency scale for the resulting spectra.
In this paper we report the first observation of multi-species detection using MUMAS in which acetylene, nitrous oxide and carbon monoxide are simultaneously detected in a mixture of all three gases.
2 Multi-mode absorption spectroscopy
The technical details of MUMAS and the signal interpretation have been presented previously and are outlined here only briefly for convenience [13, 14]. The MUMAS signature, consisting as it does of a super-position of a set of n-individual absorption spectra corresponding to that produced by each mode, can be modelled accurately provided the form of the laser mode spectrum is known and the spectra of the absorbing species are also known. Specifically, the key parameters of the laser spectrum are the mode spacing, Δν m, the individual mode line-width, δν m, the distribution of power across the modes, i.e. the intensity envelope of the mode spectrum, I(ν m) and hence the spectral range covered, Δν S, and, finally, the spectral location of the modes, ν m. The spectral location can be measured or determined more accurately using a cross-correlation of the estimated mode spectrum with the known spectrum of a particular absorbing species. The intensity envelope of the laser may also be measured using a spectrometer or approximated by a suitable function such as a Gaussian or Airy function. The individual mode line-widths may be found using a suitable spectrum analyser. However, when the mode line-width δν m is much narrower than the absorption features being probed then the actual value is not critical, since it does not contribute significantly to the observed width of the feature in the MUMAS signature. The most critical parameter in modelling the MUMAS signature is therefore the mode spacing, Δν m. In principle, this can also be measured using a spectrometer or spectrum analyser with sufficient resolution and accurately specified free spectral range. In practice, however, the best means of determining the mode spacing is by fitting a modelled MUMAS spectrum to one obtained with a known species under known conditions and using Δν m as a fitting parameter. Finally, we note that accurate values of the molecular transition wavelengths are usually available in the literature [16].
3 Experimental apparatus and procedure
3.1 Multi-mode micro-laser
The near infra-red region, in the range 1,500–1,800 nm, is rich in the overtone spectra of many small molecules and radicals and so is a promising region in which to employ MUMAS for multi-species detection. Multi-mode diode lasers that operate in this region, however, exhibit significant mode instabilities and fluctuations and so are unsuitable for MUMAS. Consequently it is better, in this case, to use a diode-pumped solid state laser where the mode frequencies and intensities are more stable and controllable. Infra-red MUMAS was first observed using such a diode-pumped Er:Yb:glass microlaser to detect C2H2 [17]. In the present work an improved version of such a diode laser-pumped Er/Yb:glass micro-cavity laser has been developed which displays a more stable and tunable mode spectrum leading to enhanced quality of MUMAS spectra. The laser was designed to emit a broad multi-mode spectrum in the region containing absorption lines of C2H2, N2O and CO around 1,565 nm.
The laser has a simple, robust structure and uses an Er:Yb-doped phosphate glass chip (Kigre QX ERSP), approximately 1 mm thick, as the active medium pumped by the fibre-coupled output of a diode laser (Roithner, RLCO-980-1000-F) at 975–980 nm. The optical system is shown schematically in Fig. 1. The pump light emerging from a 50 μm fibre is focused, through a curved mirror to a spot of diameter 50 μm in the Er:Yb:glass to match, approximately, the transverse mode diameter of the resonator. The curved mirror, with radius of curvature 25 mm, formed one cavity reflector and the cavity was completed by the reflectively coated output face of the Er:Yb:glass chip. The curved mirror was custom coated in-house and the external face of the wedged chip was custom coated with a broadband coating with reflectivity 98 % over the range 1,538–1,580 nm. Thus, the same chip could be used to provide outputs over a wide range by using curved mirrors with reflectivity designed to enhance particular regions within the overall gain spectrum. The laser used in the present work, however, used a broadband reflective coating on the curved mirror. The Er:Yb:glass chip was polished to form a wedge of 7.6° to suppress coupled-cavity lasing by reflection at the chip faces. The internal wedge face was also coated with an anti-reflection layer to give a reflectivity <0.1 % to further discourage sub-cavity formation. It seems that using a wedged chip with the small amount of dispersion at the wedged surface provided sufficient discrimination to force the laser to operate away from the peak of the gain curve. A degree of coarse wavelength tuning was achieved by selecting the angle at which the pump light entered the Er:Yb:glass wedge to define the axis of the maximum gain and hence the direction of the amplified radiation in the cavity. Refraction and dispersion at the angled face of the wedge then provided an effective wavelength filter since only a limited range of wavelengths propagated at the correct angle to be retro-reflected at the curved mirror.
The cavity elements: amplifier wedge and curved mirror were mounted in a cylindrical assembly containing an annular piezo-electric transducer, PZT, (Noliac CMAR03) which, when activated, altered the cavity length by pressing the curved mirror against a compressible “O”-ring. In this way the cavity modes could be tuned in frequency or wavelength to effect the MUMAS spectral scan. The initial mode spacing Δν m, given by c/2L where L is the cavity length for the static cavity, could be varied by suitable choice of the cylinder length holding the components or adjusted using shims to give a specific mode spacing. When pumped by 550 mW delivered to the active gain region an output power of 35 mW was typically achieved. The output was collimated using a 75 mm focal length lens to form a beam with an approximately Gaussian intensity distribution and 2 mm diameter. The full-angle beam divergence was measured to be 1.4 mrad which compares well with the estimated diffraction limit of 1.0 mrad.
The spectrum of the laser output was recorded using a 1 m Czerny–Turner spectrograph fitted with a grating, having 1,000 lines/mm, and a CCD array (Andor model DU490A-1.7). The output, shown in Fig. 2a, consists of 10 modes covering a range ΔνS = 160 GHz or 1.3 nm centred at 1,565 nm and separated by 18.38 GHz. The spectra in Fig. 2b shows the result of reducing the cavity length—the mode spacing is increased to 33.3 GHz. A small alignment difference results in a slight tuning of the overall mode envelope. By such cavity length adjustments the laser can be tailored to suit the particular absorption spectra being probed. As outlined above, the most accurate measurement of the mode spacing, Δν m, is made by fitting the MUMAS spectrum of a known gas under well specified conditions of temperature and pressure using Δν m as a fitting parameter. In this work, a MUMAS spectrum of C2H2 was used at room temperature and a pressure of 6.66 mbar. A good estimate of Δν m was first obtained by recording an interferogram of the scanning laser output using a Fabry–Perot spectrum analyser (Thorlabs SA 201) having a free spectral range of 1.5 GHz. The interferogram consists of repeating patterns of etalon transmission peaks for each mode from which an estimate of the mode spacing could be inferred. This provided a starting value to enable the fitting process to converge more quickly on the correct value. (This prior measurement is not critical since a reasonable estimate of Δν m can also be made from a measurement of the cavity length.) Using tabulated values for the line positions, with collisional widths calculated or estimated using published values of self-broadening parameters, or reasonable estimates thereof, a model MUMAS signature was calculated and fitted to the data yielding a value of Δν m = 18.38 GHz. By varying the length of the cavity, using alternative mounting cylinders for the Er:Yb:glass wedge and curved mirror, Δν m could be increased up to ~50 GHz. The relatively high reflectivity of the two cavity mirrors provides a high cavity finesse leading to narrow line-widths of the longitudinal modes. The measured mode line-width δν m was found to be limited by the resolution limit of the available spectrum analyser of ~5 MHz. As described above, this mode line-width, even with an upper limit of 5 MHz, contributes negligible broadening to the observed MUMAS spectral features and so high resolution of isolated lines can be achieved.
Spectra of the Er:Yb glass microlaser recorded with a 1 m spectrograph and a CCD array detector. The multi-mode comb is centred in the region of 1,565 nm. The mode spacing is adjustable, by varying the cavity length: a mode spacing 18.38 GHz, b mode spacing 33.3 GHz. The line-width of each mode, <5 MHz, is not resolved as it is much narrower than the instrument width
For diagnostic purposes the mode spectrum and wavelength were measured also using a Michelson interferometer (Bristol Instruments, 721-B IR). Together with the Fabry–Perot spectrum analyser and the spectrograph, these instruments allowed the mode structure and the scanning of the modes by the PZT-driven cavity mirror to be investigated but do not form part of the essential apparatus for recording or analysing MUMAS spectra.
3.2 Experimental procedure
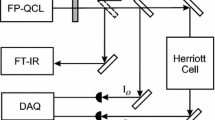
The experimental arrangement used is shown in Fig. 3 and is similar to that reported previously [13, 14]. The output of a multi-mode laser is split into a probe and reference beam, with the probe directed through a multi-pass cell containing the absorbing gases. The transmitted signal intensity is measured by a photo-diode (Thorlabs Det-410), recorded and stored using a data acquisition system (Handyscope HS4). The reference beam is similarly detected and its intensity used to normalise the signal absorption for laser intensity variations. The multi-pass cell—a White cell (Specac, Tornado 20)—provided up to 16 m of absorber path length and was evacuated before filling with different gases at low partial pressures measured by a capacitance manometer (Setra 730) with a quoted accuracy of 0.25 % down to a pressure of 0.025 mbar. Absorption spectra were recorded in the usual way as the frequency of the laser modes was scanned repetitively at rates up to 10 Hz across the inter-mode spacing, Δν m. If the frequency scan exceeds the mode spacing the pattern of absorption repeats as each absorption line is probed by the next mode in the comb. Thus, it is possible easily to confirm that a complete mode spacing has been covered and typically the scan is allowed to exceed Δν m slightly in order to ensure all the spectral features within the overall bandwidth are probed.
4 Results and analysis
4.1 Single species MUMAS
Recording and analysis of a MUMAS spectrum is illustrated in Fig. 4 by the case of N2O. The upper panel shows the absorption spectrum of N2O calculated using the literature values of line positions and strengths of the 5th harmonic of the ν 1 band, together with the modelled mode spectrum of the Er:Yb microlaser. The envelope function of the mode intensities is modelled using a truncated Gaussian function that reproduced approximately the observed spectral distribution of intensity. The lower panel shows, as the solid line, the simulated MUMAS spectrum that would result from using this mode spectrum. The spectroscopic assignments to each absorption feature is given in the stick spectrum in the lower part of the panel. The dotted line is the experimental data to which the simulated MUMAS spectrum has been fitted. We note that there are slight discrepancies in some lines and absorption features that are not reproduced in the simulation. These features may arise from weak transitions in N2O that are not tabulated in the literature owing to their low line strength or from impurity species that have not been identified.
Modelling and analysis of MUMAS data is illustrated for the case of N2O. The upper panel (top trace) shows the N2O absorption spectrum around 1,565 nm and the mode spectrum of the Er:Yb microlaser is shown below (bottom trace). In the lower panel the simulated MUMAS spectrum resulting from using this mode spectrum is shown as a solid line (black in online version). The dashed line (red in online version) is the experimental data to which the modelled N2O MUMAS spectrum is fitted. The stick spectrum in the lower panel indicates the spectral assignments of the observed transitions in the 5th harmonic of the ν 1 band of N2O
Similar spectra were recorded for each of the other two gas species, CO and C2H2, separately. Averaged MUMAS spectra were obtained from typically 20 scans at a repetition rate of up to 10 Hz. Figure 5a–c show the data for CO at 1.41 mbar, N2O at 1.80 mbar and C2H2 at 8.76 mbar, respectively. The observed transitions in CO belong to the third harmonic of the ν 1 band and those observed in C2H2 arise from several combination and overtone bands. Many of the observed transitions in C2H2 are not tabulated in the literature and the line positions and strengths were measured in the course of this work using TDLAS with a single mode laser. The figures also show fitted model MUMAS spectra calculated using the published line positions for each species and the laser parameters derived as described above [16]. Again, in some cases, the fitted spectra do not match perfectly the experimental data over the entire spectral range covered; weak features appear in the experimental data that are not reproduced by the modelled spectrum. As in the case of N2O discussed above, such discrepancies may be due to weak transitions not included in the published database or impurities present at low concentrations. Notwithstanding these small discrepancies, the fits to the multiple transitions in both N2O and C2H2 are more than adequate to identify these species. Owing to the open nature of the CO spectrum only two or three transitions fall within the bandwidth covered by the laser used in the present work, but the modelled spectrum is an excellent fit to the data.
Experimental MUMAS spectra of a CO, b N2O, c C2H2 and d a mixture of all three of these gases. The dotted lines (red in online version) indicate experimental data and the solid line (black in online version) is the temperature- and pressure-dependent modelled fit to the data, from which concentrations of the species may be derived. Note that the partial pressures of the three gases in the mixture (d) are not the same as for the individual MUMAS spectra (a), (b) and (c). In d both foreign gas broadening and self-broadening of the spectral lines affect the lineshapes and fitting of modelled spectra to the data
4.2 Multi-species MUMAS
Figure 5d shows the MUMAS spectrum of a gas consisting of a mixture of all three gases. Spectral resolution was aided by operating at a sufficiently low pressure to minimize merging of features by collisional broadening. The partial pressures as measured by the capacitance manometer were: 0.64 mbar of CO, 4.47 mbar of N2O and 6.86 mbar of C2H2. This data was recorded by averaging 20 scans at 3 Hz repetition rate. All the features in this MUMAS signature are identifiable as arising from each of these species as shown by the modelled spectrum. The partial pressures of each gas could be determined by allowing each to vary independently to achieve the best fit of the modelled spectrum to the data. Deriving the absolute number density or partial pressure of the species directly from a recorded MUMAS spectrum, obtained in the mixture, is subject to uncertainties in the absolute value of the small absorption recorded at these low pressures and also to uncertainties in the pressure broadening data. More accurate concentration values could be derived by using a calibration procedure. As a first step towards such a calibration the partial pressures of each gas derived from fits to the MUMAS experimental data were compared to values obtained by measurement using the capacitance manometer. Figure 6 shows the comparison in the case of CO. The data shows the best fit straight line to the data that is used to estimate the error that would be incurred in using this calibration. In the example of CO illustrated, the mean of the deviation in values of partial pressure from the straight line fit of each of the data points is 1.7 %. In the cases of N2O and C2H2, the mean error derived in this way was 1.9 and 0.4 %, respectively. Thus, partial pressures of each gas in the mixture could be inferred from MUMAS data with an experimental uncertainty of <2 %.
The slopes of these calibration plots deviate from exact unity. It is suspected that this discrepancy arises from uncertainty in the pressure dependent factor in the fitting of the MUMAS data. In these fits, the line broadening coefficient has been assumed to be that of air whereas, in practice, the foreign-gas broadening coefficients may not be exactly the same as that of air. As a result, the slopes of the best-fit line are either greater or less than 1.0 depending on whether the dominant broadening is greater or less than that of air. In general, it may not be possible always to have accurate values for the relevant foreign gas broadening that may be encountered in an arbitrary mix of gases. Nonetheless the present data shows clearly the linearity of the signals as a function of partial pressure, or concentration of the absorbing species, and that an approximation to the required line broadening can be made with sufficient accuracy to identify a species and estimate its concentration using such a calibration procedure.
The MUMAS signature consists essentially of a number of individual single-mode scans superposed upon a frequency scale corresponding to the interval between adjacent modes. The wide spectral coverage achieved and large number of transitions probed by the MUMAS technique can be illustrated in a more familiar fashion by plotting the molecular absorptions that contribute to the MUMAS spectra in their true spectral positions determined from the HITRAN data base and, in the case of C2H2, from our measurements of the weaker lines using TDLAS. Figure 7 shows the transitions detected in the MUMAS spectra of Fig. 5 plotted on an absolute wavelength scale and broadened to account for their temperature- and pressure-dependent line widths using partial pressures derived from the spectra in Fig. 5. In effect, Fig. 7 shows a modelled single-mode laser spectrum equivalent to the MUMAS signature in Fig. 5. Figure 7a–c show CO, N2O and C2H2, respectively, and Fig. 7d shows the spectrum of the gas mixture containing all three gases. In this particular case, the density of spectral lines leads to frequent overlap and thus not every transition is individually resolved in the MUMAS signature but their presence is inferred from the best fit to the data. The large number of lines recorded in this spectral range for C2H2 is largely responsible for the apparent background absorption in the MUMAS spectra for both pure C2H2 and the mixture, Fig. 5c and d, respectively.
Modelled spectra of the transitions that comprise the MUMAS spectra in Fig. 5 illustrating the broad spectral coverage of the MUMAS technique and the large number of transitions probed. a–c Individual species CO, N2O and C2H2 and d a mixture of all three gases. Each spectral feature is modelled using temperature- and pressure-dependent line widths and broadening coefficients obtained from the HITRAN database, or from our TDLAS measurements, and partial pressures from the fitted MUMAS spectra in Fig. 5. The density of lines in the spectrum of C2H2 in c is largely responsible for the apparently non-zero background to the MUMAS spectra in (c) and (d)
The ability to distinguish differing partial pressures (concentrations) of a specific component in a mixture was investigated by recording MUMAS in a mixture containing variable amounts of CO. In order to more accurately gauge the lowest concentration of CO to be included in the mixture an accurately measured quantity, of the order of 1.0 mbar, was introduced to an evacuated cell and diluted with N2 added to an accurately measured total pressure of order 20 mbar. Subsequently pumping the cell to a lower pressure, which was measured accurately using the capacitance manometer with a quoted error of 0.25 %, enabled a more accurate measurement of the CO partial pressure at the lowest concentrations used. A background gas, containing 13.3 mbar N2O, 11.82 mbar C2H2 and 2.24 mbar N2, was prepared in this manner into which additional partial pressures of CO were introduced in quantities that could be measured using the manometer with an uncertainty of ±0.25 %. The resulting MUMAS spectra for variable amounts of CO are shown in Fig. 8. The feature at ~6 GHz on the scan is due to CO with an additional component, giving the “shoulder” on the lower frequency side, arising from the background gas. The data shows MUMAS scans for five different partial pressures of CO: 0.193, 0.917, 1.63, 2.97 and 5.18 mbar. The plot corresponding to the highest CO concentration is clearly visible as the solid line with the strongest feature at ~6 GHz. The other plots correspond to the successively decreasing concentrations of CO with correspondingly decreasing strengths of this feature with the lowest also shown as a thick solid line. The inset to the figure shows an expanded view of the spectrum for the lowest concentrations of CO and also a background level for zero CO concentration. This background level, shown as the thin solid line (red in online version) is a modelled MUMAS spectrum for zero CO concentration fitted to the data in regions outside the feature at 6 GHz, i.e. this represents the best fit MUMAS spectrum to the data if there was no CO present and provides a “baseline” for measurement of the CO concentration.
MUMAS spectra of a gas mixture containing 13.3 mbar N2O, 11.82 mbar C2H2 and 2.24 mbar N2 with increasing concentrations of CO at partial pressures of 0.193, 0.917, 1.63, 2.97 and 5.18 mbar. The horizontal dashed line indicates the zero absorption level. The CO absorption gives rise to the feature at ~6 GHz, which increases with increasing CO concentration. The inset presents an expanded view of the spectra showing the CO absorption feature at the two lowest concentrations; 0.193 mbar (thick solid line) and 0.917 mbar (dotted line). The background absorption in the absence of CO is indicated by the thin solid line (red in online version) and is the result of a fit to the data, with zero CO concentration, outside the narrow region containing the CO feature
The detection limits of the technique have been reported previously indicating that concentrations at the ppm level can be detected using MUMAS in combination with cavity enhanced absorption spectroscopy, CEAS, and, or wavelength modulation spectroscopy, WMS [18]. The present work is not concerned with the detection limits per se but is aimed at demonstrating the ability to record, and identify unambiguously, the presence of several different species simultaneously. The signal-to-noise enhancement methods, such as WMS and CEAS, could also be applied to detection of species in gas mixtures.
Problems were encountered initially in fitting particularly the C2H2 spectra owing to a lack of published line positions for some of the weak lines observed in these spectra. To remedy this situation, the line positions and strengths for these transitions in C2H2 were measured independently by TDLAS using a single mode diode laser. Accurate line positions were obtained using calibration spectra of CO as reference data. The results of these measurements will be published elsewhere [19].
A more difficult problem arises from determining the contribution to the line-width of the lines in each species arising from foreign gas broadening by the other species present. In general this will be a problem for any arbitrary mix of different gases since the relevant line broadening data will not always be available in the literature. In the present work reasonable estimates have been used for these parameters in order to obtain an adequate fit to the data for demonstration purposes. The lack of accurate line broadening data introduces some uncertainty in the value of absorber concentration derived by using concentration as the fit variable. In situations where the dominant broadening is by gases such as nitrogen or oxygen, literature values of broadening constants will give a more accurate value of the concentrations derived in this way. Thus, in most commonly encountered situations this source of uncertainty may not be a major problem.
Finally, we consider the possibility that the MUMAS fingerprint may not be unique for a given set of partial pressures in the mixture. For example, a particular absorption “dip” may be the result of two unresolved lines arising from two different species. The same total absorption could then result from different relative amounts of the two species. This could arise if either the two absorption lines had the same transition frequency or had different frequencies that corresponded exactly to that of two different modes of the laser. In the former case, the two lines would be spectrally unresolved even by a single mode laser and in the second case the ambiguity could be removed by changing the laser mode spacing. The useful feature of MUMAS is that usually multiple lines of the two species in question are probed simultaneously. It is then likely that, even if some absorption features happen to be unresolved by the modes of the laser, there will be some individual lines of each species that provide unique features in the range covered by the entire MUMAS spectrum. Hence, fitting to all the spectral features provides a unique specification of the partial pressures of each gas in the mixture.
5 Conclusion
The work reported in this article demonstrates the principle of multi-species detection using MUMAS. Three different gases, CO, C2H2 and N2O, have been detected in a mixture at pressures in the region of 10 mbar and their partial pressures measured with an uncertainty of ±2 %. Analysis of the MUMAS spectra indicates that variation in the relative concentrations can be measured and that the signals provide a linear indication of absolute concentration of each species. In the present case variable concentrations of CO in a background gas of C2H2, N2O and N2 at a total pressure of 27 mbar, were measured down to a partial pressure of 0.2 mbar. The limits to accuracy arising from estimated values of foreign gas broadening encountered in the present work should not be a major issue in situations where the dominant broadening is by common or majority species such as nitrogen or oxygen.
It has been demonstrated also in previous work that analysis of the data can yield measurements of temperature and pressure as well as concentration [14]. Thus, MUMAS has been shown to provide a means of measuring multiple species and multiple parameters using only a single laser and a single detector. The apparatus required is relatively inexpensive, robust and compact and may be designed to detect a particular combination of species that have absorption features in the same region of the spectrum.
Extension of the technique to the mid-IR will be enabled by the next generation of inter-band cascade lasers operating in the range 3–5 μm [20]. In this spectral region, the absorption cross sections of important molecules are several orders of magnitude larger than in the near infra-red where the present work has been conducted. Thus, the detection limits of the technique for measuring concentration of these important molecules can be expected to be pushed beyond the ppm levels reported up to the present.
References
D.S. Baer, R.K. Hanson, M.E. Newfield, N.K.J.M. Gopaul, Multiplexed diode-laser sensor system for simultaneous H2O, O2 and temperature measurements. Opt. Lett. 19, 1900–1902 (1994)
B.D. Oh, M.E. Paige, D.S. Bomse, Frequency modulation multiplexing for simultaneous detection of multiple gases by use of wavelength modulation spectroscopy with diode lasers. Appl. Opt. 37, 2499–2501 (1998)
B.L. Upschulte, D.M. Sonnenfroh, M.G. Allen, Measurements of CO, CO2, OH, and H2O in room temperature and combustion gases by use of a broadly current-tuned multi-section InGaAsP diode laser. Appl. Opt. 38, 1506–1512 (1999)
K. Boylan, V. Weldon, D. McDonald, J. O’Gorman, J. Hegarty, Sampled grating DBR laser as a spectroscopic source in multi-gas detection at 1.52–1.57 μm. IEE Proc.: Optoelectron. 148, 19–24 (2001)
S.T. Sanders, “Wavelength-agile fiber laser using group-velocity dispersion of pulsed super-continua and application to broadband absorption spectroscopy”. Appl. Phys. B 75, 799–802 (2002)
L.A. Kranendonk, A.W. Caswell, A.M. Myers, S.T. Sanders, “Wavelength-agile laser sensors for measuring gas properties in engines,” SAE Paper 2003-01-1116 (2003)
N.J. Vasa, M. Singaperumal, Gas sensors based on superluminescent diodes for combustion monitoring. Appl. Opt. 48, G1–G5 (2009)
C.F. Kaminski, R.S. Watt, A.D. Elder, J.H. Frank, J. Hult, Supercontinuum radiation for applications in chemical sensing and microscopy. Appl. Phys. B 92, 367–378 (2008)
S.A. Diddams, L. Hollberg, V. Mbele, Molecular fingerprinting with the resolved modes of a femtosecond laser frequency comb. Nature 445, 627–630 (2007)
G. Somesfalean, M. Sjöholm, L. Persson, H. Gao, T. Svensson, S. Svanberg, Temporal correlation scheme for spectroscopic gas analysis using multimode diode lasers. Appl. Phys. Lett. 86, 184102 (2005)
X. Lou, G. Somesfalean, Z. Zhang, “Gas detection by correlation spectroscopy employing a multimode diode laser”, Appl. Opt. 47, 2392–2398 (2008)
P. Ewart, Y. Arita, R. Stevens, K. Richard, P. Manson, “Modes for molecules: lasers for optical diagnostics of combustion”, 4th Australian Conference on optical diagnostics for fluid dynamics and combustion, ISBN no. 0-9757855-2-4, 9-12 (2005)
Y. Arita, P. Ewart, Multi-mode absorption spectroscopy. Opt. Commun. 281, 2561–2566 (2008)
Y. Arita, R. Stevens, P. Ewart, Multi-mode absorption spectroscopy of oxygen for measurement of concentration, temperature and pressure. Appl. Phys. B 90, 205–211 (2008)
P. Grundevik, M. Gustavsson, S. Svanberg, Isotope shifts in dysprosium measured by high resolution laser spectroscopy. Phys. Lett. 56A, 25–26 (1976)
L.S. Rothman, D. Jacquemart, A. Barbe, D. Chris Benner, M. Birk, L.R. Brown, M.R. Carleer, C. Chackerian Jr, K. Chance, L.H. Couderth, V. Dana, V.M. Devi, J.-M. Flaud, R.R. Gamache, A. Goldman, J.-M. Hartmann, K.W. Jucks, A.G. Maki, J.-Y. Mandin, S.T. Massie, J. Orphal, A. Perrin, C.P. Rinsland, M.A.H. Smith, J. Tennyson, R.N. Tolchenov, R.A. Toth, J. Vander Auwera, P. Varanasi, G. Wagner, The HITRAN 2004 molecular spectroscopic database. J. Quant. Spect. Rad. Trans. 96, 139–204 (2005)
Y. Arita, P. Ewart, Infra-red multi-mode absorption spectroscopy of acetylene using an Er/Yb:glass micro-laser. Opt. Express 16, 4437–4442 (2008)
M.L. Hamilton, G.A.D. Ritchie, Y. Arita, P. Ewart, Multi-mode absorption spectroscopy, MUMAS, using wavelength modulation and cavity enhancement technique. Appl. Phys. B 100, 665–673 (2010)
J.H. Northern, M.L. Hamilton and P. Ewart, “Line positions of weak transitions in acetylene” (to be published)
I. Vurgaftman, C.L. Canedy, C.S. Kim, M. Kim, W.W. Bewley, J.R. Lindle, J. Abell, J.R. Meyer, Mid-infrared interband cascade lasers operating at ambient temperatures. New J. Phys. 11, 125015 (2009)
Acknowledgments
This work was supported by a grant from the Engineering and Physical Sciences Research Council, EPSRC (UK). HN, AT and MH are grateful to the EPSRC for personal financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Northern, J.H., Thompson, A.W.J., Hamilton, M.L. et al. Multi-species detection using multi-mode absorption spectroscopy (MUMAS). Appl. Phys. B 111, 627–635 (2013). https://doi.org/10.1007/s00340-013-5382-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-013-5382-9