Abstract
When sodium- and potassium-containing fuel additives are used in internal combustion engines, the bright fluorescence that sodium and potassium atoms emit in the burned gas zone offers a large potential for spectroscopic combustion analysis. To utilize this potential quantitatively, it is crucial to fully understand all physical and chemical processes involved. This includes (1) the temperature dependence of the fluorescence intensity due to gas-phase collisions, (2) the pressure, temperature and equivalence ratio effects on thermodynamic equilibria in the burned gas zone and (3) pressure and temperature-dependent line shapes for quantitative correction of fluorescence reabsorption. High-speed imaging of sodium and potassium fluorescence in a spark-ignited, direct injection, single-cylinder research engine was conducted under well-controlled homogeneous operating conditions at equivalence ratios ranging from 0.71 to 1.43, cylinder pressure from 3 to 15 bar and burned gas temperatures from 1,700 to 2,600 K. This study demonstrates that the influence of pressure, temperature and equivalence ratio on the fluorescence signals of sodium and potassium is understood quantitatively and establishes the potentials and limitations of this tool for burned gas temperature measurements with high temporal and two-dimensional spatial resolution in a homogeneously operated internal combustion engine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
More effective combustion measurement and analysis tools are needed to better understand the physical and chemical processes involved in engine combustion and to meet and exceed future requirements for fuel economy and emissions. Optical combustion diagnostics can provide a set of minimally intrusive measurement techniques with high temporal and spatial resolution. On the downside, the high cost and complexity of lasers and image intensifiers and the difficult interpretation of the recorded images can limit the applicability of optical measurement techniques in an industrial environment. The search for simple optical diagnostic methodologies that can overcome some of these problems is therefore an important step toward the improvement of current and future internal combustion engines. Utilizing the sodium (Na) and potassium (K) fluorescence in internal combustion engines can remove the requirement for expensive lasers and image intensifiers and can offer experimental insight into the combustion process at high temporal and spatial resolution.
The natural fluorescence of sodium and potassium atoms in flames has been investigated by numerous researchers under a variety of applications. Rassweiller et al. [1–3] and Withrow et al. [4, 5] added sodium chloride mist to the intake air to better visualize combustion in IC engines with high-speed film cameras and to obtain burned gas temperature using the sodium-line reversal method. Drake et al. [6] and Zeng et al. [7] utilized sodium luminosity in a spray-guided, SIDI gasoline engine to visualize early flame propagation. Reissing et al. [8] and Beck et al. [9] have demonstrated the use of sodium and potassium fluorescence intensity ratios to measure burned gas temperature in an optically accessible engine. Their work assumed that the sodium and potassium fluorescence ratio was only affected by the temperature-dependent population of the excited states and neglected the temperature, pressure, and equivalence ratio dependent chemical equilibrium between the Na and K atoms and their oxidation products. The self-absorption of the fluorescence light was also not considered in their work.
The purpose of the present paper is to develop the use of sodium and potassium for combustion tracing and for quantitative temperature imaging in burned gas zones. Spectroscopic and thermodynamic data are used to account for collisional energy transfer rates, thermodynamic equilibria of sodium and potassium metals and their hydroxides in the burned gas, and radiation reabsorption. High-speed imaging of sodium and potassium fluorescence arising from alkali metal additives in the fuel is obtained in an optically accessible, spark-ignited, direct injection (SIDI) single-cylinder optical engine operating under homogeneous conditions. Burned gas temperatures determined from cylinder pressure analysis using a two-zone combustion model are compared to the sodium and potassium fluorescence intensities and to Na/K fluorescence intensity ratios.
2 Quantitative assessment of alkali fluorescence
2.1 Energy transfer processes
Spontaneous emission is a physical phenomenon that involves the transfer of internal energy of atoms or molecules. When an atom is in an energetically excited state whose life time is limited, it will relax into a lower-lying energy state following the laws of quantum mechanics and one possible pathway is to emit a photon in the process. In the case of sodium and potassium fluorescence at flame temperature, the two quantum states of interest are the lowest lying excited state and the ground state. Since the energy separation between the excited state and the ground state of potassium differs from sodium, the wavelengths of the emitted light of sodium and potassium differ.
Table 1 summarizes the spectroscopic properties of sodium and potassium fluorescence taken from the National Institute for Standards and Technology (NIST) Atomic Spectra Database [10]. The letters ‘q’ and ‘p’ denote the excited state and the ground state, respectively, while the Einstein-A coefficient denotes the rate constant of spontaneous emission.
Sodium and potassium atoms at elevated temperature can get excited via collisions and undergo relaxation both via collisions and spontaneous emission of light. As illustrated in Fig. 1, the radiative processes are generally not equilibrated in a flame environment doped with alkali metals due to the absence of irradiation. This leads to a total relaxation rate being higher than the collisional excitation rate and the system is not in thermal equilibrium in the strict sense. If the radiative energy loss was significant, this would mean that the population of the excited alkali states cannot be described accurately by the Boltzmann law as a function of gas temperature. Rather, the excited state would generally be under-populated resulting in the measured excitation temperature being lower than the actual gas temperature. However, under the assumption that collisional excitation and relaxation occur on a much faster time scale than the relaxation via radiation, the rate of radiative energy loss can be neglected. In this state of approximate thermal equilibrium, the distribution of the population of internally excited states follows Boltzmann statistics with the excitation temperature being equal to the gas temperature as defined by the Maxwell law of velocity distribution.
Numerous studies have been conducted to identify and quantify the inelastic collision processes in flames doped with alkali metals and to investigate whether the Boltzmann law can be applied to measure the gas temperature via alkali metal fluorescence. The collision rate can be calculated from the collision cross-section using (1) [11].
where Q C,inel. is the inelastic collisional energy transfer rate, N is the number of gas molecules in a given volume V, σ C,inel. is the inelastic collision cross-section, k is the Boltzmann constant, T is the gas temperature and μ is the reduced mass of alkali atom and collision partner. Table 2 gives an overview of measured collisional quenching cross-sections for sodium and potassium with various collision partners. Inelastic collisions with the noble gases He, Ar, Ne, Xe, Kr as collision partners were found to be virtually zero in several studies [12–17].
Norrish et al. [23] conducted a detailed study on the inelastic collision cross-sections of sodium with various hydrocarbon molecules at a temperature of 403 K. While the unsaturated hydrocarbons showed cross-sections of up to 104 \( {\text{\AA}}^{2} \), the cross-sections for saturated hydrocarbons were below 1 \( {\text{\AA}}^{2} \). To the authors’ knowledge, no similar data are available for potassium. Considering the very low concentration of unsaturated hydrocarbons in the burned gas zone, it is valid to neglect collisional energy transfer of alkali metals with hydrocarbons in the burned gas region.
Boers et al. [24] used an atmospheric pressure propane–air flame seeded with sodium chloride (NaCl) and found that the overall inelastic collision processes can be largely attributed to collisions with nitrogen due to both the high concentration of nitrogen in the burned gas region and the large cross-section of sodium-nitrogen collisions. Assuming a total inelastic collision cross-section of 24 \( {\text{\AA}}^{2} \), they compare collisional and radiative relaxation rates and find the fluorescence quantum yield Y = A/(Q C,inel. + A) to be 0.04. Due to the small contribution of radiation to the overall energy transfer rate, they conclude that one can assume the system to be in close proximity to thermal equilibrium. At atmospheric pressure, the excitation temperature equals the gas temperature within an error of 8 K at 2,200 K and the Boltzmann law can be applied to describe the population of the excited state versus the ground state as a function of gas temperature [18, 20, 25, 26]. At elevated pressure in an internal combustion engine, the fluorescence quantum yield can be expected to be even smaller reducing the difference between excitation temperature and gas temperature down to 0.5 K at a gas temperature of 2,500 K and a pressure of 15 bar. It is therefore valid to calculate the population of the excited states of alkali atoms as a function of gas temperature using the Boltzmann distribution law \( {\text{e}}^{{ - \frac{{E_{\text{q}} - E_{\text{p}} }}{kT}}} \). The number of spontaneous emission processes is directly proportional to the population of the excited state. We can therefore expect an exponential dependence of fluorescence signal strength on burned gas temperature as illustrated in Fig. 2 for sodium, potassium and the Na/K fluorescence ratio. The dependence of the Na/K ratio on temperature can be approximated as nearly linear within the temperature range of interest, because the exponent of the Boltzmann equation is small for the Na/K ratio and the higher order term of a power series expansion can be neglected.
2.2 Influence of chemical environment on thermodynamic equilibrium
The intensity of spontaneous emission is directly proportional to the number density of atomic sodium and potassium in the burned gas zone. Chemical reactions in a flame environment affect the alkali atoms and in part convert them into their oxidation products, which are predominantly the hydroxides. When the alkali atom is bound in a molecule, no more light can be emitted at the wavelength of the atomic transition. It is therefore necessary to take chemical reactions into consideration when computing the fluorescence intensity of elemental sodium and potassium in the burned gas region at any given point during the combustion cycle.
Some studies have been conducted on the alkali reaction mechanism and kinetics in flames. Glarborg et al. [27] investigated the reaction mechanism and formation of alkali sulfates in flames. While their focus was set on the formation of the sulfates, they provide reaction pathways and kinetics data for the Na/O/H subsystem. Kaskan [28] discusses likely reaction pathways for alkali in H2/O2/N2 flames doped with sodium and potassium chloride and states that the formation of the oxidation products occurs on a time scale fast enough to assume chemical equilibrium between the elemental alkali and the oxidation product. This finding is supported by studies done by Bulewicz et al. [29] and James et al. [30], who investigated the reaction of lithium with water to produce lithium hydroxide and atomic hydrogen in a fuel-rich H2/O2/N2 flame. While Kaskan finds the three-body reaction of alkali with oxygen to be dominant over the reaction of alkali with water, it should be noted that his calculations on thermodynamic equilibrium used estimated data for the bond strength of Na–OH and Na–O of 90 and 80 kcal/mol, respectively. More recent data published by Hynes et al. [31] provide a value of 78.9 ± 2.0 kcal/mol for the Na–OH bond and 60.3 ± 4.0 kcal/mol for the Na–O bond. Hynes et al. [31] have thoroughly investigated further thermodynamic properties of alkali hydroxide, oxide and dioxide in various lean H2/O2/N2 flame environments between 1,650 and 2,400 K. They found the hydroxide to be the dominant product species containing more than 99 % of all bound metal and the alkali oxide and dioxide to only constitute a small fraction of the alkali product species.
Hynes et al. also propose a reaction mechanism that produces results in close agreement with their experimentally determined species concentration. They discuss the previous work on alkali reaction mechanisms and kinetics and address some large discrepancies in reaction rate constants among the publications cited. It must be noted that these reaction mechanisms were typically developed for H2/O2/N2 flames and Jensen et al. [32] outline the typically large uncertainties on the obtained values for the reaction rates. To the authors’ knowledge, no such reaction mechanisms have been developed for the use in internal combustion engines running on gasoline-like hydrocarbon fuel. Reliable modeling of alkali reaction kinetics in an engine requires more chemical kinetics data.
Fortunately, it is reasonable to assume thermodynamic equilibrium in the high-temperature, high-pressure burned gas regions in IC engines, based on observations in flames made by Hynes et al. [31], Bulewicz et al. [29], James et al. [30] and Kaskan [28] on the rapid equilibration in the burned gas region.
Thermodynamic properties such as enthalpy of formation and specific heat as a function of temperature are given in Gurvich et al. [33, 34], Chase [35], as well as the database of the National Institute of Standards and Technology (NIST) [36] and shown in Table 3.
The mean values provided by the NIST database [36] and the JANAF tables in Chase [35] are used here with the software Chemkin-Pro X64 Version 15101 to calculate thermodynamic equilibria in the burned gas region of an internal combustion engine as a function of temperature, pressure and equivalence ratio. Other alkali species considered in the calculations are Na+, Na−, NaO, NaO−, NaH, Na2, K+, K−, KO, KO−, KH and K2. These species are of negligible importance in this study and are, therefore, not discussed in greater detail. The fraction of atomic sodium and potassium at lean (Φ = 0.8), low-temperature and high-pressure conditions is low because of the formation of sodium and potassium hydroxide. Atomic species concentrations are higher for fuel rich (Φ = 1.4), high-temperature and low-pressure conditions (Fig. 3a, b). The Na/K atomic fraction ratio varies with equivalence ratio, pressure and temperature as a result of differing enthalpies of formation between sodium, potassium and their hydroxide (Fig. 3c, d). An analysis of the effect of the reported large uncertainties of the enthalpy of the hydroxides is carried out in Sect. 4.
a Calculated fraction of elemental sodium and potassium at thermodynamic equilibrium in the burned gas zone as a function of equivalence ratio and temperature at a constant pressure of 10 bar. b Calculated fraction of elemental sodium and potassium at thermodynamic equilibrium in the burned gas zone as a function of pressure and temperature at a constant equivalence ratio of 1.0. c Ratio of elemental Na/K in the burned gas zone as a function of equivalence ratio and temperature at a constant pressure of 10 bar. d Ratio of elemental Na/K in the burned gas zone as a function of pressure and temperature at a constant equivalence ratio of 1.0
2.3 Assessment of self-absorption of alkali fluorescence
Due to the presence of ground state alkali atoms in the burned gas region, part of the emitted light can get re-absorbed. Because collisional energy transfer is dominant, there is little chance that the absorbed photon will be re-emitted. In good approximation, it is valid to consider all light that gets reabsorbed to be lost. The observed intensity of the fluorescence is, therefore, influenced by the amount of light absorbed. The Beer–Lambert law allows for calculating the fraction of the absorbed light.
where A is the fraction of light being trapped by absorption, I 0 is the emitted intensity of light, I T the transmitted intensity of light, v the frequency of light, S(v) the spectral absorption and emission profile, κ(v) the absorptivity, n the number density of potential absorbers and l the thickness of the burned gas region. The number density n can be calculated based on the amount of fuel injected and the mole fraction of sodium and potassium elements in the burned gas after consideration of the chemical equilibrium between alkali atoms and their oxidation products. Both number density and thickness of the burned gas region change as a function of piston position, but the product of nl remains constant during the expansion stroke after the burned gas has filled the combustion chamber.
The absorptivity can be expressed as [11]:
where f pq is the oscillator strength of the transition between the excited state q and the ground state p. S(v) is determined by ways of natural line broadening, collision and Doppler broadening; the latter two are strongly affected by temperature and pressure and are dominant over natural line broadening in the engine environment.
The width of the naturally broadened transition line in frequency space, δv N, the collision broadened line width δv C and the Doppler broadened line width δv D can be expressed as [11]:
where A q,0 is the Einstein-A coefficient for the transition between the excited state and the ground state, Q C,eff is the effective collision rate, k is the Boltzmann constant, T is the temperature, m is the mass of the alkali atom, v 0 the center transition frequency and c the speed of light in vacuum.
Collision broadening can occur via both inelastic collisions, which change the quantum state and internal energy of the atom, and elastic collisions that do not induce a change of the quantum state or merely cause mixing of the degenerate, excited quantum states. The elastic collision cross-sections of both sodium and potassium in the burned gas environment exceed the inelastic cross-sections by nearly one order of magnitude and are the dominant source of collision line broadening. A variety of literature sources [11, 37–41] have conducted experimental measurements of effective (inelastic + elastic) collision cross-sections and the reported values show a spread of a factor of more than two. Hofmann et al. [39] provide a very reasonable explanation for this spread that is relating it to systematic experimental errors made by the other authors. It is therefore believed that Hofmann’s data are the most accurate and are shown in Table 4. The value \( \sigma_{{C,{\text{eff}}}} = 188\,{\text{\AA}}^{2} \) was adopted in this study for both sodium and potassium.
The three line-broadening effects can finally be combined in the Voigt line profile as [11]:
where v 0 denotes the center frequency of the transition.
2.4 Model prediction of sodium and potassium fluorescence intensity in the engine
With the understanding of dependences of sodium and potassium fluorescence intensity on the collisional energy exchange environment, the chemical equilibrium and the effect of self-absorption, the expected measured fluorescence intensities of sodium and potassium and Na/K intensity ratio can be predicted. The prediction takes into account the transmissivity of the filters and quantum efficiency of the cameras used in the experiment. The mole fractions of sodium and potassium in the fuel used in the experiments were estimated as 2,000 and 200 ppm, respectively, and these values were used in the following calculations. The three combustion parameters affecting the fluorescence intensity are temperature, pressure and equivalence ratio. The calculated Na/K fluorescence intensity ratio is plotted in Fig. 4 over a range of temperatures, pressures and equivalence ratios that can be observed during homogeneous engine operation. The Na/K fluorescence intensity ratio shows strong dependences on these quantities. The signal ratios in Fig. 4a are most strongly affected by equivalence ratio between Φ = 0.9 and 1.1 and at temperatures less than 2,100 K. While the equivalence ratio dependence continues in a less-sensitive way in the rich regime, the fluorescence intensity ratios are nearly independent on equivalence ratio between Φ = 0.5 and 0.8. In engine experiments under homogeneous operating conditions, the equivalence ratio can be obtained from fuel and air metering or analysis of the exhaust gas composition. The cylinder pressure is typically measured with a pressure transducer in the cylinder head leaving only the burned gas temperature to be measured using the Na/K fluorescence intensity ratio. However, at cylinder pressures above 4 bar and stoichiometric or lean equivalence ratios, the Na/K fluorescence intensity ratio in Fig. 4 is not strictly monotonically increasing with temperature. This is caused by the self-absorption of the fluorescence when the mole fractions of sodium and potassium differ substantially from each other and poses a severe limitation to the applicability of this tool for burned gas temperature measurements in engines, since the burned gas temperature cannot uniquely be identified by measuring the Na/K fluorescence intensity ratio even if equivalence ratio and cylinder pressure are known. Imaging sodium and potassium fluorescence is not a suitable tool for burned gas temperature measurements over the entire temperature, equivalence ratio and pressure range occurring in internal combustion engines. Fortunately, a simplified approach of this technique can be used under a limited range of operating conditions that is presented below.
The expected Na/K fluorescence intensity ratio depends strongly on temperature, pressure and equivalence ratio. The Na/K fluorescence intensity ratio is most sensitive to equivalence ratio changes between Φ = 0.9 and 1.1 and low temperature. At stoichiometric and lean equivalence ratio and a cylinder pressure of above 4 bar, a given Na/K fluorescence intensity ratio can correspond to two temperatures, which poses severe limitations to the calculation of burned gas temperature from the recorded Na/K fluorescence intensity ratio under these conditions
3 Experiments
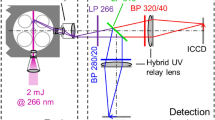
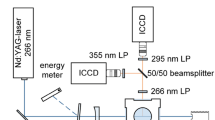
Measurements in an optical engine were conducted to obtain a range of validation data for the model that describes sodium and potassium fluorescence under engine conditions and the potential of this diagnostic tool for experimental engine research is evaluated in this study. A direct injected, 500 cm3 single-cylinder gasoline engine with optical access through a piston window was used and run at 2,000 rpm at various equivalence ratios with 8.5 ± 0.2 mg of fuel injected in each cycle at 290° before combustion top dead center (bTDC). The cylinder pressure was measured on a crank angle basis using a Kistler 6125A pressure transducer in the cylinder head. One of the two intake valves was deactivated to generate a high swirl motion in the combustion chamber to assist the mixing of air and fuel. The air-intake system allows for diluting the air with additional amounts of nitrogen to simulate the effects of exhaust gas recirculation on engine combustion. The air/fuel ratio was obtained by metering intake air and fuel using a Coriolis (Micro Motion Elite CMF010) mass flow meter, via the oxygen and carbon balance of the emissions bench, as well as via an oxygen sensor in the exhaust manifold. The air/fuel ratio obtained by the Coriolis meter was used in the calculation, and the accuracy error was determined by comparisons to the emissions bench and oxygen sensor readings as ±0.5 %. The precision error of the Coriolis meter was determined as ±1.8 % via the comparison of measured fuel mass flow from multiple engine experiments with identical injector pulse width. The total uncertainty of the measured equivalence ratio and injected fuel mass is, therefore, less than 2 %. Indolene served as the fuel instead of commercial gasoline and was doped with additives that contain sodium sulfonate and potassium carbonate dissolved in oil. Indolene itself was found to contain trace amounts of sodium that were not quantified in this study. In addition, the exact composition of the fuel additives is proprietary information of the manufacturer “Lubrizol” and the mole fraction of sodium and potassium in the fuel was approximated as 2,000 and 200 ppm, respectively, based on later experiments that are not reported here, where the concentrations were known more accurately. The fluorescence of the two sodium lines at 589.0 and 589.6 nm was monitored together with the 766.5 and 769.9 nm emission of potassium on two separate high-speed CMOS cameras (Vision Research Phantom V7.1) with quantum efficiencies of 33 and 22 % near 589 and 767 nm, respectively. Using a dichroic filter (Edmund Optics NT43-962) with 99 % reflectivity near 589 nm and 98 % transmissivity near 767 nm, the optical path of the emitted sodium and potassium light from the engine was split. This allows the cameras to share an identical line of sight into the combustion chamber to ensure good spatial overlap of the images. Narrow band-pass filters on each camera lens with center wavelength of 589.0 nm (FWHM 10 nm, 71 % peak transmission, 50-mm diameter) and 766.5 nm (FWHM 10 nm, 50 % peak transmission, 50-mm diameter), respectively, ensured that any combustion luminosity other than sodium and potassium fluorescence did not interfere with the recorded signal. A schematic of the experimental setup is shown in Fig. 5.
Figure 6 shows a series of recorded sodium and potassium images of a single cycle over a range of 54° crank angle. The images were recorded under stoichiometric, homogeneous engine operation and no nitrogen dilution. The cameras were recording at 6.0 kHz, which corresponds to one pair of images every other crank angle degree. The images show a clear spatial stratification of fluorescence signal intensity of both sodium and potassium. The fluorescence intensity of both components is obviously affected by the mass fraction of fuel burned at early points in the cycle. Furthermore, the sodium and potassium fluorescence intensity is affected by the size of the burned gas cloud extending in the third dimension, because the alkali fluorescence originates from the three-dimensional burned gas cloud and the cameras can only resolve two spatial dimensions integrating the signal along their line of sight. Since a pent-roof shaped cylinder head was used, the recorded sodium and potassium intensities are the highest near the center line of the images. If sodium and potassium signals were both affected equally by these effects, taking the ratio of the sodium to potassium signal intensity would eliminate their influence. On the other hand, sodium and potassium fluorescence are affected differently by temperature, the chemical environment and self-absorption and therefore not only the individual fluorescence signals but also the Na/K signal ratio are expected to be a function of temperature, equivalence ratio, cylinder pressure and dopant concentration.
Pairs of recorded sodium (left) and potassium (right) fluorescence images at various crank angles aTDC of a single cycle. The cylinder pressure and crank angle position are noted in the top left corner of each image. The images show large intensity gradients due to the influence of mass fraction burned and the depth of the burned gas cloud
Figure 7 shows the same series of images with the ratio of the recorded sodium to potassium fluorescence signal. These images show a much more uniform intensity distribution indicating that the effect of mass fraction burned and the depth of the burned gas cloud can largely be accounted for by ratioing of the sodium and potassium fluorescence. This is supported by Fig. 8 that shows an intensity profile of the sodium, potassium and Na/K fluorescence images of Figs. 6 and 7. The profile was taken in vertical (from exhaust side to intake side) direction immediately to the right of the masked-out spark-plug ground strap.
Image series of Na/K ratio images at various crank angles aTDC of the same cycle as in Fig. 6. The ratios are independent of mass fraction burned and burned gas cloud geometry resulting in a much stronger spatial homogeneity of the Na/K fluorescence signal ratio. The spatial variation of the Na/K fluorescence intensity ratio can be due to temperature differences or inhomogeneity in the equivalence ratio
The vertical intensity profile of Figs. 6 and 7 was taken immediately to the right of the masked-out spark plug. The fluorescence intensities of sodium and potassium show strong intensity gradients, because the thickness of the burned gas zone differs due to the pent-roof shape cylinder head. The Na/K fluorescence intensity ratio, a measure of temperature, shows the expected low spatial variation
The burned gas temperature is calculated using a two-zone ‘GT-Power’ engine combustion model. This model requires the cycle and crank-angle resolved heat-release data that were obtained from in-cylinder pressure data using a combustion analysis tool provided by General Motors. The term ‘two-zone’ refers to the fact that the model assumes a fully burned and fully unburned gas zone, with the size of these zones depending on the mass fraction burned provided by the combustion analysis tool. The calculated burned gas temperature is, therefore, assumed to be the same over the entire burned gas zone making this tool only applicable for homogeneous engine operation. The model also includes a calculation of heat transfer to the walls of the combustion chamber using a Woschni model [42]. This model includes empirical approximations of various parameters, which introduced uncertainty to the accuracy of the calculated burned gas temperature. The calculated heat-release rate was ensemble averaged over ten cycles to calculate an ensemble averaged burned gas temperature. Figure 9 shows the calculated burned gas temperature and mass fraction burned as a function of crank angle for a stoichiometric operating condition without nitrogen dilution.
It is not possible to validate the model predictions of sodium and potassium fluorescence intensity for every possible combination of pressure, equivalence ratio and temperature using engine experiments, since cylinder temperature, cylinder pressure and equivalence ratio cannot be varied independently in the experiment. However, the measured Na/K fluorescence intensity ratio can be compared to model calculations at a number of observed combinations of cylinder pressure and burned gas temperature at various equivalence ratios and nitrogen dilution rates.
The measured sodium and potassium images and their ratios were ensemble averaged over ten cycles and spatially averaged over the entire visible burned gas region and over a range of 12°–60° after combustion top dead center (aTDC). The recorded fluorescence signals and the Na/K signal ratio together with the theoretical predictions are then plotted over the calculated burned gas temperature in Fig. 10. Given the uncertainty in temperature of ±100 K the signal predictions are in quantitative agreement with the measurement. All calculated Na/K fluorescence intensity ratios had to be divided by 1.12 to match the measured data. This constant factor is believed to be due to uncertainty in the filter transmissivity and camera quantum yield and does not affect the temperature, equivalence ratio or pressure dependence of the Na/K fluorescence intensity ratio. The observed differences between the data with different rates of nitrogen dilution are largely due to minor variances in the equivalence ratio (Φ = 1.02 ± 0.02). Nitrogen dilution itself does not significantly affect the intensity of sodium and potassium fluorescence other than by affecting burned gas temperature and cylinder pressure.
a The measured cylinder pressure is plotted against the calculated burned gas temperature between 12° and 60° aTDC for engine operation at 0–30 % nitrogen dilution. b Dependence of predicted and measured Na/K fluorescence signal ratio on burned gas temperature for various rates of nitrogen dilution. The calculated data points were divided by a factor of 1.12 to match the measured data. This is believed to be due to uncertainty in the filter transmissivity and camera quantum yield, but does not change the temperature dependence of the Na/K fluorescence intensity ratio. It is important to stress that part of the apparent temperature dependence is in fact due to changes in the cylinder pressure. Nitrogen dilution does not significantly affect the Na/K fluorescence intensity ratio other than via the change in cylinder pressure. c, d Dependence of predicted and measured sodium and potassium fluorescence on calculated burned gas temperature for various rates of nitrogen dilution. The data show no dependence on nitrogen dilution within the limits of uncertainty in the burned gas temperature calculation
Figure 11 shows predicted and measured sodium and potassium fluorescence as well as Na/K fluorescence intensity ratios plotted over the calculated burned gas temperature for various equivalence ratios and no nitrogen dilution. It is important to note that the cylinder pressure changes throughout the cycle and when changing the mass intake of air to alter the equivalence ratio. The observed temperature and equivalence ratio dependence in Fig. 11 is, therefore, partly due to the pressure dependence of the Na/K fluorescence intensity ratio. The comparison of predicted and measured Na/K fluorescence intensity ratios shows agreement within an uncertainty of ±100 K, the individual sodium and potassium fluorescence signals also display an uncertainty of approximately ±100 K. A thorough analysis of experimental uncertainties in Sect. 4 addresses this discrepancy. It is important to observe that the Na/K fluorescence intensity ratios in Figs. 10 and 11 increase strictly monotonically with burned gas temperature within the range of cylinder pressure, equivalence ratio and temperature of this experiment. This makes it possible to find curve-fits that describe the dependence of the Na/K fluorescence intensity ratio on burned gas temperature for various equivalence ratios. It must be stressed that these curve-fits can subsequently only be applied to obtain the burned gas temperature via the measured Na/K fluorescence intensity ratio for engine cycles that follow a similar pressure trace as the cycles used to generate the curve-fits. Applying the curve-fits to engine-operating conditions with significantly altered pressure traces would result in large errors in the derived burned gas temperature.
a The measured cylinder is plotted against the calculated burned gas temperature between 12° and 60° aTDC for engines operation between 0.71 ≤ Φ ≤ 1.43. b Dependence of predicted and measured Na/K fluorescence signal ratio on burned gas temperature for various equivalence ratios. The seemingly strong dependence on equivalence ratio at lean operating conditions is mainly caused by the dependence of Na/K fluorescence intensity ratio on cylinder pressure. The apparent temperature dependence is also partly due to changes in the cylinder pressure. The predicted and measured Na/K signal ratios show good agreement in their dependence on both equivalence ratio and burned gas temperature within an uncertainty of less than 100 K. The calculated data points were divided by a factor of 1.12 to match the measured data. This is believed to be due to uncertainty in the filter transmissivity and camera quantum yield, but does not change the temperature and equivalence ratio dependence of the Na/K fluorescence intensity ratio. c, d Dependence of predicted and measured Na and K fluorescence signals on burned gas temperature for various equivalence ratios. Systematic discrepancy exists between predicted and measured signal strength. The intensity is generally over-predicted for lean operating conditions and under-predicted for rich engine operation
4 Discussion of measurement uncertainties and application of temperature measurement
Uncertainty in the literature data of the enthalpy of formation of the hydroxides are the primary source of uncertainty in the calculation of the chemical equilibrium. Figure 12 illustrates this effect on the predicted Na/K fluorescence ratio when the enthalpies of formation of the sodium and potassium hydroxides are varied over the full range of uncertainty (±12.6 kJ/mol). Using the value −210.4 kJ/mol for the sodium hydroxide and −220.0 kJ/mol for potassium hydroxide almost entirely removes any Na/K signal ratio dependence on equivalence ratio, whereas the values −185.2 and −245.2 kJ/mol for sodium hydroxide and potassium hydroxide, respectively, would result in a significantly larger equivalence ratio dependence and altered temperature dependence. It is believed that the mean values for the hydroxides are accurate, because the model prediction matches the experimental data as shown in Figs. 10 and 11. However, this analysis illustrates a need for more precise thermodynamics data.
Measured and predicted Na/K signal ratios as a function of temperature for equivalence ratios 0.71 and 1.43 using mean and extreme values for the enthalpies of formation of the sodium and potassium hydroxides from within the reported uncertainty of ±12.6 kJ/mol. The data display a large dependence on the selected enthalpy of formation values making it the primary source of uncertainty. The reported mean values show good agreement with the measured data
Uncertainty is introduced by incomplete information of the sodium and potassium concentration in the fuel additives. While it is not possible to provide reliable uncertainties of the values used in the analysis (2,000 ppm sodium and 200 ppm potassium concentration in the fuel), the uncertainty was assumed as ±10 % and the Na/K signal ratio was recalculated using 1,800 ppm sodium and 220 ppm potassium, as well as 2,200 ppm sodium and 180 ppm potassium. While these variations change the absolute values of the predicted Na/K signal ratio, it did not significantly affect its equivalence ratio, pressure or temperature dependence.
The assumption of a homogeneous burned gas temperature in the combustion chamber (inherent in the two-zone GT-Power model) causes additional uncertainty. Since the sodium and potassium fluorescence intensity depends exponentially on burned gas temperature, a thermal stratification along the cameras’ line of sight causes the integrated fluorescence to correspond to a temperature higher than the calculated mean. This effect would be much less pronounced for the Na/K fluorescence ratio, since its dependence on burned gas temperature is nearly linear as discussed in Sect. 2.1 and shown in Fig. 2. The accuracy of the temperature calculation is also largely affected by the heat transfer sub-model. GT-Power offers various ways to estimate the heat transfer between the two gas zones and to the walls, but all models are based on empirically determined fit parameters that are not optimized for the engine and operating conditions in this study. The “WoschniSwirl” model for heat transfer was used in this study, because it is believed to be most representative of the high swirl operating condition. The convective heat-transfer multiplier was set to 1.35 as recommended in the GT-Power documentation. The cylinder wall, cylinder head and piston temperatures were estimated as 425, 500, and 550 K, respectively. It is not possible to conduct a quantitative uncertainty analysis of the calculated burned gas temperature because of the various limitations of the GT-Power two-zone model and estimations of parameters such as wall temperatures and convective heat transfer multiplier.
In the case of burned gas temperature stratification along the line of sight, one must conclude that the observed sodium and potassium fluorescence intensities would correspond to a temperature higher than the mean due to their exponential temperature dependence. To confirm this, the theoretical prediction of the sodium and potassium fluorescence intensity was recalculated under the assumption that the measurement corresponds to a temperature that is 100 K higher than the calculated burned gas temperature. The new forecast of the sodium and potassium fluorescence intensity corresponds to the measured signal within a much smaller discrepancy of approximately 50 K, whereas the uncertainty on Na/K signal ratio increased as expected. This is because the Na/K fluorescence intensity ratio is much less sensitive to the assumed temperature heterogeneities along the line of sight of the cameras due to its nearly linear temperature dependence in the temperature range of interest. This in addition to its independence on mass fraction burned and size of the burned gas cloud early in the cycle make it a desirable tool for the two-dimensionally resolved high-speed measurement of burned gas temperature in a homogeneously operated engine.
The use of Na/K fluorescence intensity ratio for spatially resolved burned gas temperature measurements in the engine requires the assumption of spatially homogeneous equivalence ratio distribution. To best establish homogeneity, the fuel was injected early in the intake stroke at 290° bTDC to allow maximum time for the mixing process. However, the assumption of spatial homogeneity has not been verified in this study. Small differences in Φ ± 0.1 would result in large, systematic errors in the calculated burned gas temperature. It is desirable to extend the presented technique to allow for simultaneous spatially resolved equivalence ratio measurements in future experiments using a third alkali component.
5 Application of the diagnostic tool
Using the measured relations of the spatially and ensemble-averaged Na/K signal ratio to calculate burned gas temperature from curve-fits of the data in Figs. 10b and 11b, we can now deduce crank angle resolved burned gas temperatures on an individual cycle basis with two-dimensional spatial resolution. Figure 13 shows the same data set as Fig. 7 but with the Na/K ratio converted into a temperature scale. This illustrates the application of this tool for spatially resolved high-speed imaging of burned gas temperature in homogeneous combustion environments. The application of this technique is limited to engine cycles with similar pressure traces as the cycles used to generate the relations between the Na/K fluorescence ratio and the burned gas temperature. This is due to the fact that the Na/K signal ratio also strongly depends on the cylinder pressure. In addition, this tool can only be applied to homogeneous engine operation due to the strong dependence of the Na/K fluorescence intensity ratio on equivalence ratio. A spatial stratification of the equivalence ratio would result in apparent but unreal temperature gradients.
The Na/K fluorescence intensity ratio was used to obtain spatially averaged burned gas temperature for individual cycles with 0 and 30 % nitrogen dilution. The difference between an undiluted (IMEP 256 kPa) and 30 % N2 diluted (IMEP 242 kPa) individual cycle is shown in Fig. 14. Although these two cycles differ significantly in cylinder pressure, the obtained temperatures are in good agreement with the calculated burned gas temperature from GT-Power, because the pressure trace of each cycle is nearly identical to the pressure traces used in Fig. 10b, where the relation between Na/K fluorescence intensity ratio and burned gas temperature throughout the cycle was obtained. Figure 15 illustrates the limitation of this technique to cycles with considerably different pressure traces by examining three selected individual cycles from this dataset of 300 consecutive cycles at nearly stoichiometric engine operation with 30 % nitrogen dilution. Combustion at this operating point is highly unstable with the coefficient of variance (COV) of the IMEP for this data set being 16.8. The three selected cycles represent a typical “good”, “intermediate” and “bad” cycle with respect to their indicated mean effective pressures (IMEP) of 242.3, 145.8 and 107.8 kPa, respectively. The total mass fraction burned of these cycles was 100, 84 and 48 %, respectively. The three cycles show a significant difference in their burned gas temperature, which can qualitatively be explained by their differing burn rates. While all cycles start out with nearly identical measured burned gas temperatures, the well-burning cycle arrives at 50 % mass fraction burned near 21° crank angle aTDC resulting in a significantly higher burned gas temperature than the other two cycles. The measured burned gas temperature agrees well with the GT-Power calculation for this cycle differing by less than 20 K throughout most of the observed part of the cycle. The intermediate cycle shows a slow initial burn rate similar to the bad cycle, but the combustion continues on late in the cycle and reaches the 50 % mass fraction burned point near 65° aTDC, where the mass fraction burned and measured burned gas temperature differ significantly from the bad cycle. This behavior is reflected in the burned gas temperature calculation in GT-Power, although it is important to point out that the accuracy of the GT-Power calculations for partial burn cycles has not been quantified in this study. Figure 15 also demonstrates the limitation of this technique for quantitative temperature imaging. The pressure trace of the well-burning cycle is nearly identical to the data set in Fig. 10, where the relation of Na/K fluorescence intensity ratio and burned gas temperature was established. Consequently, the burned gas temperature obtained from the Na/K signal ratio is in good agreement with the calculated burned gas temperature in GT-Power. The pressure traces of the intermediate and bad cycles differ substantially from the data set used for calibration in Fig. 10. As a consequence, the burned gas temperatures obtained from the Na/K fluorescence intensity ratios are approximately 150 K too low compared to the temperature calculated by GT-Power. These systematic discrepancies have not been identified in the past and future advancement of this technique is necessary to overcome some of the present limitations.
Crank angle resolved individual cycle analysis of burned gas temperature derived from the measured Na/K fluorescence ratio shows significant differences between undiluted (IMEP 256 kPa) and 20 % N2 diluted (IMEP 242 kPa) cycles at near stoichiometric engine operation. The calculated burned gas temperature in GT-Power is in good agreement with the measurement within ±50 K
Crank angle resolved individual cycle analysis of burned gas temperature derived from the measured Na/K fluorescence ratio shows significant differences between well-burning (IMEP 242.3 kPa), intermediate (IMEP 145.8 kPa) and poorly burning (IMEP 107.8 kPa) cycles at near stoichiometric engine operation with 30 % nitrogen dilution. The three cycles differ substantially in cylinder pressure, and consequently the burned gas temperature obtained from the Na/K fluorescence intensity ratio for the intermediate and poorly burning cycle differ by approximately 150 K from the calculated temperature. Only the pressure trace of the well-burning cycle is nearly identical with the ensemble averaged pressure trace of the cycles used to establish the curve-fits in Fig. 10b, and consequently, the obtained temperature of the well burning cycle is in good agreement with the calculated burned gas temperature in GT-Power
6 Conclusions
The natural fluorescence of sodium and potassium in the burned gas region of an internal combustion engine is shown to depend on burned gas temperature, pressure, equivalence ratio, mass fraction burned and the depth of the burned gas volume. The dominant chemical and physical processes that determine the fluorescence intensity of both metals are understood quantitatively within the uncertainty of various measured and calculated quantities such as equivalence ratio, pressure and burned gas temperature. The sodium and potassium fluorescence intensities can be predicted as a function of these quantities. The spatially averaged fluorescence of both sodium and potassium can be utilized independently for the measurement of burned gas temperature, but temperature heterogeneities along the line of sight set a limit to the accuracy of the derived temperature. In addition, no spatial resolution can be obtained due to the sodium and potassium fluorescence dependence on mass fraction burned and unknown depth of the burned gas volume. The Na/K fluorescence intensity ratio can also be predicted as a function of pressure, equivalence ratio and burned gas temperature and is independent of mass fraction burned or depth of the burned gas zone. This makes the fluorescence intensity ratio particularly promising for two-dimensional, high-speed temperature measurements, because cylinder pressure and equivalence ratio are known under homogeneous engine-operating conditions. At T ≥ 1,700 K the visible sodium and potassium fluorescence has high intensity resulting in excellent signal to noise ratios even for high-speed imaging. The fact that no laser or image intensifier is needed is a significant advantage over many other optical combustion diagnostic tools making the use of this tool highly desirable for engine combustion research. However, the relation of Na/K signal ratio to temperature is not always strictly monotonically increasing or decreasing and limits the applicability of this tool. The non-monotonic relationship is caused by the self-absorption of the sodium and potassium fluorescence when the mole fractions of sodium and potassium differ largely from each other. Altering the alkali concentrations in the fuel has the potential to minimize this limitation.
While the approach of utilizing the Na/K fluorescence ratio for temperature measurements in engines was first suggested by Reissing et al. [8], the current study adds significantly to the understanding of various physical and thermodynamic parameters that influence the sodium and potassium fluorescence intensities. It is demonstrated that large errors in the obtained burned gas temperature can occur if the pressure or equivalence ratio dependences are not accounted for correctly. The Na/K fluorescence intensity ratio can be used reliably to obtain burned gas temperature on an individual cycle basis with crank angle resolution, so long as the pressure trace of the engine cycle is similar to the pressure trace of the operating conditions used to establish the relationship. This tool cannot be applied to combustion cycles with pressure traces considerably different from that which is the case for partial burn analysis or for cycles with different load or mean intake air pressure (MAP).
Utilizing the Na/K fluorescence intensity ratio also requires the assumption of spatially nearly homogeneous fuel distribution. Consequently, the tool cannot be applied to stratified engine operation, where the local equivalence ratio is unknown. Even direct injected engine operation with injection timing of 290° bTDC might result in some spatial stratification, which was not addressed in this study but might put additional limitations on the accuracy of the obtained local burned gas temperature. Using three-component alkali fluorescence imaging has the potential to allow for simultaneous burned gas temperature and equivalence ratio measurements. A future study will examine the suitability of additional alkali elements such as lithium, rubidium and cesium for quantitative combustion spectroscopy in an engine.
References
G.M. Rassweiler, L. Withrow, SAE J. Trans. 36(4), 125 (1935)
G.M. Rassweiler, L. Withrow, SAE J. Trans. 42(5), 185 (1938)
G.M. Rassweiler, L. Withrow, W. Cornelius, SAE J. Trans. 46(1), 25 (1940)
L. Withrow, G.M. Rassweiler, SAE J. Trans. 36(2), 297 (1936)
L. Withrow, G.M. Rassweiler, J. Appl. Phys. 6(6), 362 (1938)
M.C. Drake, T.D. Fansler, K.H. Peterson, Stratified ignition processes in spray-guided SIDI engines, in 9th Internationales Symposium fur Verbrennungsdiagnostik (9th Intl. Symp. on Internal Combustion Diagnostics) Baden-Baden, Germany, 2010
W. Zeng, C.A. Idicheria, T.D. Fansler, M.C. Drake, SAE Int. 2011-01-1281 (2011)
J. Reissing, J.M. Kech, K. Mayer, J. Gindele, H. Kubach, U. Spicher, SAE 1999-01-3688 (1999)
K.W. Beck, T. Heidenreich, S. Busch, U. Spicher, SAE 2009-32-0030/20097030 (2009)
NIST Atomic Spectra Database, (National Institute of Standards and Technology), http://www.nist.gov/pml/data/asd.cfm. Accessed 29 July 2012
C.T.J. Alkemade, T. Hollander, W. Snelleman, P.J.T. Zeegers, Metal Vapor in Flames (Pergamon Press, Oxford, 1982)
R. Carabetta, W. Kaskan, in Symposium (International) on Combustion, vol. 11, no. 1 (1967), p. 321
S. Tsuchiya, K. Kuratani, Combust. Flame 8, 299 (1964)
G. Copley, B.P. Kibble, L. Krause, Phys. Rev. 163(1), 34 (1967)
D.R. Jenkins, Proc. Roy. Soc. A 293(1435), 493 (1966)
J.G. Winans, Z Phys. 60, 631 (1930)
D.R. Jenkins, Proc. Roy. Soc. A 303(1475), 453 (1968)
H.P. Hooymayers, P.L. Lijnse, J. Quant. Spectrosc. Radiat. Transf. 9, 995 (1968)
P.L. Lijnse, J. Quant. Spectrosc. Radiat. Transf. 14, 1143 (1974)
J.R. Greig, Brit. J. Appl. Phys. 16, 957 (1965)
P.L. Lijnse, C. Van der Maas, J. Quant. Spectrosc. Radiat. Transf. 13, 741 (1972)
P.L. Lijnse, P.J.T. Zeegers, C.T.J. Alkemade, J. Quant. Spectrosc. Radiat. Transf. 14(10), 1079 (1974)
R.G.W. Norrish, W.M. Smith, Proc. R. Soc. Lond. A 176, 295 (1940)
A.L. Boers, C.T.J. Alkemade, J.A. Smit, Physica XXII, 358 (1956)
I.R. Hurle, J. Chem. Phys. 41, 3911 (1964)
P.J.T. Zeegers, C.T.J. Alkemade, Combust. Flames 9, 247 (1965)
P. Glarborg, P. Marshall, Combust. Flame 141, 22 (2004)
W.E. Kaskan, in 10th Symposium (International) on Combustion, vol. 1 (1965), p. 41
E.M. Bulewicz, C.G. James, T.M. Sugden, Proc. Roy. Soc. Lond. A 235, 1200 (1956)
C.G. James, T.M. Sugden, Proc. Roy. Soc. Lond. A 227, 1170 (1955)
A.J. Hynes, M. Steinberg, K. Schofield, J. Chem. Phys. 80(6), 2585 (1983)
D.E. Jensen, G.A. Jones, Faraday transactions 1: physical chemistry in condensed phases. J. Chem. Soc 78(9), 2843 (1982)
L.V. Gurvich, G.A. Bergman, L.N. Gorokhov, V.S. Iorish, V.Y. Leonidov, V.S. Yungman, J. Phys. Chem. Ref. Data 25(4), 1211 (1996)
L.V. Gurvich, G.A. Bergman, L.N. Gorokhov, V.S. Iorish, V.Y. Leonidov, V.S. Yungman, J. Phys. Chem. Ref. Data 26, 4 (1997)
M.W. Chase, J. Phys. Chem. Ref. Data Monograph No 9 (1998)
NIST Chemistry WebBook (National Institute of Standards and Technology), http://www.webbook.nist.gov/chemistry/. Accessed 29 July 2012
E. Hinnov, H. Kohn, J. Opt. Soc. Am. 47(2), 156 (1956)
E. Hinnov, J. Opt. Soc. Am. 47(2), 151 (1956)
F. Hofmann, H. Kohn, J. Opt. Soc. Am. 51(5), 512 (1961)
C. van Trigt, T. Hollander, C.T.J. Alkemade, J. Quant. Spectrosc. Radiat. Transf. 5, 813 (1965)
C.G. James, T.M. Sugden, Nature 171, 428 (1953)
G. Woschni, SAE 670931 (1967)
Acknowledgments
This work is sponsored by General Motors Company and its Collaborative Research Laboratory with the University of Michigan. The experiments were conducted at General Motors Global Research and Development while M. Mosburger was a Summer Intern. The authors would like to acknowledge fruitful discussions and contributions from Todd Fansler (GM), Xiaofeng Yang (GM), Seung-Hwan Keum (GM), Kevin Peterson (UM), and Tom Settersten (Sandia National Laboratories). William Tisler provided expert assistance in conducting the experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mosburger, M., Sick, V. & Drake, M.C. Quantitative high-speed burned gas temperature measurements in internal combustion engines using sodium and potassium fluorescence. Appl. Phys. B 110, 381–396 (2013). https://doi.org/10.1007/s00340-012-5266-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-012-5266-4