Abstract
Unusual bright red-dominant upconversion light was observed in Ho3+/Yb3+ co-doped YF3-BaF2-Ba(PO3)2 glasses excited by the 980-nm laser diode at room temperature. The integral intensity ratios of the red upconversion emission to the green one reached about 10:1 in optimized 0.125Ho3+-15Yb3+ co-doped sample. In order to find out its behind-the-scene mechanism, the optical properties and the phonon-assisted relaxations on the excited levels of Ho3+ in our samples were investigated. Additionally, the effects of the concentrations of the doping ions, excitation pump power, and temperature on the upconversion emissions were also systematically studied. These results revealed that the proper phonon frequency of fluorophosphate glasses, the efficient phonon-assisted relaxations from 5I6 to 5I7 levels (4,960 s−1), and the long lifetime of the 5I7 (about 2.8 ms) levels should be responsible for bright red upconversion emission at a much greater concentration ratio of C 3+Yb /C 3+Ho .
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent years, a number of studies have been carried out to develop rare-earth (RE) ion-doped materials capable of efficient frequency upconversion and to utilize these materials as compact solid-state lasers, biomedical diagnostic labels [1, 2], temperature sensors [3], and clean energy and color display phosphors [4]. Among the RE ions, Ho3+, Er3+, and Tm3+ were the most attractive active ions applied to infrared-to-visible upconversion fluorescence due to their favorable energy-level structures. In addition, upconversion florescence of these ions has been extensively investigated in a variety of crystalline and vitreous materials. Low-cost fluorophosphates glasses were expected to be promising and applicable hosts for the upconversion florescence of RE3+ ions because of their low melting temperature, good homogeneity, improved chemical/physical stability, and flexible optical properties [5]. On the other hand, with the commercialization of the ~980-nm laser diode (LD), Yb3+ has become a popular sensitizer for Ho3+/Er3+/Tm3+ ion to increase the optical pumping efficiency due to its larger absorption cross-section and efficient energy transfer from Yb3+ ions to the active ions.
Under the near-infrared excitations, bright blue- or green-upconversion light was usually dominating in most RE3+ single- or multi-doped materials. Red upconversion emission (~650 nm) can be theoretically generated by the 4F9/2 → 4I15/2 radiative transition of Er3+, 5F5 → 5I8 of Ho3+, and 1G4 → 3F4 of Tm3+. However, red upconversion emission was not so strong when compared with the green ones of Er3+ (2H11/2, 4S3/2 → 4I15/2) and Ho3+ (5F4, 5S2 → 5I8), and the blue one of Tm3+ (1G4 → 3H6). Therefore, bright red-dominant upconversion light was only reported in a few literatures [6–10]. In these cases, red upconversion light was usually caused by the relatively high concentration Ho3+ that leads to the cross-relaxations among different levels of Ho3+ and therefore inhibits green upconversion emission [8–10]. Here we will report a red-dominant upconversion light in YF3-BaF2-Ba(PO3)2 glasses where the intensity ratios of the red emission to the green one were greater than 9:1, even when the concentration ratios of C 3+Yb /C 3+Ho were great as 600:1. In order to investigate this unusual red-dominant light in the present case, the structures of the glasses were evaluated to determine the cut-off frequency of phonons through IR or Raman spectra. The radiative transitions probabilities and the relaxation rates of Ho3+ were calculated through using the Judd–Ofelt theory. The temperature effect on the decay time of the green down-conversion emission of Ho3+ was studied to investigate the efficient phonon-assisted relaxation in the glass. The effects of excitation pump power, the concentration of doping ions, and temperature were also studied.
2 Experimental
The composition of samples used in this paper can be expressed as (in mol %) follows:
where x = 0.05, 0.075, 0.1, 0.125, 0.2, 0.25, 0.3, 1.0, and y = 0, 5, 10, 15, 20, 25, 30. Starting powders were thoroughly ground with agate mortar and pestle, kept at 450 °C for 1 h for the pre-reaction, and then melted at 1,050–1,150 °C for 15 min in a corundum crucible in ambient atmosphere. The melts were poured on a preheated stainless steel plate and then annealed to room temperature. The obtained glasses were cut and polished into 2-mm thick slabs for optical property measurements.
Density was measured according to Archimedes’ principle using distilled water as the immersion liquid. Refractive index was measured on an Abbe refractometer using monobromonaphthalene as adhesive coating. The density and refractive index of YBPF: 1Ho-0Yb sample were determined to be 4.602 g/cm3 and 1.593, respectively. The infrared (IR) absorption spectra of YBPF: 0Ho-0Yb and ABPF glass were measured on a Nicolet/Nexus 670 FT-IR Analyzer using KBr pellets in 1,500–500 cm−1 range. The Raman spectrum in the 300–3,000 cm−1 range was obtained using a Renishaw inVia Microscope with a 514-nm laser excitation.
UV/Vis/NIR Absorption spectrum of YBPF: 1Ho-0Yb in 30,000–5,000 cm−1 range was recorded by a Varian Cary 5000 UV/Vis/NIR spectrophotometer at the scan step of 10 cm−1. Visible up- and down-conversion signals were recorded by an Edinburgh FSP920 Combined Time Resolved and Steady State Fluorescence Spectrometer equipped with thermo-electric cooled red-sensitive PMT. A 450-W Xe900 steady-state xenon lamp (ozone free) and a μF920H flash lamp were used as the excitation sources in steady and dynamic down-conversion luminescence measurement, respectively. The pulse repetition rate of μF920H was 100 Hz and the pulse width was about 1.5–3.0 μs. A ~980-nm centered continue wave (CW) LD with a maximum power of 1 W was used for the excitation source for upconversion luminescence. For the temperature-dependent measurement, the sample was mounted in a 77- to 500-K Oxford Optistat DN-V liquid nitrogen optical cryostat with an ITC601 temperature controller.
3 Results and discussions
3.1 IR and Raman spectra of YBPF glasses
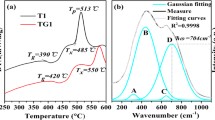
The IR spectrum of the YBPF: 0Ho-0Yb glasses is shown in Fig. 1a and that of ABPF glasses is also presented in for comparison, since the glass-forming ability of Y3+ is similar to that of Al3+ [5], and the structures of Al3+-base (AlF3 and/or Al(PO3)3) fluorophosphate glasses have been investigated more frequently. According to previous studies[11–13], for fluorophosphates glasses containing 10–25 mol % (PO) −3 , the broad band ranging from 800 to 1,400 cm−1 can be ascribed to the stretching and asymmetric vibrations of P–O–P, O–P–O, F–P–F, and/or O–P–F bonds, and the broad band ranging from 500 to 700 cm−1 can be ascribed to the non-bridging bond in M[O]4,6, M[F]4,6 and/or M[O,F]6 anion units (M = Al3+ or RE3+). The ~735-cm−1 band can be ascribed to P–O–P stretching vibration [11, 12], but it may be also ascribed to the non-bridging band in anion units M[O]4 and M[O,F]4 [13]. Due to the larger radius, RE3+ ions incline to form anion units RE[O]6, RE[F]6, and/or RE[O,F]6. And this will break the F–P–F long chain in the glasses, then -P- connect to -O- to form PO2 −/PO3 − units. So, for YBPF samples, their ~910 and 735 cm−1 bands ((P–O–P) as , and (P–O–P) ss ) are more distinct when compared with the ABPF glasses.
Raman spectra of YBPF: 0Ho-0Yb glass are shown in Fig. 1b and six bands are labeled as a–f. According to previous literatures [11, 14, 15], band a centered at 340 cm−1 may relate to the overlapping vibration of the skeletal deformation vibration of metaphosphate chains and PO2 deformation vibration of pyrophosphate segments. Broadband b from 400 to 690 cm−1 and the band c centered at 745 cm−1 are ascribed, respectively, to the group vibration of anion units RE[F]6/RE[O,F]6 and the stretching vibration of P–O–P, consisting of the IR spectra. Band d can be ascribed to the P–F vibration in PO2F2 − unit. The strong band e at 1,045 cm−1 can be ascribed to the symmetry stretching vibration of O–P–O(F) in P2(O,F)7 dimer, since the F−/PO3 − reaches about 9:2 in our sample. And the shoulder band f around 1,130 cm−1 is related to the vibration of O–P–O in metaphosphate tetrahedron.
3.2 UV–Vis–NIR absorption and Judd–Ofelt calculation
The room temperature UV–Vis–NIR absorption spectrum of YBPF: 1Ho-0Yb glass (l = 2.42 mm) is shown in Fig. 2. The absorption bands are attributed to f → f transitions from the ground state 5I8 to the different excited states [16]. The experimental oscillator strengths of these transitions are calculated from
where m and e are the electron mass and charge, respectively, c is the speed of light, l is the thickness of sample, N is the number of absorbing ions in the unit volume, v is the wave number, and OD(v) is the optical density. By a least square fitting of the experimental and theoretical oscillator strengths, the Judd–Ofelt parameters are obtained: Ω 2 = 2.95 × 1020, Ω 4 = 2.61 × 1020 and Ω 6 = 2.15 × 1020 cm−2, respectively.
These J–O parameters are important for the investigation of the local structure and bonding in the vicinity of RE ions. Previous works [17–19] reveal that the Ω 2 is sensitive to symmetry of the RE ions site and the covalence between RE ions and ligand ions, and the Ω 6 is affected by the electronic density of RE3+ [20, 21]. For comparison, the Ω 2, 4, 6 of Ho3+ obtained from YBPF: 1Ho-0Yb and some other hosts are tabulated in Table 1. It can be seen that the Ω2 of our glass as well as other glasses are larger than that of crystalline materials, mainly attributed to its lower symmetry. Among glasses, Ω2 of our glass is smaller than that of pure oxide glass but larger than that of pure fluoride glass, since the covalence of Ho–O band becomes weak due to the strong attraction of F− ions for the electrons of Ho3+. On the other hand, the attraction of F− also decreases the shielding effect of 6s electrons of Ho3+ on the 5d ones, and thus increases the electronic density of the 5d orbit, resulting in an increment on the value of Ω 6. Furthermore, in fluorophosphate glasses, π-electron donation of non-bridging P–O bond [21] to the 5d orbital of Ho3+ also greatly augments the Ω 6.
On the other hand, with the experimental intensity parameters Ω 2, 4, 6, the spontaneous radiative transition probabilities from an initial manifold \( \left. {\left| {(S',L')} \right.J'} \right\rangle \) to a final manifold \( \left. {\left| {(\overline{S} ,\overline{L} )\overline{J} } \right.} \right\rangle \)can be calculated from:
where \( \left\langle {\left\| {U^{(\lambda )} } \right\|} \right\rangle \) are the reduced matrix elements. And then the spontaneous emission life time τ rad of the initial manifold can be yielded as:
The radiative transition probabilities (A ed, A md) and radiative lifetimes (τ rad) of the 5I7, 5F5, and 5S2,5F4 levels are listed in Table 2.
3.3 Phonon-assisted relaxation on excited levels of Ho3+ in YBPF glass
Figure 3a presents the visible down-conversion excitation and emission spectra of YBPF: 0.05Ho-0Yb. The strongest excitation band centered at 450 nm can be ascribed to the 5I8 → 5G6, 5F1 absorption. The emission bands peaks at 547 and 657 nm are assigned to the 5F4, 5S2 → 5I8 and 5F5 → 5I8 radiative transitions, and the emission bands centered at 596 and 749 nm can be assigned to 5F3 → 5I7 and 5F4, 5S2 → 5I7 radiative transitions, respectively. The integral intensity ratio of the red emission to the green one (I657nm/I547nm) is about 0.24. The decay curve of the green emission is depicted in the Fig. 3b. It shows a single-exponential behavior and gives the experimental decay time τexp of 17.1 μs. In 0.05 % Ho3+ singly doped sample, the cross-relaxation among the excited levels can be neglected, and the relaxation rate W R of the 5F4,5S2, and 5F5 levels can be calculated from
At room temperature, the W R of the 5S2, 5F4 level is 50,553 s−1 and it is much greater than the A rad of 5S2, 5F4 level listed in Table 2. This should relate to the high cut-off frequency phonon of the fluorophosphate glasses. As Raman spectra (Fig. 1b) shows, YBPF glasses have the strongest absorption at 1,045 cm−1, which has been ascribed to O–P-O(F) SS in P2(O,F)7 dimer. Meanwhile, the UV–Vis-NIR absorption spectra (Fig. 2) shows that the energy gap between the 5S2, 5F4, and 5F5 levels is about 2,770 cm−1. By coupling with the vibrations in the glass and emitting about three phonons, Ho3+ in the 5S2, 5F4 state relaxes fast to 5F5 and then generates red emission. It must be noted that the down-conversion fluorescence is still green-dominant due to the greater A ed of the 5S2 + 5F4 → 5I8 radiative transition. Meanwhile, the multi-phonon-assisted relaxation (MPR) on the 5F5 level works also efficiently (τexp = 19.7 μs, W R = 46,805 s−1) and further reduces the red emission intensity [26, 27].
In order to prove the 3-phonon-assisted relaxation on the 5S2, 5F4 level, the temperature effect on the decay time τexp and multi-phonon-assisted relaxation rate (W MPR) of the 5S2, 5F4 level are investigated as shown in Fig. 4a, b, respectively. As temperature increases from 77 to 490 K, the τexp decreases from 17.2 to 14.5 μs while the W MPR increases from 50,258 s−1 to 61,084 s−1. According to previous works of M. J. Weber and H. W. Moos et al. [28–32], the relation between the W MPR of an excited level and temperature can be written as
a Temperature dependence of the experimental decay time of the 547 nm down-conversion emission; b Temperature dependence of the multi-phonon relaxation rate of the 5S2, 5F4 level. Solid line is the temperature-relaxation model obtained from Eq.(5) assuming hω = 1,045 cm−1 and n = 3
where h is the Planck constant, k is the Boltzmann constant; ΔE is the energy gap to the next-lower level, ω is the cut-off frequency of the phonon, n is the order of the MPR process required to bridge the energy gap, and n = ΔE/hω, W R(0) is the decay rate at 0 K, but here we use the rate at 77 k. For the 5S2, 5F4 level of Ho3+ in our sample, Fig. 4b shows the observed data well fit to the model calculated from Eq.(5) by assuming three 1,045 cm−1 phonons were engaged.
These phonon-assisted processes are also efficient for the relaxations with the energy gaps wider than 3,000 cm−1. However, when the energy gaps are wider than 5,000 cm−1, the W MPRs of the relaxation become comparably small since five or more phonons are needed to bridge the gaps. The dependence of the relaxation rate W MPR on the energy gap ΔE to the next-lower level can be expressed as [29–31]
where the W 0 is the relaxation rate when ΔE = 0, and α is host-dependent constant. The data on the W MPR of some excited levels of Ho3+, Er3+, and Tm3+ in YBPF glass at room temperature are plotted semi-logarithmically versus ΔE in Fig. 5, and the α is fitted to be 1.97 and the W 0 to be 3.64 × 106 s−1. This linear plot is helpful to study the relaxations on some levels when the actual lifetimes of these levels cannot be experimentally obtained, such as the 5I6 and 5I7 levels of Ho3+ in our case. According to Eqs.(6) and (4), the W MPR of the 5I6 levels is estimated to be close to about 4,600 s−1 (ΔE = 3,540 cm−1) and thus the level lifetime decreases from 4.0 ms (τrad) to about ~210 μs, while the W MPR of the 5I7 level (ΔE = 5,150 cm−1) is estimated to be about ~220 s−1, and the level lifetime reaches about ~3.0 ms.
3.4 Red-dominant upconversion light of Ho3+/Yb3+ co-doped samples
Figure 6a shows the photograph and upconversion emission spectrum of YBPF: 0.125Ho-15Yb excited by a 980-nm CW LD with the pump power (P ex ) about 450 mW/mm2 at room temperature. The green emission band peaking at 542 nm corresponds to the 5F4, 5S2 → 5I8 transition and the red emission peaking at 657 nm corresponds to the 5F5 → 5I8 transitions. The weak blue broad emission centered at 476 nm is ascribed to the 5F3 → 5I8 transition in many previous reports, but here it more probably comes from the cooperative upconversion of Yb3+ pairs in Yb[F]6/Yb[O,F]6 cluster [33] since it is also observed in the samples singly doped with Yb3+. As the photograph shows, YBPF: 0.125Ho-15Yb gives off bright red-dominant upconversion light, the intensity ratio of I657nm/I542nm reaches 10:1. This red light could be readily seen with the naked eye at pump power as low as 60 mW/mm2. The effect of concentration of Ho3+ and Yb3+ on the intensities of upconversion emissions is shown in Fig. 6b, c. Since the concentration of Ho3+ is not high enough to induce intense cross-relaxations among different levels of Ho3+, the effects of increasing Ho3+ on the emissions intensities are not very notable. On the other hand, due to the strong absorption of Yb3+ around 980 nm and the efficient energy transfer from Yb3+ to Ho3+, increasing Yb3+ concentration benefits the populations on the excited levels of Ho3+ and thus greatly enhances the emission intensities before the intense concentration quenching (C 3+Yb = 30 mol %). For all the co-doped glasses, the red emission is much stronger and I657nm/I542nm increases from about 9:1 to 13:1 as the concentration ratio of C 3+Yb /C 3+Ho decreases from 600:1 to about 16:1.
In frequency upconversion processes, the upconversion emission integral intensity I UP will be proportion to the nth power of the pump power P ex, i.e.,
where n is the number of pump photons required to excite the emitting state. Such plots for both the green (5F4, 5S2 → 5I8) and red (5F5 → 5I8) emissions in YBPF: 0.05Ho-15Yb and YBPF: 0.5Ho-25Yb samples are presented in Fig. 7. The values of n obtained are all close to two, indicating that two-photon absorption processes are responsible for three upconversion emission bands. Due to a lower doping concentration of Ho3+, no obvious inflections in these plots are observed, thus ruling out a photon avalanche upconversion mechanism [34, 35].
According to the concentration and pump-power dependences of the green and red upconversion emissions, the two-photon upconversion mechanisms for these emissions are briefly illustrated in Fig. 8. Firstly, Yb3+ ions are excited from the 2F7/2 to 2F5/2 level by absorbing laser photons due to their large absorption cross-section around 980 nm. Because the 2F5/2 level of Yb3+ mismatches the 5I6 level of Ho3+, then ground Ho3+ is promoted to the 5I6 levels by receiving a phonon-assisted transfer from Yb3+. For the green emission, Ho3+ on the 5I6 level is promoted to the 5F4,5S2 levels directly by absorbing energy of laser photons (excited state absorption, ESA) or excited Yb3+ ions (energy transfer upconversion, ET). For the red emission, two possible upconversion channels are responsible for the population of the 5F5 level: (I) The previously mentioned MPR from the 5F4,5S2 to 5F5 level. Apparently, this channel will result in a green-dominant upconversion light according to Fig. 3a. (II) The promotion from the 5I7 level that populated via the MPR from the 5I6 level. According to the discussion in Part 3.3, this 5I6 → 5I7 relaxation is very fast, and thus makes the 5I7 level efficiently populated after ground Ho3+ is excited. Meanwhile, the lifetime of the 5I7 level is estimated to be about 3.0 ms, and it is long enough for the promotion to the 5F5 level via ESA and/or ETU. In summary, in our upconversion case, the channel II is considered to be the major channel for the red upconversion emission. Moreover, this also will explain the much larger intensity ratio of I657nm/I542nm in the upconversion case, because the channel II generates only the red emission throughout whole upconversion process.
Simplified energy-level diagram of Ho3+ and Yb3+ ions and upconversion luminescence mechanism for our samples. Solid straight lines with upward and down arrows denote pumping and upconversion transitions, and radiation transitions; dot lines denote energy transfer; MPR, ESA, ETU and PET are the acronyms for the multi-phonon-assisted relaxation, excited state absorption, energy transfer upconversion and phonon-assisted energy transfer, respectively
3.5 Temperature effect on the red-dominant upconversion light
Figure 9 and inset show the effects of temperature on the intensities of the green and red upconversion emissions in YBPF: 0.125Ho-15Yb (P ex ≈ 150 mW/mm2). Due to the thermally enhanced non-radiative relaxations on all the excited levels of Ho3+, both the green and red emissions intensities decrease drastically as temperature increases−. On the other hand, increasing temperature facilitates the 5I6 → 5I7 and 5S2, 5F4 → 5F5 MPRs, and then augments the intensity ratio of I657nm/I542nm from about 6:1 to 18:1 as Fig. 10 shows. This increment on the intensity ratio in the upconversion case is much greater than that in down-conversion case, further confirming that the bright red upconversion is mainly decided by the MPR relaxation on the 5I6 level. Moreover, this increment is also greater than that induced by the concentration variations of Ho3+ and/or Yb3+, indicating the red-dominant light of samples is mainly decided by the proper phonon energy of the host but not the doping concentrations of RE3+ ions.
Increasing temperature also affects the spectrum shape of the red emission. Upconversion spectra in Fig. 9 inset show that the ratio of the signal intensity at 643 nm to that at 657 nm increases from about 0.60–0.84 as temperature increases from 100 to 460 K. This ~643 (657) nm upconversion signal that can be ascribed to the 5F5 → 5I8 transitions starting from the higher (lower) crystal-field components of the 5F5 level and ending at lower (higher) crystal-field components of the 5I8 level. And because the energy difference between each components in the 5F5 and 5I8 states are very small (a few kT), the higher crystal field components of the 5F5 levels can be populated from the lower ones through the thermal agitation. Furthermore, the full width at half maximum (FWHM) increases from about 21.3 to 25.2 nm as temperature increases, and this can be related to the thermally enhanced electron–phonon interaction [36, 37].
4 Conclusions
Ho3+ singly doped and Ho3+/Yb3+ co-doped YBPF glasses were fabricated and characterized. The experimental intensity parameters Ω 2, 4, 6, spontaneous radiative transition probability and radiative decay time were calculated using Judd–Ofelt theory. The temperature dependence of relaxation rate of the 5S2, 5F4 → 5F5 was studied and the result revealed the 5S2, 5F4 → 5F5 relaxation was a 3-phonon-assisted process. For all Ho3+/Yb3+ co-doped samples excited by a 980 nm CW LD at room temperature, a weak green emission peaking at 542 nm and a strong red emission peaking at 657 nm were recorded. This strong red emission could be interesting for its applications on the biomedical labels and color display. Power-dependent studies revealed that both green and red emissions of the samples result from 2-photon absorption processes. All the results showed the bright red-dominant upconversion light of YBPF: Ho3+/Yb3+ were decided by proper phonon energy of the samples: (1) The fast phonon-assisted relaxation from the 5I6 level was favorable to the population on the 5I7 level. (2) The lifetime of 5I7 level is long enough to receive energy for the promotion to the 5F5 level, from where the bright red emission originates.
References
S.A. Wade, S.F. Collins, G.W. Baxter, J Appl Phys 8, 4743 ((2003))
F. Vetrone, R. Naccache, V. Mahalingam, C.G. Morgan, J.A. Capobianco, Adv Funct Mater 19, 2924–2929 (2009)
N.K. Giri, D.K. Rai, S.B. Rai, J. Appl Phys. 104, 113107 (2008)
J. Yang, C. Zhang, C. Peng, C. Li, L. Wang, R. Chai, J. Lin, Chem Eur J 15, 4649 (2009)
F.X. Gan, Optical Glass, Bejing Science Press 301 (1985)
L. Feng, J. Wang, Q. Tang, L. Liang, H. Liang, Q. Su, J Lumin 124, 187 (2007)
L. Feng, Q. Su, Y. Li, C. Zheng, C. Wang, H. Du, Spectrochim Acta Part A 73, 41 (2009)
W. Xiong, P. Yang, J. Liao, S. Lin, J Cryst Growth 280, 212 (2005)
H. Wang, C. Tu, Z. You, F. Yang, Y. Wei, Y. Wang, J. Li, Z. Zhu, G. Jia, X. Lu, Appl Phys B 88, 57 (2007)
N.M. Sangeetha, F.C.J.M. van Veggel, J Phys Chem C 113, 14702 (2009)
B. Karmakar, P. Kundu, R.N. Dwivedi, J Non-Crystal Solids 289, 155 (2001)
R. Lebullenger, L.A.O. Nunes, A.C. Hernandes, J Non-Crystal Solids 284, 55 (2001)
A.M. Efimov, J Non-Crystal Solids 209, 209 (1997)
M.S. Liao, Y.Z. Fang, H.T. Sun, L.L. Hu, Opt Mater 29, 867 (2007)
L. Zhang, L. Wen, J. Zhang, L. Hu, Mater Chem Phys 91, 166 (2005)
W.T. Carnall, P.R. Fields, K. Rajnak, J Chem Phys 49, 4424 (1968)
M.J. Weber, J Non-Crystal Solids 74, 167 (1985)
C.K. Jørgensen, B.R. Judd, Mol Phys 8, 281 (1964)
S. Tananbe, T. Ohyagi, N. Soga, T. Hanada, Phys Rev B 46, 3306 (1992)
S. Tanabe, T. Ohyagi, S. Todoroki, T. Hanada, N. Soga, J Appl Phys 73, 8451 (1993)
H. Ebendor-Heidepriem, D. Ehrt, M. Bettinelli, A. Speghini, J Non-Crystal Solids 240, 66 (1998)
Q. Su, Q.Y. Wang, S.X. Wu, Chin Chinese J Lasers 166, 12 (1989)
B. Peng, T. Izumitani, Opt Mater 4, 797 (1995)
K. Tanimura, M.D. Shinn, W.A. Sibley, Phys Rev B 30, 2429 (1984)
R. Van Deun, K. Binnemans, C. Görller-Walrand, J.L. Adam, J Alloys Compd 283, 59 (1999)
T. Suhasini, B.C. Jamalaiah, T. Chengaiah, J. Suresh Kumar, L. Rama Moorthy, Physica B 407, 523 (2012)
D. Kasprowicz, M.G. Brib, A. Majchrowski, E. Michalski, A. Biadasz, J Alloys Compd 509, 1430 (2011)
E.D. Reed Jr, H.W. Moors, Phys Rev B 8, 980 (1973)
W.D. Partlow, H.W. Moos, Phys Rev 157, 252 (1967)
L.A. Riseberg, H.W. Moos, Phys Rev 174, 429 (1968)
M.J. Weber, Phys Rev B 8, 54 (1973)
C.B. Layne, W.H. Lowdermild, M.J. Weber, Phys Rev B 16, 10 (1977)
F. Auzel, D. Meichenin, F. Pellé, P. Goldner, Opt Mater 4, 35 (1994)
F. Lahoz, I.R. Martín, J.M. Calvilla-Quintero, Appl Phys Lett 86, 051106 (2005)
V. Lavín, F. Lahoz, I.R. Martín, U.R. Rodríguez-Mendoza, J.M. Cáceres, Opt Mater 27, 1754 (2005)
M. Sendova-Vassileva, M. Iliev, A.V. Chadwick, J Phys Condens Matter 3, 5407 (1991)
G. K. Liu; Spectroscopic Properties of Rare Earths in Optical Materials (Springer, Berlin, 2005)
Acknowledgments
This work was supported by the National Nature Science Foundation of China (20971130, 20501023), the project of the combination of Industry and Research by the Ministry of Education and Guangdong Province (2008B090500027), and the Nature Science Foundation of Guangdong (5300527) and the Science and Technology Project of Guangzhou (2005Z2-D0061).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lai, B., Feng, L., Zhang, J. et al. Multi-phonon-assisted relaxation and Yb3+ sensitized bright red-dominant upconversion luminescence of Ho3+ in YF3-BaF2-Ba(PO3)2 glass. Appl. Phys. B 110, 101–110 (2013). https://doi.org/10.1007/s00340-012-5256-6
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-012-5256-6