Abstract
In this study, we have investigated the principal role of Y2O3 on the emission features of Tm3+ ion and up-conversion phenomenon in Tm3+ and Yb3+ co-doped Li2O–Y2O3–SiO2 glass system. The concentration of Y2O3 is varied from 0 to 5 mol% while that of Yb3+ and Tm3+ is fixed. When the glasses are doped with Tm3+ ions, the intense blue and red emissions were observed, whereas Yb3+ doped glasses exhibited NIR emission at about 980 nm. When the glasses are co-doped with Tm3+ and Yb3+ ions and excited at 900 nm, the blue and red emission lines were observed to be reinforced and strengthened with increase in the concentration of Y2O3. The IR emission band detected at about 1.8 μm due to 3F4 → 3H6 transition of Tm3+ ions is also observed to be strengthened due to co-doping. The reasons for enhancement in the intensity of various emission bands due to co-doping have been identified and discussed with the help of rate equations for various emission transitions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The short wavelength solid-state lasers are being used for a variety of applications such as color displays, high density optical data storage and reading, biomedical diagnostics, infrared laser viewers and indicators, biomedicine, etc.
One of the effective ways of producing short wavelength lasers is the frequency up-conversion process using rare earth ion-doped glass materials; this process is based on conversion of near-infrared photons into visible photons via multiphoton [1]. The pioneering work of Auzel [2] on energy transfer demonstrated the advantages of an activator–sensitizer combination in laser hosts for improving the quantum yield of photons at the lasing wavelength. The rare earth host materials with low phonon energy can reduce non-radiative losses due to the multi-phonon relaxation and thus offer suitable environment for up-conversion process [3–5]. Further, it is well-known that the rare earth doped oxide glasses are of particular interest for the photoinduced nonlinear optics. In fact, rigorous studies along these lines on several glass systems were reported earlier by Kassab et al. [6, 7].
Among different rare earth ion-doped glasses, the Tm3+ ion-activated glasses have gained much attention because of the fact that Tm3+ ion possesses two meta-stable excited levels viz., 1D2 and 1G4, in its energy level structure for displaying blue emission either from its normal emission or by up-conversion luminescence process [8, 9].
Further, among different rare earth ions, Yb3+ ion is considered as a promising sensitizer for Tm3+ ions in glass matrices due to its significantly high absorption cross-section in the NIR region and favourable energy level structure. The ground state of trivalent ytterbium ion (Yb3+) is 2F7/2. The doublet splitting of this state is approximately 10,000 cm−1 with the 2F5/2 component lying above the ground state, 2F7/2, with the average radiative lifetime ~0.28 ms. Yb3+ ion is reported to act as a donor or alternately as an energy transfer bridging ion between a donor and an acceptor ion in several glass systems [10]. In fact, efficient energy transfer processes from Yb3+ to Tm3+ ions [11] when excited at the wavelength corresponding to 2F7/2 → 2F5/2 transition of Yb3+ ion, intense R, G and B emissions were reported in different kinds of glass matrices [12–17].
For lasing ions, it is well-known that the combination of the energy gaps between the excited level and the one just below it and the maximum phonon energy (MPE) plays an important role in the non-radiative relaxation rate that influences luminescence efficiency significantly. For Tm3+ ions, different host materials show a great difference in the MPE value. Two most common host materials used for Tm3+ ions are silica and fluoride glass. Their MPE differs about several times, being 1,100 cm−1 (silicate) and 550 cm−1 (fluorides), respectively [18]. Large MPE of the silicate glasses limits its infrared transparency range less than 2.2 μm and improves its multi-phonon relaxation rates.
The thermo-physical, chemical and mechanical stability of the lithium silicate glasses can be improved by adding sesquioxides like Y2O3. In fact, the addition of Y2O3 to this type of glass systems is reported to enhance refractive index and to lower the phonon energy considerably. For these reasons, the glasses mixed with Y2O3 were proved to be high efficient luminescence materials [19, 20] and are being considered as good candidates for integrated optics, photonic and biomedical applications [21]. Further, solid-state laser materials consisting of Y2O3, were proved to have the efficient operation both in continuous wave operation and in pulsed regimes [22].
The principal goal of the present work is to study the fluorescent features of Tm3+ and Yb3+ ions and the up-conversion phenomenon involved in the Li2O–Y2O3–SiO2 glass system co-doped with Tm3+ and Yb3+ ions as a function of Y2O3 concentration. The variation of Y2O3 content in the glass network is expected to inculcate the structural modifications and local field variations around Ln3+ ion embedded in the glass network which influence luminescence transition probabilities.
2 Experimental
The following composition contents were chosen for the present study:
(49−x) Li2O–xY2O3–50SiO2: 1.0Tm2O3, (49−x) Li2O–xY2O3–50SiO2: 1.0Yb2O3 and (49−x) Li2O–xY2O3–50SiO2: 0.5Tm2O3 + 0.5Yb2O3 with x = 1.0–5.0 mol%.
The details of the composition are presented in Table 1.
Appropriate amounts of analytical grade reagents of Li2CO3, SiO2, Y2O3, Tm2O3 and Yb2O3 powders (Metall, China), all in mol%, were thoroughly mixed in an agate mortar and melted in a platinum crucible in the temperature range of 1,400–1,450 °C in an automatic temperature-controlled furnace for about 1 h. The resultant bubble-free melt was then poured in a brass mould and subsequently annealed at 400 °C. The samples prepared were ground and optical polished to the dimensions of about 1 cm × 1 cm × 0.2 cm. The density d of the bulk samples was determined (to an accuracy of ±0.0001) by the standard principle of Archimedes’ using o-xylene (99.99 % pure) as the buoyant liquid. The mass of the samples was measured to an accuracy of 0.1 mg using Ohaus digital balance, Model AR2140 to evaluate the densities. The refractive index (n d) of the samples was measured (at λ = 589.3 nm) using Abbe refractometer with mono bromo naphthalene as the contact layer between the glass and the refractometer prism. There is a provision in this apparatus to measure the refractive index up to an accuracy of ±0.0001. The methods adopted for recording optical absorption, photoluminescence were similar to those reported in our earlier papers [23, 24]. The fluorescence decay curves were recorded using spectrofluorometer Fluorolog-3 (HORIBA Jobin Yvon) fitted with pulsed xenon lamp of 450 W.
3 Results
From the measured values of the density and average molecular weight M of the samples, various other physical parameters such as rare earth ion concentration N i, mean rare earth ion separation R i and polaron radius for all the glass samples were evaluated and presented in Table 2. The dependence of density d on the concentration of Y2O3 for all the titled glasses shows slight enhancement with increasing Y2O3 content. The increasing density can be understood due to the replacement of Li2O (molecular weight 29.88) by Y2O3 (molecular weight 225.81).
The optical absorption spectra (Figs. 1, 2) of Tm3+ doped Li2O–Y2O3–SiO2 glasses mixed with different concentrations of Y2O3 recorded at room temperature in the wavelength range 400–1,800 nm, have exhibited the following absorption bands: 3H6 → 1G4, 3F2, 3F3, 3H4, 3H5 and 3F4. Among these, 3H6 → 3H5, 3F4 transitions are found to be in the near-infrared region, 3H6 → 1G4 in the blue region, whereas 3H6 → 3F2, 3F3, 3H4 transitions are in orange and red spectral regions. As shown in the Fig. 1, due to strong absorption of the host glass in the ultraviolet range, the absorption bands at wavelength shorter than 400 nm (viz., transitions to the multiplets above 1G4) could not be observed. Further, the NIR band corresponding to 3H6 → 3H5 transition is found to be relatively sharper in the spectra of all the glasses. The influence of Y2O3 content on the strength of absorption on each band could clearly be seen from these spectra.
Experimental oscillator strengths (OS) of the absorption transitions are estimated from the absorption spectra in terms of the area under an absorption peak. The theoretical OS have been evaluated using the modified Judd–Ofelt theory as proposed in reference [25]. The OS of the electric dipole transition between two states have been calculated using the J–O theory, with the conventional equation [26, 27]. The \( \left\| {U^{{{\uplambda}}} } \right\| \) reduced matrix elements used for evaluating OS have been re-calculated, using recent literature data on Tm3+ ions. Hamiltonian parameters were taken from Ref. [28]. The procedure of fitting of the calculated OS to those evaluated from the experimental spectra is described in [29]. A set of matrix equations (which includes the U2, U4, and U6 matrices, the matrices of the experimental OS and the energies of the corresponding transitions) have been solved to minimize the difference between the calculated \( f_{\text{calc}} \) and observed \( f_{\exp } \) OS. The quality of fitting determined by the root mean squared deviation is presented in Table 3. The values of Ωλ are found to be in the following order: Ω2 > Ω4 > Ω6. The comparison of the data on Ωλ parameters of Tm3+ ions in various other glass matrices [30–35] indicated the similar trend. The detailed values of Ωλ for the Tm3+ doped glasses mixed with different concentrations of Y2O3 are presented in Table 4. The Ω2 parameter is observed to decrease considerably with the concentration of Y2O3.
Figure 3 represents the optical absorption spectra of Yb3+ doped Li2O–Y2O3–SiO2 glasses recorded at room temperature in the wavelength region 800–1,200 nm. The spectra have exhibited an absorption band with a degeneracy corresponding to localized 2F7/2 → 2F5/2 transition of Yb3+ ion spreading over a spectral range covering 900–1,000 nm [36]. In the absorption spectra of co-doped glasses (Fig. 4), the bands due to transitions of both the ions are present. However, with the increase in the concentration of Y2O3 a significant increase in the intensity of all absorption bands is observed.
The luminescence spectra of all the studied glasses recorded at room temperature in the visible and NIR regions are shown in (Figs. 5, 6), respectively. The spectra exhibited the following prominent emission bands:
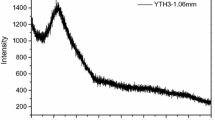
The luminescence spectra of Yb3+ solely doped Li2O–Y2O3–SiO2 glasses excited at 900 nm exhibited a broad luminescence band corresponding to standard 2F5/2 → 2F7/2 transition of Yb3+ ions (Fig. 7). As the concentration of Y2O3 is increased, the spectral intensity of this band is found to increase.
Figure 8 represents the luminescence spectra of Tm3+ and Yb3+ co-doped Li2O–Y2O3–SiO2 glasses excited at 400 nm. The spectra exhibited all emission bands due to both the ions including 2F5/2 → 2F7/2 transition of Yb3+ ions.
In Fig. 9, the luminescence spectrum for one of the co-doped Li2O–Y2O3–SiO2 glasses (viz., TmYbY5) excited at 900 nm is presented. The spectrum exhibited following intense R, B, G and NIR emission bands due to up-conversion phenomenon.
As the concentration of Y2O3 is increased the intensity of these three bands is observed to increase. In addition, the band due to 2F5/2 → 2F7/2 transition of Yb3+ ions is also observed in the spectra of all the glasses.
Figure 10 represents the energy level diagram containing the up-conversion transitions for one of the co-doped glasses (viz., TmYbY5) excited at 900 nm. Using these spectra and Ωλ parameters, the radiative transition probability, radiative life time \( \tau \) of upper level and the branching ratio \( \beta_{{{\text{JJ}}'}} \) for each transition are evaluated and presented in Table 5. Following the shapes of spectral lines, we have estimated approximately scattering losses and found that these are less than 4 % as such do not disturb the principal peaks.
4 Discussion
Among various constituents of Li2O–Y2O3–SiO2 glass composition, SiO2 is a well-known glass former and participates in the glass network with tetrahedral [SiO4/2]0 units and all the four oxygens in SiO4 tetrahedral are shared. On addition of modifiers like Li2O, the Si–O–Si linkage is broken and form Si–O− termination. Thus, the structure is depolymerised and there will be formation of meta, pyro and ortho-silicates viz., [SiO4/2]0, [SiO3/2O]−, [SiO2/2O2]2−, [SiO1/2O3]3− and [SiO4]4− as per the following equilibrium [37]:
In general, Y2O3 participates in the glass network with tetrahedral (YO4) and octahedral (YO6) occupancy. The YO4 tetrahedra alternate with SiO4 groups, whereas octahedral yttrium ions occupy interstitial positions [38] and induce bonding defects in the glass network.
The concentration of tetrahedral and octahedral positions of yttrium ions depends upon the content of Y2O3 in the glass network [39, 40]. The ytterbium ion is expected to be coordinated by three SiO4/YO4 tetrahedral ligands. The evidence for six-fold coordination of Yb3+ in different glasses was derived from the application of crystal field theory on the basis of optical absorption and emission spectra by Robinson et al. [41]. The YbO6 spheres can be assumed to be surrounded by yttrium ions of different cationic field strengths and polarizabilities. Although such specific coordination number cannot be assigned to Tm3+ ions, the variation in the concentration of yttrium ions also causes variations in the crystal field strength around Tm3+ ions. Such variations in the concentration of different structural units of Y2O3 and silicate groups around Ln3+ ions might be responsible for the observed differences of the luminescence intensity of various transitions of these samples.
Out of the three J–O parameters, the values of Ω4 and Ω6 are expected to be influenced strongly by the vibrational levels associated with the central rare earth ions bound to the ligand atoms, whereas Ω2 depends on the covalence and distortion related to structural changes in the neighbourhood of rare earth ions (short-range effect).
The observed decreasing trend of Ω2 parameter with the increase in the content of Y2O3 indicates decreasing interaction of rare earth ions with the ions of the host materials due to increasing distortion or structural change in the vicinity of rare earth ions. As the concentration of Y2O3 is increased, there may be an increasing concentration of YO6 structural units that induce bonding defects. Hence, the larger the concentration of modifying ions, the larger will be the average distance between silicates, inter lithium silicates and Si–Y chains. This ultimately leads to the increase of average bond length of Tm–O causing weaker field around Tm3+ ion. This leads to the decreasing value of Ω2 with increase in the concentration of Y2O3.
For Tm3+ doped glasses (TmY5), the value of β (branching ratio) of the blue emission (1D2 → 3F4 and 1G4 → 3H6) is found to be ~50 %, whereas for the red emission (1G4 → 3H5) it is ~39 %; a slight variation in these values could be observed with Y2O3 concentration.
The fluorescence decay curve of the blue emission corresponding to 1G4 → 3H6 transition of Tm3+ doped glass (TmY5) is shown in (Fig. 11); the curve viewed to be single exponential. The decay curves recorded for other glasses have also exhibited similar behavior. The radiative life time τ, evaluated from these curves for the transition 1G4 → 3H6 is presented in Table 6.
The radiative decay rate for the given upper level is defined as \( \frac{1}{{\tau_{\text{m}} }} = A_{\text{rad}} \). In addition, energy transform multi-phonon decays (harmonic and anharmonic) may also contribute to the above term. Overall, for the studied samples the radiative life time is observed to increase with the increase in concentration of Y2O3 indicating decreasing trend of vibrational losses with increase in the concentration of Y2O3. Thus, the measurements on radiative life times also support the viewpoint that there is a decreasing tendency of interaction between the rare earth ions and glass network with increase in the concentration of Y2O3.
Up-conversion mechanism involved Yb3+ and Tm3+ co-doped glasses can be understood as follows: With 900 nm excitation of the co-doped glasses, the Yb3+ ion goes to 2F5/2 excitation level and de-excites to the 2F7/2 level. The resulting emitted photon excites the Tm3+ ions from 3H6 to 3H5, 3F4 to 3F3 and 3H4 to 1G4. The 1G4 level is further populated by non-radiative decays and internal excitations viz., three-photon absorption processes shown as a, b and c in Fig. 10. Subsequent transitions from 1G4 to 3H5, 3F4 and 3H6 give rise to blue, green and red emissions, respectively.
The corresponding rate equations for the Tm3+ ion under co-doping conditions can be written as:
For Tm3+ ions:
For Yb3+ ions:
Solving Eqs. (1–6) as per the procedure reported in our earlier papers [23] the population of the energy level 5 (1G4) is estimated as
In the above equations n 0 and n i represent the populations of the ground state and the excited level i, respectively, W ij represents transitions between levels i and j of the rare earth ion, τ i indicates the life time of level i of rare earth ion and C i represents the energy quenching rates. The absorption cross-section for each transition is denoted by V ij , whereas the incident pumping flux is indicated by φ.
The total population of 1G4 level represented by Eq. (7) depends on up-conversion from ground state of Yb3+ ions and also from its own levels due to step wise up-conversion mechanism as explained in the text. The energy transfer efficiency parameter (η eff) from Yb3+ to Tm3+ can be written as:
The value of η eff for the emission from 1G4 level for the glass, TmYbY5, is found to be 38 %. The variation of η eff indicated an increasing trend with increasing the concentration of Y2O3 (Table 7).
5 Conclusions
We have established principal role of Y2O3 on the features of the photoluminescence spectra of Tm3+, Yb3+ ions in Li2O–Y2O3–SiO2 glass system. With increase in the concentration of Y2O3 a slight red shift in the peak positions with considerable increase in the intensity of various emission bands could clearly be seen. When Tm3+ and Yb3+ are present together in the glass, the incident radiation 2F5/2 → 2F7/2 (900 nm) excites both the ions. For Tm3+ ion, the excitation takes place from 3H6 to 1G4 level by absorbing three photons and transitions takes place to 3H6, 3H4 and 3F4 levels giving rise to blue, green and red emission.
The observed decreasing trend of Ω2 parameter with the increase in the content of Y2O3 indicates decreasing interaction of rare earth ions with the ions of the host materials. This is due to the increasing distortion or structural change in the vicinity of rare earth ions due to the growing presence of octahedral yttrium ions that act as glass modifiers.
The 3H4 level of Tm3+ ions is populated due to the non-radiative relaxation from 3F2 or 3F3 levels and de-excited to 3F4 level. In view of these, more intense 3H4 → 3F4 (~1.5 μm) NIR emission in the co-doped samples is observed in the glasses mixed with higher concentration of Y2O3.
References
Z. Pan, S.H. Morgan, A. Loper, V. King, B.H. Long, J. Appl. Phys. 77, 4688 (1995)
F.E. Auzel, Proc. IEEE 61, 758 (1973)
R. Naccache, F. Vetrone, J.C. Boyer, J. Capobianco, Mater. Lett. 58, 2207 (2004)
K. Hirao, S. Kishimoto, K. Tanaka, S. Tanaba, J. Non-Cryst Solids 139, 151 (1992)
S. Tanaba, S. Yoshii, K. Hirao, N. Soga, Phys. Rev. B 45, 4620 (1992)
L.P.R. Kassab, R.D.A. Pinto, R.A. Kobayashi, M. Piasecki, P. Bragiel, I.V. Kityk, J. Phys. D Appl. Phys. 40, 1642 (2007)
L.R.P. Kassab, K.J. Plucinski, M. Piasecki, K. Nouneh, I.V. Kityk, A.H. Reshak, R. de A Pinto, Optics Commun, 281, 3721 (2008)
G. Poirier, F.C. Cassanjes, C.B. de Araujo, V.A. Jerez, S.J.L. Ribero, Y. Messaddeq, M. Poulain, J. Appl. Phys. 93, 3259 (2003)
J. Li, X. Wang, Z. Jiang, Optics Commun. 282, 4249 (2009)
N.K. Giri, A.K. Singh, D.K. Rai, S.B. Rai, Opt. Commun. 281, 3547 (2008)
S.F. Leon-Luis, J. Abreu-Afonso, J. Pe˜na-Martinez, J. Mendez-Ramos, A.C. Yanes, J. del-Castillo, V.D. Rodriguez, J. Alloys Compd. 480, 706 (2009)
S.J. Zeng, G.Z. Ren, C.F. Xu, J. Alloys Compd. 509, 2540 (2011)
A.S. Aleksandrovsky, I.A. Gudim, A.S. Krylov, A.V. Malakhovskii, V.L. Temerov, J. Alloys Compd. 496, 18 (2010)
P.V. dos Santos, M.V.D. Vermelho, E.A. Gouveia, M.T. de Arau′jo A.S. Gouveia-Neto, F.C. Cassanjes, S.J.L. Ribeiro, Y. Messaddeq, J. App. Phys. 90, 6550 (2001)
S. Ye, B. Zhu, J. Luo, J. Chen, G. Lakshminarayana, J. Qiu, Opt. Express 16, 8989 (2008)
X. Pei, Y. Hou, S. Zhao, Z. Xu, F. Teng, Mater. Chem. Phys. 90, 270 (2005)
X. Li, S. Bo, X. Shengchun, L. Jingwen, J. Rare Earths 28, 194 (2010)
I.T. Sorokina, K.L. Vodopyanov (eds.): Solid-state mid-infrared laser sources, topics. Appl. Phys. 89, 219 (2003)
Ch. Srinivasa Rao, T. Srikumar, Y. Gandhi, V. Ravikumar, N. Veeraiah, Philos. Mag. 91, 958 (2011)
H.R. Fernandes, D.U. Tulyaganov, A. Goel, J.M.F. Ferreira, J. Euro, Ceram. Soc. 32, 291 (2012)
Y.B. Saddeek, M.S. Gaafar, S.A. Bashier, J. Non-Cryst. Solids 356, 1089 (2010)
J. Kong, D.Y. Tang, C.C. Chan, J. Lu, K. Ueda, Opt. Lett. 32, 247 (2007)
Y. Gandhi, M.V. Ramachandra Rao, Ch. Srinivasa Rao, T. Srikumar, I.V. Kityk, N. Veeraiah, J. Appl. Phys. 108, 023102 (2010)
P. Raghava Rao, N. Venkatramaiah, Y. Gandhi, V. Ravi Kumar, I.V. Kityk, N. Veeraiah, Spectrochim. Acta 86, 472 (2012)
E.B. Dunina, A.A. Kornienko, L.A. Fomicheva, Eur. J. Phys. 6, 407 (2008)
B.R. Judd, Phys. Rev. 127, 750 (1962)
G.S. Ofelt, J. Chem. Phys. 37, 511 (1962)
W.T. Carnall, G.L. Goodman, K. Rajnak, R.S. Rana, J. Chem. Phys. 90, 3443 (1989)
B.M. Walsh, “Judd–Ofelt Theory: Principles and Practices” in Advances in Spectroscopy for Lasers and Sensing, ed. by B. Di Bartolo, O. Forte (Springer, Netherlands, 2006) p. 403
R. Balda, L.M. Lacha, J.M. Fernández-Navarro, Opt. Mater. 27, 1771 (2005)
T. Satyanarayana, I.V. Kityk, M.G. Brik, V. Ravi Kumar, N. Veeraiah, Phys. B 405, 1872 (2010)
G. Lakshminarayana, M. Mao, J.R. Qiu, M.G. Brik, Phys. B 404, 3348 (2009)
G.A. Kumar, E. De la Rosa, H. Desirena, Opt. Comm. 260, 601 (2006)
L.R.P. Kassab, W.G. Hora, B.W. Lozano, M.A.S. de Oliveira, G.S. Maciel, Opt. Comm. 269, 356 (2007)
Q. Qian, Y. Wang, Q.Y. Zhang, G.F. Yang, Z.M. Yang, Z.H. Jiang, J. Non-Cryst. Solids 354, 1981 (2008)
G. Wang, J. Zhang, S. Xu, S. Dai, L. Hu, Z. Jiang, J. Lumin. 109, 193 (2004)
K.J. Rao, Structural Chemistry of Glasses (Elsevier, Amsterdam, 2002)
G. Kaur, M. Kumar, A. Arora, O.P. Pandey, K. Singh, J. Non-Cryst. Solids 357, 858 (2011)
M. Sundara Rao, Ch. Srinivasa Rao, B.V. Raghavaiah, G. Sahaya Baskaran, V. Ravikumar, I.V. Kityk, N. Veeraiah, J. Mol. Struct. 1007, 185 (2012)
N. Purnachand, T. Satyanarayana, I.V. Kityk, N. Veeraiah, J. Alloys Compd. 492, 706 (2010)
C.C. Robinson, J.T. Fournier, J. Phys. Chem. Solids 31, 895 (1970)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramesh Babu, N.C., Srinivasa Rao, C., Brik, M.G. et al. Manifestation of up-conversion in Yb3+/Tm3+ doped Li2O–Y2O3–SiO2 glass system. Appl. Phys. B 110, 335–344 (2013). https://doi.org/10.1007/s00340-012-5247-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-012-5247-7