Abstract
The BaGd2−x O4:xDy3+ (0 ≤ x ≤ 0.08) phosphors were synthesized at 1,300 °C in air by the solid-state reaction route. The as-synthesized phosphors were characterized by X-ray powder diffraction, photoluminescence excitation spectra, photoluminescence (PL) spectra, X-ray excited luminescence (XEL) spectra, and thermoluminescence (TL) spectra. It is found that the quenching concentration of Dy3+ ions in BaGd2O4 host is dependent on the selected excitation wavelength. The optimal PL intensity for the investigated BaGd2−x O4:xDy3+ phosphors is found to be x = 0.01, 0.02, and 0.04, upon excitation by 234, 277, and 350 nm ultraviolet light, respectively. The energy transfer among Dy3+ ions upon excitation by 350 nm is confirmed to be an electric dipole–dipole interaction mechanism based on the fitting of Huang’s rule. In addition, the intensive XEL from BaGd2O4:Dy3+ phosphor is observed by the naked eyes at room temperature, and TL properties of the investigated phosphors are analyzed and discussed. All the results imply that the investigated phosphors could be a promising scintillating phosphor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Potential and technological application in optical and optoelectric domains, such as scintillators and laser hosts, have received intensive research interest in rare-earth doped materials [1–5]. Scintillating materials, which convert ionizing radiation, such as X-rays, α-, β-, and γ-rays, or UV incident beams into visible or IR light, have attracted special attention. A good scintillator is characteristic with high-luminescence efficiency, high density, fast decay, high radiation damage threshold, desirable chemical and physical stability [2–4]. Up to date, many scintillating crystals and glasses, such as Bi4Ge3O12, PbWO4, and SCG-1 glass [4, 5], have been deeply explored and practically applied. Recently, some promising scintillating materials such as Lu2Si2O5:Ce3+ (LSO:Ce), LaBr3:Ce3+, and CaI2:Ce3+ exhibit the potential application in the field of medical image [6–8]. However, the expensive cost of Lu2O3 and the hygroscopic nature of LaBr3 or CaI2 are challenging obstacles for their scale application. Therefore, the search for excellent scintillating materials is still going on.
Binary rare earth oxides AR2O4 (for A = Ca, Sr, Ba, and R = rare earth) within CaFe2O4 structure have been found many potential applications due to their special properties, especially in the luminescent and magnetic aspects. As a photoluminescence (PL) adopter, the structure characteristic and luminescent property of SrY2O4, SrGd2O4, SrLu2O4, BaY2O4, and BaGd2O4 have been investigated extensively [9–19]. In addition, the potential application of SrY2O4 and BaY2O4 as the thermal barrier coating material was proposed, and their thermophysical and mechanical properties were also explored [20, 21]. It is worthy to note that the scintillating properties for this kind of materials are rarely reported except for the vacuum ultraviolet investigation on SrGd2O4:Eu3+ phosphor by Wang et al. [22]. Compared with the SrGd2O4 host, rare-earth-doped BaGd2O4 host may be expected to have a better scintillating nature due to the following factors: (1) Both dense BaO and Gd2O3 are attributive to improving the density of the host. The reported density of BaGd2O4 is about 7.68 g/cm3 [23], which is slightly higher than of the promising LSO scintillating crystal with density of 7.4 g/cm3. (2) The BaGd2O4 host is very stable up to 1,860 °C, which is of significance for its practical application [24]. (3) The same valance state and similar ion radii of lanthanide ions have greatly facilitated for the incorporation of luminescent ions such as Ce3+, Tb3+, and Eu3+ into the Gd3+ site of the BaGd2O4 host. (4) And more importantly, the sensitizer ions of Gd3+ in BaGd2O4 host can efficiently transfer its absorbed energy to the incorporated luminescent centers, which results in the enhancement of light yield.
The first report on the luminescent properties of BaGd2O4:Eu3+ phosphors was focused on application as mercury lamp phosphors, and its quantum efficiency was found to be about 40 % by Blasse [25]. Later, this phosphor activated with both Tb3+ and Sm3+ ions was found to be highly effective in X-ray storage filed [23]. With the emergence of field emission displays and plasma plane display technologies, more attention have been paid on the synthesis and luminescent properties of rare-earth-doped BaGd2O4 in recent years [17–19]. To our knowledge, there is no report on scintillating properties of rare-earth-doped BaGd2O4 phosphors. Herein, in the present study, the structure and luminescent properties of the BaGd2O4:Dy3+ scintillating phosphors are investigated by X-ray diffraction (XRD), PL, X-ray excited luminescence (XEL) spectra, and thermoluminescence (TL) spectra in detail.
2 Materials and methods
Powder samples with stoichiometric composition of BaGd2−x O4:xDy3+ (x = 0.0, 0.005, 0.01, 0.02, 0.04, 0.06, and 0.08) phosphors were synthesized by the high temperature solid-state route. Raw materials of BaCO3 (Shanghai Chemical Reagent, A.R. Grade), Gd2O3, and Dy2O3 (Ganzhou Qiandong Industrial Group Co. Ltd., 99.99 %) were mixed thoroughly in an agate mortar. The amount of BaCO3 was excess in the range of 1–9 mol% to compensate its evaporation losses during the synthesis process. The well-ground mixtures were pre-fired at 900 °C for 180 min and subsequently calcined at 1,300 °C for 600 min in air, finally cooled down naturally to room temperature.
The phase purity of the as-synthesized phosphors was investigated by XRD with a X-ray diffractometer (Brucker D8) with Cu-Kα radiation at 40 kV and 40 mA. The XRD patterns were collected in the range of 25º ≤ 2θ ≤ 80º. The photoluminescence excitation (PLE) and PL spectra were recorded on a fluorescence spectrophotometer (Hitachi F-7000), equipped with a 150 W xenon lamp as the excitation source. The XEL of the investigated phosphors were examined using an X-ray excited spectrometer (Fluormain) in which an F-30 X-ray tube (W anticathode target) was used as the X-ray source operating at 80 kV and 4 mA. All the investigated phosphors were irradiated for 5 min by an 254 nm ultraviolet lamp with 5 W before TL measurement, and the TL curves were collected at a linear rate of 1 °C/s in the temperature 25–350 °C using a TL system (FJ427A, Beijing Nuclear Instrument Factory). All the measurements were carried out at room temperature except for the TL spectra.
3 Results and discussion
3.1 Crystal structure and XRD analysis
It is reported that the crystal structure of BaGd2O4 is isostructural to CaFe2O4-related structure, which is composed of a double octahedral Gd2O4 2− framework and alkaline earth-ion reside within the framework. As shown in Fig. 1, Gd3+ ions have two non-equivalent Gd sites [10, 12, 15, 22], and both Gd sites are coordinated by six oxygen atoms and have Cs symmetry. The Gd(I) site is nearly octahedral, while the Gd(II) site is in a more distorted coordination environment. The Ba-site in this host is eight-coordinates and also exhibits Cs symmetry. It is well-known that an acceptable percentage difference in ion radii between the doped and substituted ions must not exceed 30 % [26], which suggests that Dy3+ ions (0.912 Å, CN = 6) may prefer to substitute Gd3+ (0.938 Å, CN = 6) cation ions rather than Ba2+ (1.42 Å, CN = 8) ions in the BaGd2O4 host. Therefore, the general formula of the investigated phosphors is described as Ba2Gd2−x O4:xDy3+, where x denoting the substitution ratio of Dy3+ for Gd3+ ions.
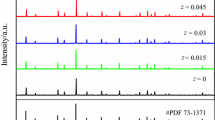
The effect of excess BaO on the structure evolution of BaGd2O4 host is illustrated by XRD patterns shown in Fig. 2, and the standard PDF files of both Gd2O3 (JCPDS: 86-2477) and BaGd2O4 (JCPDS: 82-2320) are also displayed for comparison. It is obvious that the BaGd2O4 phase begins to form, when the excess BaO is 3 %. However, minor Gd2O3 impurity phase labeled as blue arrow can also coexist in the investigated BaGd2O4 host. This impurity can be reduced or deleted by adding more BaO, and the purity phase BaGd2O4 host is finally obtained in the case of excess 9 % BaO.
3.2 Luminescence properties
3.2.1 PL spectra
The PLE spectra of Ba2Gd2−x O4:xDy3+ phosphors are shown in Fig. 3a, b when monitoring Dy3+ 580 nm and Eu3+ 614 nm emission, respectively. As shown in Fig. 3b, there exhibits an additional broad excitation band located at 235 nm when the 614 nm emission of Eu3+ ions is monitored, which is tentatively identified as the charge-transfer state of Eu3+ ions being a trace element of the starting Gd2O3 raw materials [27, 28]. The charge transfer excitation of Eu3+ ions is an electronic transition from the ground-state to the excited state of the 4f shell, and the corresponding band position in excitation spectrum is mainly determined by the covalency of Eu–O bond and the coordination environment of Eu3+ ions as well. In the Eu–O couple, the excitation energy for charge transfer can be estimated by the following Jorgensen’s equation [29],
where v in cm−1 denotes the position of the charge transfer band, χ(O) and χ(Eu) are the optical electronegativity of the oxygen and europium ions, respectively. Taking χ(O) = 3.2 and χ(Eu) = 1.75 [30], the band position for Eu–O charge transfer is calculated to be at 238 nm, which is in line with our results. In addition, the typical excitation peaks at 277 and 313 nm are assigned to optical transition of Gd3+ 8S7/2 → 6IJ, and 6IJ, respectively. All the PLE intensity of these peaks decrease with the increasing substitution ratios of Dy3+ for Gd3+ ions. The appearance of Gd3+ excitation peaks indicates that energy transfer from Gd3+ to Eu3+ ions may occur.
To study the luminescent property of Dy3+ in BaGd2O4 host, the excitation spectra of Ba2Gd2−x O4:xDy3+ phosphors monitoring at 580 nm of Dy3+ ions emission is shown in Fig. 3a. Except for the characteristic excitation peaks of Gd3+ ions in the vicinity of 276 and 313 nm, there exists a strong broad band situated at 230 nm and a weak line shape peak at 350 nm. The former superposed bands may be associated with the charge transfer states of both O2−−Dy3+ and O2−−Eu3+ ions [23], while the latter one is assigned to the 6H15/2 → 4M15/2, 6P7/2 transitions of Dy3+ ions. It is also interesting that the strongest excitation intensity of 234, 277, and 350 nm is x = 0.01, 0.02, and 0.04, respectively.
Figure 4 shows the PL spectra of BaGd2−x O4:xDy3+ phosphors under 234, 277, and 350 nm excitation, respectively. As shown in Fig. 4c, the PL spectrum of Dy3+ ions consists of three emission regions: blue, yellow, and weak red emission regions. For Dy3+ ions, the blue emission between 435 and 500 nm is assigned to the optical transition of 4F9/2 → 6H13/2, the yellow emission in the wavelength region 555–595 nm corresponds to the 4F9/2 → 6H13/2 transition, while the weak red emission (640–680 nm) is associated with the 4F9/2 → 6H11/2 transition. As shown in Fig. 4a, b, upon BaGd2−x O4:xDy3+ phosphors under both 234 and 277 nm ultraviolet light excitation, the characteristic emissions of Eu3+ ions are also observed at 595 and 613 nm, which belong to the 5D0 → 7F1 and 5D0 → 7F2 transitions of Eu3+ ions, respectively. The characteristic emission of Gd3+ 313 nm only exists in the case of 277 nm excitation. With the elevated Dy3+ content, the characteristic PL intensity of both Gd3+and Eu3+ ions reduce remarkably, which imply the energy transfer may happen from Gd3+ and Eu3+ to Dy3+.
The emission intensity of Dy3+ 580 nm as a function of both Dy3+-doping concentration and the selected excitation wavelength are comparably presented in Fig. 5. The results suggest that the optimal content of Dy3+ ions emission in BaGd2−x O4:xDy3+ phosphors depends on the selected excitation wavelength. The quenching concentration of Dy3+ in the investigated phosphors is x = 0.01, 0.02, and 0.04 under 234, 277, and 350 nm, respectively. They are in accordance with the PLE spectra in Fig. 3a.
The concentration quenching of the luminescence is due to the energy transfer from one activator to another until all the energy is consumed. For this reason, it is necessary to obtain the critical distance (R c) that is the critical separation between donor (activators) and acceptors (quenching site). The critical distance R c of the energy transfer between the same activators Dy3+ in BaGd2−x O4:xDy3+ could be estimated according to the following equation [31]:
where x c is the critical concentration, N is the number of Gd3+ ions in the unit cell, and V is the volume of the unit cell. By taking the experimental and analytic values of x c, N, and V (0.04, 8 and 451.804 Å3, respectively), the critical transfer distance of Dy3+ in BaGd2−x O4:xDy3+ phosphors is estimated to be about 13.92 Å.
According to Huang’s rule [32, 33], the relationship between the integral luminescent intensity I and doping concentration x can be expressed as follows:
where γ is the intrinsic transition probability of sensitizer, s is index of electric multipole, which is 6, 8, and 10 for electric dipole–dipole, electric dipole–quadrupole, and electric quadrupole–quadrupole interaction, respectively. If s = 3, the interaction type is an exchange interaction. d is the dimension of the sample, here d = 3. A and X 0 are the constants and Γ(1 + s/d) is a Γ function. From Eqs. (3) and (4), it can be derived that
where f is independent of the doping concentration.
Figure 6 shows the log(I/x)–logx plot for the 4F9/2 → 6H15/2 transitions of Dy3+ ions in BaGd2−x O4:xDy3+ phosphors. According to Eq. (5), using the linear fitting to deal with the experimental data in the region of high concentrations, the value of the slope parameter −s/d is obtained to be −1.65 for the 4F9/2 → 6H15/2 transition. The slope parameter is approximately to −2. Therefore, the index of the electric multipole energy transfer is 6. The result means that the electric dipole–dipole interaction mechanism is dominant by the energy transfer of Dy3+ ions in the investigated phosphors.
The energy levels of Gd3+, Dy3+, and Eu3+ ions, and visible PL emission and energy transfer of BaGd2O4:Dy3+ phosphors are depicted in Fig. 7 according to the calculated data of energy levels by Carnall et al. [34–36]. When the 234 nm UV light is used to excite the BaGd2O4:Dy3+ phosphors, the electron is excited into the 6DJ level of Gd3+ and the charge transfer states of both Dy3+ and Eu3+ ions. The initial excited electrons relax quickly to their lower energy levels until it arrives at the 6PJ of Gd3+ ions, 4F9/2 of Dy3+ ions, and 5D0 of Eu3+ ions by phonon-assisted process, respectively. Then they give rise to the responding characteristic emissions. However, the energy transfer occur for Gd3+−Dy3+ and Gd3+−Eu3+ ion pairs due to the matched energy levels between Gd3+ 6PJ levels and Dy3+ 4K15/2 or Eu3+ 5HJ levels. In additional, the energy transfer may happens from 5HJ of Eu3+ ions to 6P7/2 of Dy3+ ions due to the matched energy levels, and the population of Dy3+ 4F9/2 level may also be enhanced by the so-called cross-relaxation process of 5HJ (Eu3+) + 4H9/2 (Dy3+) → 6H9/2 (Dy3+) + 5D0 (Eu3+) [37, 38]. So both characteristic emissions of Gd3+ 313 nm and Eu3+ 614 (593) nm are observed to be decreased remarkably, especially under excitation at 277 nm shown in Fig. 4b. It is interesting that the BaGd2O4:Dy3+ phosphor is excited by 350 nm UV light, there only exists Dy3+ characteristic emission without any Eu3+ emission. It is this phenomenon that energy transfer cannot happen from Dy3+ to Eu3+ ions. For Dy3+ ions, the energy gap between 4F9/2 and 6H9/2 levels matches well with that of the 6F1/2 and 6H15/2 levels indicating that the energy transfer occurs via 4F9/2 (Dy3+) + 6F1/2 (Dy3+) → 6H9/2 (Dy3+) + 6H15/2 (Dy3+) cross-relaxation process by electric dipole–dipole interaction in nature. The probability of the energy transfer gets higher with the increasing Dy3+ ions content, particularly in the BaGd2−x O4:xDy3+ phosphors with high concentration of Dy3+ ions, which results in the depopulation of the 4F9/2 level and the decrease of the blue and yellow emissions.
3.2.2 XEL spectra
The representative XEL spectra of BaGd2−x O4:xDy3+ (x = 0.0, 0.02, and 0.06) phosphors under X-ray excitation are shown in Fig. 8. These spectra show similar characteristics as those under ultraviolet light excitation. Namely, the undoped BaGd2O4 phosphors is observed with red emission of Eu3+ ions, which originates from the purity of Gd2O3 raw materials because the impurity Eu3+ ions is frequently present in Gd2O3 raw material [27, 28]. While the strongest XEL intensity of Dy3+ ions in the blue and yellow regions is observed when the content of Dy3+ is 0.02 mol by the naked eyes at room temperature. The XEL intensity reduce dramatically when the content of Dy3+ is 0.06 mol due to concentration quenching. The results indicate that the BaGd2O4:Dy3+ phosphor is of significance for scintillating filed.
3.3 TL glow curves
The TL glow curves of BaGd2−x O4:xDy3+ phosphors after 254 nm UV light irradiation for 5 min are shown in Fig. 9. They are fitted to a good approximation by a set of three Gaussian TL glow curves assuring the lowest χ 2 value [39, 40]. The fitted peaks 1, 2, and 3 locate about 55, 85, and 120 °C, respectively. The inset of Fig. 9 shows the representative three Gaussian fitting for the x = 0.0 and 0.01 phosphors. With the elevated Dy3+ content, the TL intensity of both peaks 2 and 3 are always reduced, while the peak 1 increases until x = 0.01 and decreases dramatically due to concentration quenching of Dy3+ ions. There are no additional peaks in glow curves indicates the introduction of Dy3+ into BaGd2O4 host cannot create any new kinds of traps, but resulting in the change of concentration of traps.
The TL glow-peaks indicate the existence of shallow as well as deep traps for the investigated phosphors which are responsible for the trapping of charge carries produced by the irradiation process. The Gaussian curve fitting procedure suggests the possibility of having a second order mechanism for TL process [39]. In this case, the kinetic parameters can be obtained by using the following equations.
First, the depth traps E t (eV) or activation energy can be calculated by the equation [41]:
where k is the Boltzman constant, T m (K) is the thermal peak temperature, and w is the full width at half maximum of the thermal glow-peak. The lifetime τ(s), is the time to empty the traps, at temperature T m is given by [41]:
where s (s−1) correspond to the frequency factor. This factor is associated to the freed of the trapped electrons from each trap and increase or decrease depending of the trap. It is related with the activation energy through the general equation valid to any order [42],
where β is the heating rate, and b determines the kinetic order process, being 1 and 2 for the first and second order, respectively.
The calculated parameters, such as peak position T, depth trap E t, frequency factors s, and lifetime τ are listed in Table 1. The analyzed results imply the peak positions of both peaks 2 and 3 are little changed with the introduction of Dy3+ ions, while the corresponding peak 1 is shifted towards the lower temperature side. It is noteworthy that the lifetimes to empty the traps are similar in the vicinity of 10 s. However, the frequency factor is changed over a wide range in more than nine orders of magnitude.
4 Conclusions
In summary, novel BaGd2−x O4:xDy3+ scintillating phosphors have been successfully synthesized and their luminescent properties have been investigated. The purity phase BaGd2O4 host is obtained by adding excess 9 mol% BaO. The optimal quenching concentration for strongest Dy3+ emission depends on the selected excitation wavelength, and it is found to be x = 0.01, 0.02, and 0.04 under 234, 277, and 350 nm, respectively. The energy transfer from Gd3+ to both dopant Dy3+ and impurity Eu3+ ions, and energy transfer from Eu3+ to Dy3+ ions are simultaneously observed by the PL spectra. The deconvolution of TL spectra by Gaussian fitting suggests any new traps cannot create with the introduction of Dy3+ ions. The bright XEL intensity for x = 0.02 samples is observed by the naked eyes at room temperature. All the results imply that the investigated phosphor may be of significance for scintillating application.
References
C. Feldmann, T. Justel, C.R. Ronda, P.J. Schmidt, Adv. Funct. Mater. 7, 511–516 (2003)
M.J. Webber, J. Lumin. 100, 35–45 (2002)
S.E. Derenzo, M.J. Webber, E. Bourret-Courchesne, M.K. Klintenberg, Nucl. Instr. Meth. Phys. A 505, 111–117 (2003)
M. Nikl, Meas. Sci. Technol. 17, R37–R54 (2006)
A.D. Bross, Nucl. Instr. Meth. Phys. A 247, 319–326 (1986)
P.A. Cutler, C.L. Melcher, M.A. Spurrier, P. Szupryczynski, L.A. Eriksson, IEEE Trans. Nucl. Sci. 56, 915–919 (2009)
J.T.M. de Haas, P. Dorenbos, IEEE Trans. Nucl. Sci. 55, 1086–1092 (2008)
N. Roy, R. Hawrami, A. Burger, L.A. Boatner, W.S. Choong, W.W. Moses, IEEE Trans. Nucl. Sci. 56, 873–880 (2009)
T.L. Barry, R. Roy, J. Inorg. Nucl. Chem. 29, 1243–1248 (1967)
R. Atkins, A.L. Diaz, J. Lumin. 128, 1463–1470 (2008)
L. Zhou, J. Shi, M. Gong, Mater. Res. Bull. 40, 1832–1838 (2005)
H. Li, S. Zhang, S. Zhou, X. Cao, Mater. Chem. Phys. 114, 451–455 (2009)
M. Taibi, E. Antic-Fidancev, J. Aride, M. Lemaitre-Blaise, P. Porcher, J. Phys. Condens. Matter 5, 5201–5208 (1993)
M. Taibi, J. Aride, E. Antic-Fidancev, M. Lemaitre-Blaise, P. Porcher, Phys. Stat. Sol. A 144, 453–459 (1994)
Y. Doi, W. Nakamori, Yukio Hinatsu, J. Phys. Condens. Matter 18, 333–344 (2006)
H. Kobayashi, H. Ogino, K. Nakamura, T. Mori, H. Yamamura, T. Mitamura, Jpn. J. Ceram. Soc. 102, 583–596 (1994)
L. Zhou, J. Shi, M. Gong, J. Phys. Chem. Solid 68, 1471–1475 (2007)
A.R. Bojja, S. Mudavat, S. Karnati, V. Muga, B.M. Cheng, Proc. SPIE 7784, 77841E−1:8 (2010)
B. Mari, K.C. Singh, M. Sahal, S.P. Khatkar, V.B. Taxak, M. Kumar, J. Lumin. 131, 587–591 (2011)
K. Fukuda, H. Matsubara, J. Am. Ceram. Soc. 88, 3205–3206 (2005)
T. Maekawa, K. Kurosaki, S. Yamanaka, Mater. Lett. 61, 2303–2306 (2007)
J. Zhang, Y. Wang, L. Guo, Y. Huang, J. Am. Ceram. Soc. 94, 1–7 (2011)
K.D. Sieber, B.R. Sever, L.B. Told, European Patent 0626437
D. Kolar, S.D. Skapin, D. Suvorov, Acta Chim. Slov. 46, 193–202 (1999)
G. Blasse, J. Chem. Phys. 45, 2356–2359 (1966)
X.Y. Sun, L.W. Lin, W.F. Wang, J.C. Zhang, Appl. Phys. A 104, 83–88 (2011)
Z. Tian, H. Liang, B. Han, Q. Su, Y. Tao, G. Zhang, Y. Fu, J. Phys. Chem. C 112, 12524–12529 (2008)
G. Dominiak-Dzik, W. Ryba-Romanowski, R. Lisiecki, P. Solarz, B. Macalik, M. Berkowski, M. Gzowacki, V. Domukhovski et al., Cryst. Growth Des. 10, 3522–3530 (2010)
C.K. Jorgensen, Prog. Inorg. Chem. 12, 101–158 (1970)
L. van Pieterson, M. Heeroma, E. de Heer, A. Meijerink, J. Lumin. 91, 177–193 (2000)
G. Blasse, Philips Res. Rep. 24, 131–144 (1969)
S.H. Huang, L.R. Lou, Chin. J. Lumin. 11, 1–7 (1990)
Q.Y. Meng, B.J. Chen, W. Xu, Y.M. Yang, X.X. Zhao, W.H. Di, S.Z. Lu, X.J. Wang, J.S. Sun, L.H. Cheng, T. Yu, Y. Peng, J. Appl. Phys. 102, 093505 (2007)
W.T. Carnall, P.R. Fields, K. Rajnak, J. Chem. Phys. 49, 4424–4442 (1968)
W.T. Carnall, P.R. Fields, K. Rajnak, J. Chem. Phys. 49, 4443–4446 (1968)
W.T. Carnall, P.R. Fields, K. Rajnak, J. Chem. Phys. 49, 4450–4455 (1968)
S. Buddhudu, M. Morita, S. Murakami, D. Rau, J. Lumin. 83–84, 199–203 (1999)
L.R. Singh, R.S. Ningthoujam, J. Appl. Phys. 107, 104304 (2010)
Z. Ju, R. Wei, J. Zheng, X. Gao, S. Zhang, W. Liu, Appl. Phys. Lett. 98, 121906 (2011)
R.A. Rodriguez, E. De la Rosa, L.A. Diaz-Torres, P. Salas, R. Melendrez, M. Barboza-Flores, Opt. Mater. 27, 293–299 (2004)
R. Chen, S.W.S. McKeever, Theory of Thermoluminescence and Related Phenomena (World Scientific Publishers, Singapore, 1997)
D. Jia, W.M. Yen, J. Electrochem. Soc. 150, H61–H65 (2003)
Acknowledgments
We wish to acknowledge our gratitude to the financial support provided by the National Natural Science Fund of China (Grant No. 11165010), the Natural Science Foundation of Jiangxi Province (Grant No.20114BAB202002), the Scientific Project of Department of Education in Jiangxi Province (Grant No. GJJ12462), the Key Subject of Atomic & Molecular Physics in Jiangxi Province (2011–2015), the Open Fund of Key Laboratory for Ultrafine Materials of the Ministry of Education, East China University of Science and Technology, and the Program for Young Excellent Doctors in Jinggangshan University (JZB007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, XY., Zhou, YZ., Yu, XG. et al. Synthesis and luminescent properties of BaGd2O4:Dy3+, an novel scintillating phosphor. Appl. Phys. B 110, 27–34 (2013). https://doi.org/10.1007/s00340-012-5246-8
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-012-5246-8