Abstract
We investigate upconversion emissions in alkali metal ions (Li+, Na+, and K+) and Er3+-codoped Y2O3 nanocrystals. By introducing Li+, upconversion intensity is significantly enhanced, while Na+ and K+ hardly have this influence. FT-IR data give evidence that the main mechanisms of the enhanced upconversion emission cannot be attributed to the decreased surface defects. EXAFS data and variations of enhanced upconversion intensities in different samples indicate that Li+ can occupy the interstitial sites in lattice and thus arouse large site asymmetry. In addition, locations in the samples and effects on the upconversion emission of Na+ and K+ are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Infrared-to-visible upconversion (UC) of rare-earth (RE) ion-doped nanocrystals (NCs) has been studied extensively in the past few years [1, 2], due to their potential applications in the fields of solid-state lasers [3], light emitting diodes [4], 3-D display [5, 6], fluorescence labeling [7], and temperature sensor [8, 9], etc. Comparing with bulk counterpart, NCs have some particular characteristics, such as surface effect and quantum size effect, etc., which attract special concerns in photoluminescence (PL) process. RE ions with abundant energy levels, long lifetime of excited states and good chemical durability, are suitable for the UC-PL processes.
It should be noted that UC applications are greatly restricted by the low efficiency at the current stage. There are different ways to increase UC efficiency, such as reducing phonon energy, thermally inducing, core–shell structure, and coating Ag film, etc. [10–14]. It has been reported that UC-PL intensity of RE3+ can be greatly enhanced by codoping Li+ in some oxide NCs [15–17]. Mechanisms of the enhancement are attributed to the decreased symmetry around RE3+ in lattice and neutralization of OH group outside the NCs. Besides, it is proposed that Na+ and K+ also enhance the UC-PL intensity [17]. Recently, some experimental results contradict both of the analysis of the enhancement mechanisms of doping Li+ and the effects of Na+ and K+ on the UC-PL intensity. To clarify the enhancement mechanisms by doping method, the effects of alkali metal (AM) ions on UC-PL should be investigated in more detail.
In this paper, we report UC-PL properties of AM+/Er3+ (AM = Li, Na, and K)-codoped Y2O3 NCs. By contrasting structures and spectra of different samples, mechanisms of the effects on the UC-PL intensity of the doping AM+ are investigated.
2 Experimental section
Samples were prepared by a sol–gel process: Y(NO3)3·6H2O, Er(NO3)3·6H2O, Li2CO3, NaNO3, and KNO3 with predetermined concentrations dissolved in de-ionized water at 80 °C. Citric acid was added to the solution with the mole ratio of cation to citric acid to be 1:3 and then ammonia was added to adjust the pH value to about seven. The solution was dried in air at 130 °C for 20 h to form a black bulk, which was further calcined in air at 800 °C for 2 h and finally cooled to the room temperature.
A diode laser emitting at 976 nm with full width at half maximum of 2 nm was used to irradiate samples, and the maximum excitation power used in the experiment was about 2.6 W. UC-PL spectra were collected by a SPEX1000M spectrometer attached by a photomultiplier tube, and all the spectra measurements were performed at room temperature.
Structures of samples in powder form were identified by X-ray diffraction (XRD) using an XRD-6000 diffractometer. The 2θ angle of XRD spectrum was recorded from 20° to 60° at a scanning rate of 8°/min. Fourier transform infrared (FT-IR) spectra were measured with a SPECTRUM100 infrared spectrometer via the potassium bromide (KBr) pellet technique. In making the KBr pellets, 1 mg of samples after heating at 110 °C for 2 h was mixed with 100 mg of KBr powder. Extended X-ray absorption fine structure (EXAFS) was performed at the beamline of U7C at the National Synchrotron Radiation Laboratory (NSRL) in Hefei, China; the detail description is shown in Ref. [16]. Inductively coupled plasma-mass spectrometry (ICP-MS) measurements which confirm the element contents were performed by a XSeriesII mass spectrometer; 0.5 g samples were dissolved in 500 ml diluted hydrochloric acid for spectral measurement.
3 Results and discussion
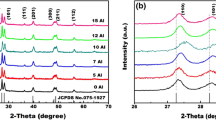
Concentrations of Er3+ and Li+ doped into Y2O3 NCs in this study are chosen to be 2 and 3 mol %, respectively, on the basis of the published data to achieve higher efficiency [18]. In order for horizontal comparison, concentrations of Na+ and K+ are chosen to be the same value of Li+, which is only a wishful task since some AM+ are lost in the synthesized procedure based on the ICP-MS measurements. The powder XRD patterns (Fig. 1) show that all of the diffraction peaks from samples are in good agreement with the standard values for the highly crystalline cubic Y2O3 crystal (JCPDS No. 05-0574). No evident changes show on lattice parameter (see Table 1). The crystalline sizes of the NCs are calculated by the Scherrer’s equation, average radii of crystallites are all around 20 nm for Er3+-doped, Er3+/Na+-codoped, and Er3+/K+-codoped Y2O3 NCs. As for Er3+/Li+-codoped Y2O3, average radius is around 35 nm.
Figure 2 shows UC-PL spectra of AM+/Er3+:Y2O3 NCs, from which it can be seen that UC-PL intensity is increased by Li+ by a factor of about ten, while Na+ and K+ decrease the UC-PL intensity with varying degrees. Generally speaking, larger size of NCs brings smaller specific surface area, which means less surface defects exist in larger NCs. It is well known that surface defects promote multiphonon nonradiative decay efficiently. Thus, it seems that Li+ can enhance the UC-PL intensity due to the decrease of defects outside the NCs. To verify this opinion, FT-IR spectra of AM+/Er3+:Y2O3 NCs are measured. As shown in Fig. 3, C–O bond centered at ~1,500 cm−1 and O–H bond centered at ~3,500 cm−1 substantially unchange in different samples which is consistent with the observation in Er3+/Yb3+/Li+:NaGdF4 NCs [19], and means surface defects remain approximately identical. Thus, it can be concluded that the enhancement of UC-PL intensity cannot be attributed to the increasing crystalline size. FT-IR data also deny the neutralization of Li+ which was considered as one enhancement mechanism of doping Li+ since O–H bond hardly changes by Li+. Thus, the enhancement mechanisms of Li+ should be searched from other directions.
Inset of Fig. 3 shows slight peak shifts of Y–O bond in Er3+/Li+:Y2O3 and Er3+/Na+:Y2O3 compared with that of Er3+/K+:Y2O3 and Er3+:Y2O3, which indicates a weak structural distortion in Er3+/Li+- and Er3+/Na+-codoped sample. Also, it can be verified through the EXAFS measurement. EXAFS is employed to study the local structure around Er3+. Based on the photoelectron backscattering theory, XAFS oscillation due to a single species of scattering atom, χ, is given by [20]:
where S 20 is the amplitude reduction factor accounting for the many-body effect, N j the coordination number, F j (k, π) the backscattering amplitude, R j the nearest-neighbor distance, λ the mean free path of the photoelectron for inelastic scattering, 2σ 2 the Debye–Waller factor, which describes the level of static and dynamic disorder, k the wave vector of the photoelectron, and \( \varphi_{j} \left( k \right) \) is the phase shift.
Figure 4 shows the Fourier transform of k 3-weighted χ(k) of Er3+ in Y2O3 NCs. After doping AM+, average bond length of Er–O substantially unchange in Er3+/K+:Y2O3 compared with that in Er3+ singly doped Y2O3, whereas it slightly shortens in Er3+/Li+:Y2O3 and Er3+/Na+:Y2O3, which indicates that samples with Li+ and Na+ possess lower symmetric environment and it is also consistent with the FT-IR data.
It is well known that the f–f transitions arising from forced electric dipole are parity forbidden and become partially allowed when the ion is situated at the low symmetry site. As a result, the optical transition probabilities of RE3+ increase in a low symmetric matrix, and thus sample with Li+ ion has stronger UC-PL emission than Er3+-doped and Er3+/K+-codoped Y2O3 in the present work. However, it seems that Er3+/Na+-codoped Y2O3 NCs have a comparable structural distortion with Er3+/Li+:Y2O3 on the basis of the FT-IR and EXAFS measurements, thus the next logical question must be why only Li+ enhance the UC-PL intensity whereas Na+ not?
To investigate the effects of AM+ on the UC-PL emission of Er3+, we should confirm the locations of Er3+ in the samples firstly since its luminescence is our subject. Based on the knowledge of crystal chemistry, the doped impurities in the NCs possess three types of locations, i.e., (1) outside the lattice in the grain boundary, (2) substitution of cations in lattice, and (3) occupation of interstitial sites in lattice. The dopants with large size are usually located outside the lattice (type 1) while those with small size which can easily penetrate into the lattice often occupy the interstitial sites (type 3), and those with both similar size and chemical nature with the cations in lattice tend to substitute the cation sites (type 2). It should be noted that type 1 impurities hardly change the host structure since they cannot penetrate into the lattice, and type 3 impurities cause larger structural distortion than type 2 ones because of the larger space of the interstitial in lattice.
Based on the aforementioned discussion, it can be concluded that Er3+ (0.89 Å) with almost identical size and similar chemical nature with Y3+ (0.90 Å) can easily substitute cation sites in lattice (type 2). Li+ (0.76 Å) with small size mostly occupy the interstitial sites in lattice (type 3), K+ (1.38 Å) are mostly located outside the NCs due to their relatively large size (type 1), and we suppose that Na+ (1.02 Å) are located at both type 1 and type 2 sites because of its similar but slightly larger size compared with Y3+.
These discussions answer the previous question. In particular, Li+ (type 3) that occupy the interstitial sites enhance UC-PL intensity by decreasing the local symmetry; K+ (type 1) outside the NCs hardly affect the lattice environment and thus cannot enhance the UC-PL emission. On the contrary, the outside K+ also substitute some Er3+ sites on the surface of grain, thus further decrease the number of luminescent center on the surface which efficiently participate in the UC-PL processes, and finally decrease the UC-PL intensity. As for Na+, the type 1 Na+ decrease the UC-PL emission like K+ and type 2 Na+ increase it like Li+, it can be concluded that the effects of type 1 Na+ are stronger than that of type 2 Na+ since the actual intensity of Er3+ is decreased by doping Na+.
At the current stage, it is considered that AM+ located at type 2 and 3 sites have the similar effects on UC-PL intensity [21]. However, based on the aforementioned discussions, UC-PL intensity should be much more enhanced by the type 3 ions than that of type 2 since type 3 ions arouse more local asymmetry. Since Li+ in the interstitial sites arouse more structural distortion than Na+ which occupied cations sites, the FT-IR and EXAFS data should show a larger structural change in Li+-doped sample than that in Na+ doped. However, structural variations of Er3+:Y2O3 codoped with Li+ and Na+ are comparable. This phenomenon indicates that there is a larger content of Na+ than that of Li+ in the lattice. To verify this opinion, ICP-MS measurements are performed and the results are listed in Table 1, from where it can be seen that Er3+ content substantially unchange in various samples which confirms our experimental accuracy. Li+ and K+ contents are obviously smaller than the pre-doped concentration which indicates that some contents are lost in the synthesized procedure. Na+ content is obviously larger than that of Li+, which is consistent with our previous discussion.
In addition, it has been proved that codoping Zn2+ into Er3+:Gd2O3 NCs can enhance UC-PL intensity by a factor of about three [22], which is consistent with above analysis since radius of Zn2+ is 0.74 Å and of Gd3+ is 0.94 Å, which is rather similar to the situation of Li+/Er3+:Y2O3 NCs.
In order to investigate the UC-PL mechanisms of the specimens, excitation power dependences of UC-PL intensity were employed. As shown in Fig. 5, photon numbers needed in the UC processes are 1.67 and 1.32 for green and red emission in Er3+:Y2O3 NCs, respectively, and hardly change in other samples (data not shown). Under this circumstance, energy levels of Er3+ are drawn out in Fig. 6 and UC processes are also labeled. It should be noted that there is rather weak ultraviolet peak located at ~409 nm, which comes from the absorption of Er3+ 4F9/2 level, and this path is too weak to influence the green and red emission, hence green and red emissions in two samples are mainly two photon processes.
4 Conclusions
In summary, by comparing the structural data and UC-PL spectra of AM+/Er3+-codoped Y2O3 NCs, we confirm that the enhanced UC-PL intensity aroused by doping Li+ is mainly attributed to Li+ occupying the interstitial sites in lattice rather than neutralizing the O–H group outside the NCs, which renews the understanding of effects of doping Li+ in Y2O3 NCs. Besides that, the effects of Na+ and K+ located at different sites on the UC-PL emission are also investigated.
References
Z.Q. Li, Y. Zhang, Nanotechnology 19, 345606 (2008)
F. Wang, D.K. Chatterjee, Z.Q. Li, Y. Zhang, X.P. Fan, M.Q. Wang, Nanotechnology 17, 5786 (2006)
M.F. Joubert, Opt Mater 11, 181 (1999)
Z.Y. Lin, X.L. Liang, Y.W. Ou, C.X. Fan, S.L. Yuan, H.D. Zeng, G.R. Chen, J Alloys Compd 496, L33 (2010)
J. Ding, Q. Zhang, J.M. Cheng, X.F. Liu, G. Lin, J.R. Qiu, D.P. Chen, J Alloys Compd 495, 205 (2010)
C.F. Xu, Q.B. Yang, G.Z. Ren, Y.X. Liu, J. Alloys Compd 503, 82 (2010)
G. Seisenberger, M.U. Ried, T. Endress, H. Buning, M. Hallek, C. Brauchle, Science 294, 1929 (2001)
F. Sidiroglou, S.A. Wade, N.M. Dragomir, G.W. Baxter, S.F. Collins, Rev Sci Instrum 74, 3524 (2003)
B. Dong, D.P. Liu, X.J. Wang, T. Yang, S.M. Miao, C.R. Li, Appl Phys Lett 90, 181117 (2007)
A.S. Oliveira, M.T. de Araujo, A.S. Gouveia-Neto, A.S.B. Sombra, J.A. Medeiros Neto, N. Aranha, J Appl Phys 83, 604 (1998)
A.S. Oliveira, M.T. de Araujo, A.S. Gouveia-Neto, J.A. Medeiros Neto, A.S.B. Sombra, Y. Messaddeq, Appl Phys Lett 72, 753 (1998)
P.V. dos Santos, E.A. Gouveia, M.T. de Araujo, A.S. Gouveia-Neto, A.S.B. Sombra, J.A. Medeiros Neto, Appl Phys Lett 74, 3607 (1999)
Q. Lü, A.H. Li, F.Y. Guo, L. Sun, L.C. Zhao, Nanotechnology 19, 145701 (2008)
T. Aisaka, M. Fujii, S. Hayashi, Appl Phys Lett 92, 132105 (2008)
Y.F. Bai, Y.X. Wang, K. Yang, X.R. Zhang, Y.L. Song, C.H. Wang, Opt Commun 281, 5448 (2008)
Y.F. Bai, Y.X. Wang, K. Yang, X.R. Zhang, Y.L. Song, Z.Y. Pan, C.H. Wang, J Phys Chem C 112, 12259 (2008)
G.Y. Chen, H.C. Liu, G. Somesfalean, Y.Q. Sheng, H.J. Liang, Z.G. Zhang, Q. Sun, F.P. Wang, Appl Phys Lett 92, 113114 (2008)
Y.F. Bai, K. Yang, Y.X. Wang, X.R. Zhang, Y.L. Song, Opt Commun 281, 2930 (2008)
Q. Cheng, J.H. Sui, W. Cai, Nanoscale 4, 779 (2012)
J.J. Rehr, R.C. Albers, Rev Mod Phys 72, 621 (2000)
G.Y. Chen, H.C. Liu, H.J. Liang, G. Somesfalean, Z.G. Zhang, J Phys Chem C 112, 12030 (2008)
L.L. Xu, Ph. D. Thesis (Harbin Institute of Technology, 2008) (unpublished)
Acknowledgments
We thank Bo He and Fengchun Hu of the National Synchrotron Radiation Laboratory for helpful technical assistance of EXAFS experiment. We also thank the Program for New Century Excellent Talents in University (NCET-04-0333), and the Excellent Youth Fund of Heilongjiang Province (JC-04-04) for providing financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, L., Wang, Y., Bai, Y. et al. Effects of alkali metal ions on upconversion photoluminescence intensity of Er3+-doped Y2O3 nanocrystals. Appl. Phys. B 110, 111–115 (2013). https://doi.org/10.1007/s00340-012-5257-5
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-012-5257-5