Abstract
A real-time, in situ CO sensor using 2.3 μm DFB diode laser absorption, with calibration-free wavelength-modulation-spectroscopy, was demonstrated for continuous monitoring in the boiler exhaust of a pulverized-coal-fired power plant up to temperatures of 700 K. The sensor was similar to a design demonstrated earlier in laboratory conditions, now refined to accommodate the harsh conditions of utility boilers. Measurements were performed across a 3 m path in the particulate-laden economizer exhaust of the coal-fired boiler. A 0.6 ppm detection limit with 1 s averaging was estimated from the results of a continuous 7-h-long measurement with varied excess air levels. The measured CO concentration exhibited expected inverse trends with the excess O2 concentration, which was varied between 1 and 3 %. Measured CO concentrations ranged between 6 and 200 ppm; evaluation of the data suggested a dynamic range from 6 to 10,000 ppm based on a minimum signal-to-noise ratio of ten and maximum absorbance of one. This field demonstration of a 2.3 μm laser absorption sensor for CO showed great potential for real-time combustion exhaust monitoring and control of practical combustion systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Carbon monoxide (CO) is one of the important targets in monitoring combustion processes as it is a product of the incomplete combustion of hydrocarbon fuel and a key indicator of combustion efficiency. It is also a toxic pollutant that is regulated by stringent emission mandates [1]. This has led to an increasing need to develop CO sensors as part of a reliable control system for the electric-power industry, such as a coal-fired power plant. Utilities have continuous emission monitoring (CEM) systems at the atmospheric exhaust that typically monitor NO x and SO2 emission [2]. However, many coal-fired utilities combine the exhaust from multiple boilers long before where the CEM monitor is located. Thus, high emissions are difficult to attribute to individual boiler malfunctions. Often a point measurement of O2 is available at the economizer outlet, and this measurement would be much more valuable with simultaneous CO data. Monitoring CO and O2 in the flue gas of coal-fired boilers before the exhaust of multiple combustors is combined for treatment by a single scrubber and would allow the air/fuel distribution and combustor performance to be optimized on an individual burner basis. Such optimization will enable lower excess oxygen operation with reduced NO x emissions and improved unit heat rate, while maintaining acceptable CO emissions and minimizing fly ash or unburned carbon levels. Monitors placed closer to the combustion zone will also have a faster response time that is advantageous for real-time control systems.
Tunable-diode-laser absorption spectroscopy (TDLAS) has been demonstrated to be one of the most promising candidate gas-sensor technologies for practical combustion monitoring with fast, sensitive, and accurate measurements of important combustion parameters (e.g., temperature, pressure, and gas composition to determine combustion efficiency as well as selected pollutant targets) [3–6]. Such monitors can provide non-intrusive, in situ, line-of-sight (LOS) integrated data for exhaust gases containing moisture and fly ash at elevated temperatures. These in situ sensors avoid the problems of sample extraction such as condensation and chemistry in the extraction lines and/or time delays.
Laser absorption-based CO sensors have been reported for applications in a number of different practical combustion system applications [7–18]. Teichert et al. [7] have demonstrated in situ CO, H2O and temperature sensing across a 13 m path in the combustion chamber of a coal-fired power plant using telecommunication diode lasers in the 1.4–1.6 μm range, and they achieved a CO detection limit of 200 ppm after 30 s averaging. With a similar diode laser near 1,560 nm to access the second overtone band of CO, Deguchi et al. [8] performed real-time measurements of in-furnace CO and O2 concentrations for combustion control of incinerator facilities. Other efforts have been focused on using the stronger first overtone band of CO near 2.3 μm [9–12, 16–18], where reliable commercial laser sources have become more readily available in recent years. Early work by Wang et al. [9, 10] used prototype InGaAsSb Fabry–Perot diode lasers to detect CO in laboratory combustion exhaust with wavelength-modulation-spectroscopy (WMS). Ebert et al. [11] used more robust commercial DFB diode lasers to measure CO in a rotary kiln-based hazardous waste incinerator using a direct absorption (DA) strategy. More recently, Chen et al. [12] developed a VCSEL-based CO sensor at 2.3 μm with an in-line reference cell using the WMS strategy, targeting applications such as fire detection and combustion optimization.
Previously, we developed a CO sensor using a 2.3 μm DFB diode laser (Nanoplus) with WMS detection of absorption, and validated it in well-controlled environments of heated cells and laboratory combustion exhaust from a McKenna-type flat flame burner [19]. For the current power plant sensor, a higher-power (2mW) fiber-coupled laser was incorporated into a CO sensor and demonstrated in the boiler exhaust of a 300 MW unit of a pulverized-coal-fired power plant. The utility boiler unit is described in Sect. 2 to illustrate the challenges of the environment and the engineering solutions needed. Section 3 then describes the sensor design, followed in Sect. 4 by representative results of continuous CO monitoring in boiler exhaust. Finally, Sect. 5 briefly discusses the effect of particulate loading.
2 Overview of sensor installation in a coal-fired power plant
The CO sensor was demonstrated in the reheat furnace of a 300 MW tangential-fired boiler unit in a 1.2 GW pulverized-coal-fired power plant. Figure 1 shows a schematic of the boiler unit, with the sensor location at the economizer exit highlighted. The pulverized coal is mixed with air and initially burnt in a fuel-rich zone with a temperature of about 2,000 K, followed by addition of overfire air to produce a net excess oxygen of a few percent in the exhaust. The hot combustion gas heats the steam and water in a forest of heat exchanger tubes as the gas flows from the combustor and through the economizer, exiting at a temperature of about 600 K. After the economizer region, the combustion gases from multiple boilers are combined before exhausting from the stack, for subsequent cleaning in a chemical plant and scrubber.
The laser sensor was installed at the exit of the economizer region, where a temperature of about 624 K (660 °F) and pressure of 0.978 atm (kept slightly below atmospheric pressure for safety concerns) were measured. Sampling measurements of O2 were available a few meters downstream of the CO sensor LOS to gauge the excess air level in the exhaust. Figure 2 shows a diagram of the details of the flanged ports and window mounting designs. With a flue gas velocity of about 9 m/s (30 feet/s) at the economizer outlet, the time of flight between the two measurement locations would be a fraction of a second and could thus be neglected when comparing the measured results. Openings on opposite sides of the duct were available for the optical LOS, and as shown in the figure, two NEMA-4 enclosures to house the laser transmitter (pitch side) and detector optics (catch side) were mounted onto 2½” ANSI 150 flanges with 1.5 m (5′)-long inserts that served to constrain the open path length exposed to flue gas from the nominal 6.1 m (20′) duct dimension to 3.05 m (10′). This reduced the absorbance signal by half, but it kept the measurement LOS away from boundary layer uncertainties and fluctuations. Thus, the sensor LOS monitored the center portion of the gas flow that is representative of the bulk composition. The transmitter and detector enclosures were mounted with a 0.32 cm (1/8″) washer between the ANSI flange faces, producing an open annulus that drew ambient air to purge the two shield tubes and to keep the optical windows on the transmitter and detection boxes free from fouling or condensation of the flue gas. A purge gas flow rate on the order of 10 l/s was estimated based upon the dimensions of the flange components and the pressure difference between the inside and outside of the duct.
This prototype sensor made use of laboratory equipment (lasers, optical mounts, control and data acquisition electronics, etc.), allowing an assessment of the measurement scheme without the investment needed to develop a compact, fully ruggedized field-hardy sensor package with custom electronics, mounts, and lasers. At the same time, the harsh environment at the measurement location required equipment to operate at elevated ambient temperatures [up to ~49 °C (120 °F)] with significant dust, dirt, and fugitive coal dust. Thus, the equipment required enclosures and temperature control beyond that needed in the laboratory.
A detailed layout of the optical and electronic components of the prototype sensor is shown in Fig. 3. The sensor was housed in three NEMA enclosures: the electronics cabinet, the laser transmitter optics enclosure, and the detector optics enclosure. The design peak ambient temperature was ~50 °C (~120 °F) and the measured peak temperature in the transmitter enclosure and electronics cabinet ranged from 38 °C (100 °F) to more than 43 °C (110 °F) in the catch side sensor box. The electronics cabinet was an air-conditioned NEMA enclosure (28″ × 28″ × 60″), and the peak temperature observed inside the cabinet was less than 29 °C (85 °F). The smaller transmitter and detector enclosures were cooled with vortex coolers [20] driven by compressed air providing 5,000 Btu/h cooling. In addition, the laser and the detectors were mounted on water-cooled plates.
Wedged 5 cm (2″) diameter CaF2 windows were mounted on the exit port of the transmitter and detector enclosures. Initial alignment used a visible green laser beam collinear with the 2.3 μm IR beam. An adjustable collimator was used to expand the CO laser beam to as large as 3 cm in diameter at the collector side to minimize fluctuations from particulate scattering across the duct, and a 2″ off-axis parabolic mirror was used to refocus the diverging beam onto the detector (1 mm2 active area).
3 Sensor design and sensing strategy
The tunable diode laser absorption sensor for CO using transitions R(10) near 4,297.7 cm−1 and R(11) near 4,300.7 cm−1 in the first overtone band (Δv = 2) around 2.3 μm was reported earlier [13]. These transitions were selected for their minimum interference with water vapor absorption features, and relatively strong line strengths at the typical combustion exhaust temperature of 500–600 K. The sensor used a calibration-free WMS strategy with second-harmonic detection and first-harmonic normalization (WMS-2f/1f) [21–24] to achieve better noise rejection and lower detection limit. The sensor was validated in the laboratory against a conventional NDIR gas sampling analyzer in combustion exhaust gases from a laboratory ethylene–air flame and known mixtures in a heated cell [19]. The prototype absorption sensor for CO in the coal-fired boiler was designed using the results and the lessons-learned from these laboratory experiments [19]. A DFB laser with fiber-coupled output of ~2 mW (Nanoplus) was tuned to the selected transitions.
A brief introduction of the fundamentals of laser absorption spectroscopy and WMS measurements is provided here to describe the sensor design and define units and terms. Reference [19] provides more details on the spectroscopic validation and refs [19, 23] describe the laser characterization needed for calibration-free WMS.
Laser absorption spectroscopy is based on the Beer–Lambert Law, which relates the fractional transmission of monochromatic light to the flow parameters T, P, and absorber mole fraction, assumed here to be uniform along LOS:
where the transmission τ ν is defined as the ratio of transmitted intensity I and incident light intensity I 0 at frequency ν, and absorbance α is defined as the product of absorption line strength S (cm−2 atm−1), total pressure P (atm), mole fraction χ of the absorbing species i, the lineshape function ϕ ν (cm), and the optical path length L (cm). Note that S is a function of T only, whereas ϕ ν is a function of both T and P.
The nominal flue gas conditions (i.e., temperature, pressure, and the mole fractions of the major species in the gas) can be used to estimate the absorption signals. The flue gas temperature at the economizer exit ranges from 630 ± 20 °F (332 ± 11 °C) at full load to 555 ± 20 °F (290 ± 11 °C) at minimum load. The unit is ‘balanced draft’ which produces a duct pressure at the measurement location slightly below the atmospheric one [i.e., −10 inches of water column (0.978 atm)]. Typical flue gas composition at 3 % excess oxygen is described in Table 1, based on a mass balance for Mingo Logan Central Appalachian coal. Expected CO levels were less than 100 ppm at the economizer outlet for ‘tuned’ operation, with possible excursions between 100 and 500 ppm during ‘upsets’ or ‘untuned’ operating periods.
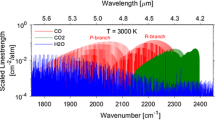
Combining the results of the laboratory measurements of CO at these flue gas measurement conditions, the expected absorption spectrum of the combustion flue gas near the candidate CO transitions was simulated to evaluate the interference from the major constituents of water vapor and carbon dioxide. As noted in Ref. [19], the 2.3 μm overtone band of CO does not overlap with any CO2 absorption bands, but has major interference from the v 1 and ν 3 fundamental bands of H2O. Figure 4 shows the simulated spectrum with HITRAN [25] that includes a portion of the CO band R branch, together with the water vapor absorption spectrum. The targeted CO lines of R(10) and R(11) lie in the valley between strong water absorption transitions and are the two strongest transitions within the branch at T = 600 K with the corresponding lower state energy E″ of 211.40 and 253.67 cm−1, respectively. At the temperature of the measurement LOS near 650 K, the selected transitions have a temperature sensitivity dS/dT/S ~ 0.07 % K−1, making the sensor insensitive to temperature uncertainties in the measurements. They have also been selected to be free of interference from the other species present in the exhaust stream such as those listed in Table 1. Thus, these are the best candidate transitions in this band for CO sensing in combustion exhaust with its abundant moisture level. Other transitions with higher E″ [e.g., R(15), R(30)] have been studied in other works [9, 10] for applications at higher temperatures in the combustion zone.
The above estimates find a CO signal free of interference with ~0.8 % absorption for 100 ppm CO in the boiler duct. Calibration-free, normalized WMS offers three advantages over DA in the exhaust from a coal-fired burner. First, the normalization offered by current-modulated tunable diode lasers provides for robust sensing free of interference from non-absorption losses such as scattering from the fly ash in the exhaust gases. Second, WMS lends itself to rapid real-time data analysis, which is needed for any eventual combustion control applications. Third, WMS has better rejection of low-frequency noise common in large-scale gas flows. The laser optical frequency ν(t) was modulated at 10 kHz by the injection current, producing simultaneous modulation of the output intensity and laser intensity I 0(t). Signals at each harmonic of the modulation frequency nf are selected by lock-in amplifier and narrow-band filter. The 1f signal is proportional to the laser intensity and the 2f signal is proportional to the product of laser intensity and absorbance in the optically thin limit. Thus, the 2f/1f signal is independent of laser intensity. Using the 1f signal to normalize the 2f signal cancels out the unknown or site-dependent factors including non-absorption losses, drift of the detector gain, light scattering, window-fouling, and variation in light collection.
As the characteristic drifts and fluctuations in the flue gas composition typically occur on at least a multiple-second time scale for boiler-wide phenomena (or multiple-minute for burner operations such as mechanically adjusting coal-feed or air-damper settings), long-term, continuous monitoring is more important in this application than a fast time resolution. Therefore, a scanned-WMS scheme was employed rather than a fixed-wavelength scheme. A 100 Hz scan rate was used with a 10 kHz sinusoidal modulation. A glass cell (see Fig. 3) filled with low-pressure CO gas was on a reference path with ZnSe beam splitter to serve as a simultaneous wavelength calibration for the scanned-wavelength sensor and account for long-term drift or fluctuations of the laser wavelength. For long-term, unattended monitoring, this absorption peak position acquired from the reference path can also be used as a feedback signal to the laser temperature control circuit to automatically readjust the laser center wavelength in real-time, using custom-designed circuits, or software algorithms. Note that as a calibration-free WMS-2f/1f strategy was used, this reference path does not need to provide the laser intensity reference for the data interpretation.
4 Results of continuous CO monitoring in boiler exhaust
The sensor evaluation was performed during a full-load running period of the utility boiler unit. The optical alignment and installation process was successfully completed with the unit online and without disruption. The calibration-free WMS strategy required neither on-site calibration nor purged optical path for the absorption-free background measurement. Also note that with the window tube design discussed above, no fouling of the optical windows was observed.
To test the sensor performance, the intake air flow of the burner was varied, which resulted in changes in the excess air level and CO concentration in the boiler flue gas. The changes in excess air level were captured by the O2 sensors installed near the economizer exit. The known inverse correlation (O2 goes up if CO goes down) of the concentrations was used to validate the behavior of the CO sensor. Figure 5 shows a representative period of 7 h during which CO and O2 were continuously monitored. Note that the 100 Hz CO measurement rate was averaged for 30 s for the data shown in the figure, and the separately measured flue gas conditions of T ~ 624 K and P ~ 0.978 atm were used in the data processing to interpret the gas mole fraction. During the first ~110 min, the intake air level was reduced, decreasing the excess O2 concentration to a minimum point ~1.4 %. At this point, the CO levels had risen to high values (150–200 ppm) and large temporal fluctuations were observed. These fluctuations resulted from the huge change in CO for fuel-rich versus fuel-lean combustion. The excess O2 concentration was then increased, and the CO concentration reflected this change by rapidly declining. At the highest excess air (~3 %) the CO was less than 10 ppm; these low values are also shown in the inset of Fig. 5 to illustrate the fidelity of the measurement. At approximately 265 min, the air flow was adjusted to a normal operation level and the measured CO first rose to about 100 ppm, and then stabilized at a nominal concentration of 25 ppm, with a characteristic oscillation period of about 15 min.
These results illustrate the sensitivity of the WMS-2f/1f scheme as the maximum absorbance at 200 ppm CO concentration was only 1.5 %. Continuous, real-time variation in the measured CO level with time was also captured, demonstrating the time resolution capability of the laser sensor. A detection limit of 0.6 ppm (or 1.8 ppm-m) with 1 s averaging was estimated from the background noise level for a signal-to-noise ratio (SNR) of one. For practical CO measurements, it is typically recommended that a SNR greater than ten be maintained, resulting in an estimated detection limit of 6 ppm, or 18 ppm-m with 1 s averaging. Note that the major noise source was laser intensity fluctuation due to beam steering and mechanical vibration. Thus, this limit could be improved upon through increased signal averaging. The data in Fig. 5 were averaged for 30 s and the detection limit was estimated to drop to ~1.1 ppm. This estimate of the CO sensor detection limit was validated by the fidelity of the low values of ~6 ppm in the inset for times between 200 and 250 min.
These field results demonstrated CO concentration measurements with a dynamic range of ~40, with a maximum absorbance observed in these experiments of only ~1.5 %. By extrapolating the sensor range to an optical depth of one for the CO absorbance, the upper concentration limit of the sensor would be 1 % CO, or 10,000 ppm. This measurement range would provide a dynamic range of CO concentration of more than 2,000. Thus, the CO sensor design tested provides the large CO dynamic measurement range needed for combustion system monitoring. The demonstrated CO sensitivity and one-second time response illustrate the potential of in situ laser sensing of combustion gases for closed-loop optimization of combustion. It is also important to note that even with combustion flue gases containing fly ash particulate and moisture at elevated temperatures, the prototype CO sensor was capable of unattended real-time monitoring. Even without optimization of the sensor package, no operator intervention was needed to make optical adjustments over a period of several hours, which was limited only by time available on that particular test day and not by a need to make adjustments. Based on these field tests, an optimized CO sensor designed specifically for harsh combustion applications could provide routine real-time, in situ CO monitoring of coal-fired boiler flue gas.
5 Discussion on particulate loading effect on light transmission
In the fly ash-laden combustion exhaust of a pulverized coal-fired unit, much of the laser light directed through the flue gas was attenuated by scattering from the fly ash particulate. This scattering loss can significantly vary with the size distribution of the scattering particles, as observed in two separate campaign measurements at the same measurement location. In the limit where the scattering particles are small compared to the wavelength of the light, the scattering falls in the Rayleigh scattering regime, which has a strong 1/λ 4 dependence on the laser wavelength λ. As a result, the 2.3 μm laser light encountered stronger scattering losses compared to a mid-IR 5.2 μm NO sensor developed and tested in the same boiler environment [26]. But the 2.3 μm CO laser was attenuated ten times less than a near-IR (NIR) 1.65 μm CO sensor. In the opposite limit, where the scattering particles are large compared to λ, the scattering is known as Mie scattering and is independent of λ.
Two different coals were burned in the utility boiler, each producing a different particulate-size distribution. Without analysis of the fly ash, quantitative data such as the mean particle size or size distribution were not available. However, we observed quite different fractional transmission at different wavelengths for the two coals. When a high-quality, clean coal (100 % central Appalachian coal) was burned with limited fouling (i.e., secondary particulate formation), a fly ash particle size distribution was produced with a large number of very small particles (1–2 μm) and the 2.3 μm sensor was in the Rayleigh limit. In this case, the 2.3 μm CO sensor transmission was ~1 % that of the 5.2 μm NO sensor. The second campaign used a coal (blend of 75 % Illinois Basin and 25 % Central Appalachian coals) known for its lower ash fusion temperature and convective pass-fouling tendencies that produced particulate scattering with similar fractional transmission for 2.3 and 5.2 μm, indicating Mie-limit behavior. Based on this limited data set, it is likely that laser absorption diagnostics with longer wavelengths in the extended near-IR and mid-IR will be suitable for a much wider range of combustor conditions and coal quality than sensors using transitions with shorter wavelengths.
6 Summary
A 2.3 μm laser absorption-based CO sensor, developed and validated earlier in a laboratory environment, was refined and tested for continuous, real-time, in situ measurement in the boiler exhaust of a 300 MW unit of a pulverized-coal-fired power plant. The measurement location was near the economizer exit, across an optical pathlength of 3.05 m where the gas temperature was 624 K. A scanned-wavelength 1f-normalized WMS-2f strategy using an injection-current-modulated DFB laser was used for its better noise-rejection capability, potential for real-time analysis, and robust behavior in a scattering environment. Despite the associated harsh combustion environment involving high temperature and moisture levels, mechanical vibration and confined spaces, as well as particulate scattering and low light transmission levels, continuous CO monitoring was successfully demonstrated. The measured CO levels followed the expected trends as the excess O2 in the boiler was varied from 1 to 3 % during a period of 7 h. The noise-limited CO detection limit in the nominal 624 K gases for the 3 m pathlength was ~0.6 ppm for 1 s averaging; a dynamic range of 6–10,000 ppm was extrapolated from these test results. The sensor performance is expected to improve with more robust and compact packaging suitable for long-term, 24/7 operation in the boiler environment. These results suggest this sensor has potential for use in a combustor control system for real-time optimization of furnace efficiency. This successful field demonstration provided direct evidence of the suitability for real-time, in situ, laser absorption sensors in harsh environments such as the coal-fired boiler exhaust.
References
US environmental protection agency, carbon monoxide national ambient air quality standards: scope and methods plan for health risk and exposure assessment, April 2009, available at http://www.epa.gov/ttn/naaqs/standards/co/s_co_index.html
US environment protection agency, continuous emissions monitoring fact sheet, available at http://www.epa.gov/airmarkt/emissions/continuous-factsheet.html
R.K. Hanson, Proc. Comb. Inst. 33, 1 (2011)
K. Kohse-Höinghaus, J.B. Jeffries (eds.), Applied Combustion Diagnostics (Taylor and Francis, London, 2002)
M.G. Allen, Meas. Sci. Technol. 9, 545 (1998)
K. Kohse-Höinghaus, R.S. Barlow, M. Aldén, J. Wolfrum, Proc. Comb. Inst. 30, 89 (2005)
H. Teichert, T. Fernholz, V. Ebert, Appl. Opt. 42, 2043 (2003)
Y. Deguchi, M. Noda, M. Abe, Proc. Comb. Inst. 29, 147 (2002)
J. Wang, M. Maiorov, D.S. Baer, D.Z. Garbuzov, J.C. Connolly, R.K. Hanson, Appl. Opt. 39, 5579 (2000)
J. Wang, M. Maiorov, J.B. Jeffries, D.Z. Garbuzov, J.C. Connolly, R.K. Hanson, Meas. Sci. Technol. 11, 1576 (2000)
V. Ebert, H. Teichert, P. Strauch, T. Kolb, H. Seifert, J. Wolfrum, Proc. Comb. Inst. 30, 1611 (2005)
J. Chen, A. Hangauer, R. Strzoda, M.C. Amann, Appl. Phys. B 102, 381 (2011)
B.L. Upschulte, D.M. Sonnenfroh, M.G. Allen, Appl. Opt. 38, 1506 (1999)
Q.V. Nguyen, B.L. Edgar, R.W. Dibble, A. Gulati, Combust. Flame 100, 395 (1995)
A.R. Awtry, B.T. Fisher, R.A. Moffatt, V. Ebert, J.W. Fleming, Proc. Comb. Inst. 31, 799 (2007)
K. Sun, R. Sur, X. Chao, J.B. Jeffries, R.K. Hanson, Combust. Inst. 34 (in press) http://dx.doi.org/10.1016/j.proci.2012.05.018
X. Chao, J.B. Jeffries, R.K. Hanson, Combust. Inst. 34 (in press) http://dx.doi.org/10.1016/j.proci.2012.05.024
K. Sun, X. Chao, R. Sur, J.B. Jeffries, R.K. Hanson, Appl. Phys. B, accepted
X. Chao, J.B. Jeffries, R.K. Hanson, Meas. Sci. Technol. 20, 115201 (2009)
D.T. Cassidy, J. Reid, Appl. Opt. 21, 1185 (1982)
X. Zhu, D.T. Cassidy, J. Opt. Soc. Am. B 14, 1945 (1997)
G.B. Rieker, J.B. Jeffries, R.K. Hanson, Appl. Opt. 48, 5546 (2009)
H. Li, G.B. Rieker, X. Liu, J.B. Jeffries, R.K. Hanson, Appl. Phys. B 87, 169 (2007)
X. Chao, J.B. Jeffries, R.K. Hanson, Appl. Phys. B 106, 987 (2012)
Acknowledgments
Support was provided by the Electric Power Research Institute with Mr. Richard Himes as program manager and the Air Force Office of Scientific Research with Dr. Chiping Li as program manager. EPRI also arranged access to the coal-fired power plant in Charlotte, NC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chao, X., Jeffries, J.B. & Hanson, R.K. Real-time, in situ, continuous monitoring of CO in a pulverized-coal-fired power plant with a 2.3 μm laser absorption sensor. Appl. Phys. B 110, 359–365 (2013). https://doi.org/10.1007/s00340-012-5262-8
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-012-5262-8