Abstract
We demonstrate a real-time inline calibration method for an open-path ammonia sensor using a quantum cascade laser (QCL) at 9.06 μm. Ethylene (C2H4) has an absorption feature partially offset from the ammonia absorption feature, and the ethylene signal serves as a reference signal for ammonia concentration in real time. Spectroscopic parameters of ammonia and ethylene are measured and compared with the HITRAN database to ensure the accuracy of the calibration. Multiple harmonic wavelength modulation spectroscopy (WMS) signals are used to separate the ambient ammonia and reference ethylene absorption signals. The ammonia signal is detected with the second harmonic (2f), while it is calibrated simultaneously with a high-harmonic (6–12f) signal of ethylene. The interference of ambient ammonia absorption on the ethylene reference signal is shown to be negligible when using ultra high-harmonics (≥6f). This in situ calibration method yields a field precision of 3 % and accuracy of 20 % for open-path atmospheric ammonia measurements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Atmospheric ammonia (NH3) is a key component in the global nitrogen cycle. As the dominant alkaline atmospheric species, ammonia reacts readily with atmospheric acidic species such as sulfuric acid (H2SO4) and nitric acid (HNO3) to form ammoniated aerosols, with strong implications for regional air quality and global radiative forcing [1–3]. Ammonia also plays an important role in the deposition of reactive nitrogen in sensitive ecosystems [4]. Despite the importance of atmospheric ammonia, its spatial and temporal variability is poorly characterized due to its low atmospheric concentration and high reactivity [5].
Traditional ammonia measurements utilize passive filters and denuders with long integration times, and they are usually labor-intensive in operation and maintenance [6]. State-of-the-art techniques include chemical ionization mass spectrometry (CIMS) [7–9], tunable laser absorption spectroscopy [10–12], photoacoustic spectroscopy [13], and cavity ring down spectroscopy [14]. All of these techniques need to sample ammonia into a closed-path system and thus involve direct contact with sampling surface to which ammonia readily adsorbs. Closed-path measurements of ammonia are complicated by significant backgrounds, unknown buffering of large changes in concentration, and ambiguity between ammonia and ammonium due to phase transitions in sampling lines [5]. For field deployments where conditions can change rapidly, the simplicity and automation of calibration needs improvement at typical ambient mole fractions [parts per billion by volume (ppbv)].
To address the sampling issue of closed-path techniques, we have developed an open-path ammonia sensor using a quantum cascade laser (QCL) operating at 9.06 μm for atmospheric measurements. Wavelength modulation spectroscopy (WMS) is used to enhance the signal to noise ratio (SNR) and resolve air-broadened absorption lines. Given the complexity of WMS systems, calibrations with reference samples are widely used to make accurate measurements. However, the same problem with the calibration of a closed-path ammonia sensor remains for an open-path sensor: one needs to introduce a known concentration of ammonia for calibration. Ethylene (C2H4) has an abundance of absorption lines in the ν7 band near the ammonia ν2 band in mid-IR. Previous research has already shown that ethylene can be used in ammonia sensors as a reference of laser wavelength at 10.34 μm [15] and as a reference for ammonia concentration calibration at 9.06 μm [16]. In this study, we present a new in situ calibration method with an inline ethylene reference cell using multi-harmonic WMS. Ethylene is a stable, relatively inert gas and has line strengths two orders of magnitude smaller than ammonia near 9.06 μm. Thus ethylene does not cause interference at typical atmospheric mixing ratios (sub-ppbv) [17], which are comparable to ammonia mixing ratios. At a low pressure (<100 hPa), high gas concentration (1 %), and short path length (~10 cm), ethylene shows a stable absorption signal partially offset from the ammonia absorption feature, and the ethylene signal can serve as a reference for ammonia concentration in real time. This calibration method can also compensate for the effect of laser drifting by line locking to the sharp ethylene peak instead of the air-broadened ammonia peak and is particularly useful near the detection limit.
Comparing with conventional WMS, this technique has advantages in accuracy, frequency, simplicity, and automation. The ammonia concentrations are retrieved by fitting the second harmonic (2f) spectra, so theoretically the precision should be the same as traditional 2f detection. The accuracy is ensured by experimental calibrations of the spectroscopic parameters of both ammonia and ethylene, which are independent of ammonia concentrations. However, the accuracies of conventional calibration methods are limited by the uncertainties of ammonia standards, which can be quite large at ambient levels (ppbv) due to the adsorption effects of the gas delivery system [5]. In long-term field measurements, frequent calibrations are usually needed to account for system drift. The traditional solution is by periodically calibrating the system with some standards, which can be expensive, labor-intensive, or subject to loss of measurement points. By checking the absorption signals of a fixed concentration reference cell, this in situ calibration method enables continuous and unattended measurements, which are very important in rapidly changing conditions in the field.
2 Experimental and model details
2.1 Experimental
The experimental setup is depicted in Fig. 1. A TE-cooled DFB QCL from Alpes Lasers is used in the experiment. The laser temperature is stabilized by a thermoelectric temperature controller (Wavelength Electronics, HTC3000) and operated in continuous wave mode with a low noise laser diode driver (Wavelength Electronics, QCL500). The laser injection current is scanned across the absorption feature by a sawtooth ramp at 33 Hz and sinusoidally modulated at 10.37 kHz. The modulation depth of the QCL is calibrated using the method reported by Tao et al. [18]. The laser beam travels through two gas cells in series and is focused onto a TE-cooled photovoltaic MCT detector (Vigo). Two wedged ZnSe windows are put in the optical train to suppress etalons and avoid saturating the detector. The analog detector signal is then passed to a digital lock-in amplifier that can demodulate at three different harmonics simultaneously (Zurich Instruments, HF2). The WMS signal output from the lock-in amplifier is collected on a National Instrument DAQ system (NI USB 6251, 16-bit, 1 MS/s). One glass cell (L = 10 cm) is filled with 1 % ethylene in nitrogen (Air Liquide, accuracy ±2 %). The other glass cell (L = 20 cm) is filled with 150 ppmv ammonia in nitrogen (Air Liquide, accuracy ±10 %) and can be diluted with dry nitrogen. The pressure of either cell can be controlled by a vacuum pump and is measured by a pressure gauge (MKS, Inc.) with a full-scale reading of 1315 hPa (1000 Torr) and an accuracy 0.5 % of reading. Either cell can be readily removed from the system to measure ethylene or ammonia absorption signals individually (e.g., used in Sect. 3). The open-path ammonia sensor prototype described in Sect. 5 differs only in that the ammonia cell is replaced by an open-path cylindrical multi-pass cell [19] with a path length of 40 m.
2.2 Simulation of WMS signals
In order to interpret and predict the multi-harmonic signal from the reference cell, we have developed a numerical model based on the general WMS theories [20–23]. The equations are rewritten to involve more variables for open-path atmospheric measurements. An infinite impulse response (IIR) filtering algorithm enables a direct comparison between the model and the signal output from a lock-in amplifier.
The injection current to the QCL can be written as a function of time, with a DC value:
where \( {\text{R}}(2\pi f_{\text{R}} t) \) represents the sawtooth ramp function with frequency f R. i R and i m are the amplitude of the current ramp and sinusoidal modulation (with a frequency \( f_{\text{m}} \gg f_{\text{R}} \)), respectively.
The current modulation leads to modulation of the laser light frequency near a constant frequency νo. The laser light frequency, ν(t), is then given by
Where \( \eta_{\text{R}} \) and \( \eta_{\text{m}} \) are the current-to-frequency tuning rate at the ramp frequency and modulation frequency, and \( \phi \) represents the phase difference between the modulated laser frequency and laser intensity. \( i_{\text{m}} \eta_{\text{m}} \) defines the modulation depth. \( \eta_{\text{R}} \), \( \eta_{\text{m}} \), and \( \phi \) are measured experimentally using the methods described in Tao et al. [18].
According to the Beer-Lambert law, the laser intensity on the detector is
where \( \tau_{i,j} ({{\upnu}}) \) represents the optical depth generated by absorption line j of absorber i, I 0(t) is the laser intensity, simulated by a polynomial function of injection current, and ν = ν(t) (see Eq (2)). For a specific absorption line, the optical depth τ is given by
Here n is the number density of the absorber, L is the optical path length, and S is the line strength of this absorption line. f(ν) is the Voigt line shape function, following the formula given by Schreier [24].
where \( x = \frac{{\sqrt {ln2} ({{\upnu}} - {{\upnu}}_{ 0} )}}{{\gamma_{\text{Dop}} }} \), \( y = \sqrt {ln2} \frac{{\gamma_{\text{col}} }}{{\gamma {\text{Dop}}}} \), and Re[W(z)] denotes the real part of the complex error function. \( \gamma_{\text{Dop}} \) is the Doppler line width (HWHM), which is a function of temperature and molecular weight. \( \gamma_{\text{Col}} \) is the collision line width (HWHM) which is dependent on temperature, pressure, and foreign gas. When collision broadening is dominant, the modulation index is calculated as the ratio between modulation depth and the collision line width. Voigt line width is used for ethylene absorption at low pressure.
Substituting equation (2), (4), and (5) into Eq. (3), we derive the simulated detector signal I 1, which is only a function of time. Then we simulate the function of a lock-in amplifier by multiplying the detector signal with a reference sinusoidal signal at different harmonics of modulation frequency (Nf) to shift the targeted harmonic components to DC [25]. An IIR low-pass Butterworth filter is then applied to acquire the Nth harmonic WMS signal. The filter order and bandwidth need to be deliberately chosen to eliminate as much noise as possible and avoid signal distortion in the meantime. The simulated in phase Nf signal is thus given by
For a single absorption line, the line center value of the Nth harmonic WMS signal is then denoted by X(line center, N).
3 Spectroscopic calibration
In order to use ethylene as a reference absorption signal to calibrate ammonia, precise knowledge of the absorption cross-sections of both ammonia and ethylene is critically important. For instance, a variation in the relative line strengths of ammonia or ethylene of 10 % leads to a direct variation of 10 % on the ammonia concentration retrieval. The spectroscopic parameters that determine the absorption cross-section are given by the HITRAN database [26]. However, HITRAN data can have large uncertainties and sometimes differ significantly from experimental validation [27]. For example, the uncertainties of HITRAN ammonia line strengths are estimated to be 10–20 % [28, 29], and there are no reported uncertainties for the parameters of ethylene. We remeasure the spectroscopic parameters for both ethylene and ammonia precisely using direct absorption, 2f, and 4f signals.
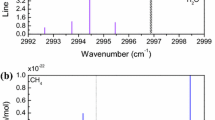
The absorption features of six ammonia lines at atmospheric pressure and two nearby ethylene lines at reduced pressure are shown in Fig. 2 based on HITRAN spectroscopic parameters. The ethylene absorption line centered at 1103.3635 cm−1 (9063.2 nm) is used as an inline reference absorption signal. An adjacent ethylene line centered at 1103.8174 cm−1 (9059.5 nm) is also studied in the spectroscopic calibration. The ammonia absorption feature, which is a composite of six individual lines on the R branch of the ν2a band, sR(6,1)–sR(6,6), is located between these two ethylene lines. Each individual ammonia line can be resolved and calibrated at low pressure (≤30 hPa).
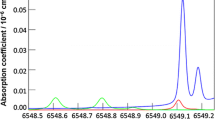
A nonlinear least squares fitting method was used to acquire the line shape parameters of ethylene. The ethylene absorption features of interest can be easily isolated by reducing the pressure below 500 hPa. First, the experimental direct absorption spectra are obtained by subtracting the absorbed signal I 1 (t) from the background signal I 0 (t) obtained by purging the cell with dry nitrogen. Only the current ramp is applied to the laser to sweep across the absorption line. A Germanium Fabry-Perot etalon signal with a free spectral range of 0.04913 cm−1 is used to calibrate the laser wavelength scale. During the Voigt fitting procedure using Eq. (5), the experimental parameters (temperature, pressure, mixing ratio, path length) are fixed to the measured or stated values. The spectroscopic parameters that have little impact on the experimental spectra (self broadening, temperature dependency exponent, lower state energy) are adopted from HITRAN 2008 database [26]. Only collision line width and line strength are fitted. An experimental ethylene spectrum at 200 hPa with the Voigt fitting is shown in Fig. 3. The ethylene line strengths extracted from the fit fully agree with HITRAN values with residuals <2 % of the peak absorption. However, the collision line widths are 15 % smaller than the air-broadened collision line width given by HITRAN 2008.
Comparing to direct absorption spectra, WMS signals can resolve congested absorption features and reveal more detailed line shape structure, especially for higher harmonics [30, 31]. The line strength and collision line width are measured in two steps. First, the pressure in the cell is reduced to 2 hPa. At this pressure, ammonia lines are essentially in Doppler line shape and resolved to the largest extent. Uncertainties coming from collision line widths are negligible in this case, which is shown in Fig. 4a. To demonstrate this, the collision line width of the absorption line centered at 1103.4412 cm−1 (sR(6,3)) is changed by ±30 % artificially, but the 4f peak to trough height only changes by ±3 %. Since the Doppler line width is well known, line strength is the only parameter to be characterized. Under this experimental condition, the contribution of line strength to WMS signal magnitude is essentially linear, so the line strength calibration is straightforward. Once the line strengths are measured, the collision line width is the only parameter to be determined. In the second step, the pressure in the cell is set to 30 hPa, at which the collision broadening is significant and the six lines are still resolved to a large extent. A similar sensitivity study is shown in Fig. 4b. When the collision line width of the same transition (sR(6,3)) is changed by ±10 % artificially, the 4f peak-trough height changes by ±15 %. In other words, any discrepancies in collision line width are amplified on 4f signal under this experimental condition.
a Red: simulation of ammonia 4f signal at 2 hPa by the numerical WMS model based on HITRAN 2008 parameters; blue and green: the collision line width of the sR(6,3) line centered at 1103.4412 cm−1 is changed by ±30 % artificially. b Red: simulation of ammonia 4f signal at 30 hPa; blue and green: the collision line width of the sR(6,3) line centered at 1103.4412 cm−1 is changed by ±10 % artificially
The experimental ammonia 4f spectra at these two pressures are presented in Fig. 5, compared with model simulation. The ammonia line strengths and collision line widths are adopted from HITRAN 2008 database. At 2 hPa, the simulation agrees with the experimental 4f spectrum within 5 % for the six ammonia lines. At 30 hPa, the simulation agrees with the experimental 4f spectrum within 10 % for the six ammonia lines.
The ethylene and ammonia spectroscopic calibrations generally agree with the HITRAN 2008 database. The only exception is the ethylene collision line width, which we measure to be 15 % lower than HITRAN for both lines. The uncertainties for line strength measurements mainly come from the uncertainties of the concentration of the gas mixture we use (2 % for ethylene and 10 % for ammonia). The accuracy of this calibration method is 20 %, according to propagation of errors of the gas concentration and spectral fitting.
4 Ammonia calibration using an inline ethylene reference cell
An inline calibration cell with a reference gas has been used in laser spectroscopy with isolated lines [32], but there are significant challenges when the reference absorption line overlaps with the target absorption line. As shown in Fig. 2, one ethylene absorption line (even under reduced pressure) sits on the shoulder of the ammonia absorption feature. Due to the constraint of QCL tuning rate, we also need to use this ethylene line for calibration.
Figure 6 shows the simulated line center values of the Nth in phase harmonic signal [X (line center, N)] as a function of modulation index. The Voigt line width of the reference ethylene signal below 100 hPa is more than 10 times smaller than the Voigt line width of ambient ammonia absorption. Hence for the same modulation depth, the modulation index for ethylene under reduced pressure is more than 10 times larger than that of ambient ammonia. By deliberately choosing the modulation depth and the reference cell pressure, we can maximize the ethylene and ammonia signal at the same time using different harmonics. A general rule of thumb is that the line center value of the Nth harmonic reaches its maximum at modulation index around N. For example, the modulation index to maximize 2f signal is 2.2. However, constrained by the limited tuning rate of the QCL (~0.006 cm−1/mA) and the broad/congested atmospheric pressure ammonia peaks (HWHM ~ 0.1 cm−1), we cannot reach the optimal modulation index for ammonia. In this study we only use a modulation index of less than 0.5 for ammonia, which still gives a sufficient ambient ammonia 2f signal. Note that in Fig. 6, at a modulation index of 0.5, the ambient ammonia high-harmonic signals are close to 0 for N ≥ 6. In the meantime, the modulation index for the ethylene line under reduced pressure is much larger and can be controlled through reference cell pressure to maximize the high-harmonic signals. The 2f ethylene signal is extremely over-modulated and flattened, yielding little interference with 2f ambient ammonia signal.
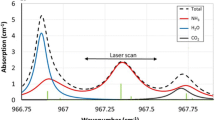
Figure 7 illustrates the 2f and 6f signals of ambient pressure ammonia and the reduced-pressure ethylene cell. In the experiment spectra shown in a and b, the pressure in the ethylene reference cell is changed from 0 to 90 hPa. The modulation index is 0.25 for ammonia and 6 for ethylene when reference cell pressure is 50 hPa. On the 2f spectra, the ethylene signals are obvious but they only perturb one trough of the ammonia 2f spectra. Since the ethylene signals are constant in 2f, they can be readily addressed by the spectral fitting routine. The 2f line center values of ammonia are not influenced by ethylene in any case. On the 6f spectra, the ammonia signals are completely invisible, but the ethylene signals are very clear.
In c and d of Fig. 7, we use the numerical WMS model to simulate the same conditions as the experiment. The concentration and pressure/temperature of ammonia and ethylene are fixed at the measured or stated values. The spectroscopic parameters are those from HITRAN 2008 except where we use our ethylene collision line width determined previously. The simulated 2f spectra are scaled to the same magnitude of the experimental 2f spectra, and the simulated 6f spectra are scaled using the same factor. The excellent agreement between experiment and simulation shows that the model can capture the relative values of different harmonic signals. It also indicates that we can fit the multi-harmonic spectra with the simulation results and retrieve ammonia concentrations.
Figure 7 demonstrates that we are able to detect the ambient ammonia signal at 2f and the reference ethylene signal at high harmonic (6f in this case). In order to compare different high harmonics, we define r(N) as the ratio between the line center value of ethylene signal and the line center value of ammonia signal at the Nth harmonic:
The ratio r(N) depends on the pressure of the ethylene reference cell and the modulation depth. We evaluate the ammonia signal at one harmonic \( \mathop N\nolimits_{{\mathop {\text{NH}}\nolimits_{ 3} }} \), and the ethylene reference signal at another harmonic N ref. To ensure that changes in ambient ammonia do not influence the reference signal, it is necessary to minimize r(N NH3) and meanwhile maximize r(N ref). Here \( {\mathop N\nolimits_{{\mathop {\text{NH}}\nolimits_{ 3} }} }\)= 2 and N ref is one of the higher harmonics.
First we consider N ref = 6. Figure 8a and b show r(2) and r(6) as a function of the modulation depth and pressure of ethylene reference cell. Note that in Fig. 8a, the vertical axis is reversed because we want to minimize r(2). The maximized r(6) can be found near a modulation depth of 0.025 cm−1 and an ethylene cell pressure of around 50 hPa. r(2) does not have a minimum for practical conditions, but at this modulation depth and ethylene cell pressure r(2) is small enough to not influence the ammonia signal.
r(N) as a function of modulation depth and ethylene cell pressure for N = 2 (a), N = 6 (b), and N = 12 (c). The other parameters (ambient temperature, ethylene/ammonia concentration) are fixed at the experimental values in the simulation. The vertical axis of a is reversed because we want to minimize r(2) but maximize r(N) for N ≥ 6
The maximum of r(6) occurs at a relatively small modulation depth, but Fig. 8a shows that r(2) keeps decreasing if the modulation depth increases. This implies that we can use even higher harmonics for the ethylene reference signal, so that the r(N ref) is larger and reaches its maximum at larger modulation depth. Figure 8c investigates the ethylene/ammonia ratio at 12f. r(12) is about 400 under optimal conditions, indicating that the ammonia signal is vanishingly small compared to the ethylene reference signal at ultra high harmonics. At the same time, r(2) is much smaller when r(12) is maximized than it is when r(6) is maximized. This indicates a general trend that higher harmonic gives better separation between the ambient ammonia and reference ethylene signals. However, it will be ultimately limited by signal-to-noise ratio since the intensity decreases as N increases. Generally, we use 6–12f depending on sensor configurations.
According to the simulations shown in Fig. 8, the modulation depth and ethylene cell pressure were set to 0.05 cm−1 and 50 hPa in the experiment, where r(12) is optimized. The ammonia cell was first purged by nitrogen and then filled with 150 ppmv ammonia. Ammonia concentrations are retrieved based upon spectral fitting using a LabVIEW-based program. Higher harmonic reference signals are fitted simultaneously with 2f, and signal amplitude obtained from the fitting is used as a scale factor to account for system drift. The outlet of the cell is open to the air to maintain ambient pressure. The lock-in amplifier can output three harmonics simultaneously, so 10f signals are also recorded. As shown by Fig. 9, when the ammonia concentration changes from 0 to about 150 ppmv (~13 % absorption), the amplitudes of both 10f and 12f signals change by less than 2 %. The precisions of both 10f and 12f reference signals are less than 1 % (1σ). Since ambient ammonia absorption rarely reaches such a high level, we can conclude that the interferences of ambient ammonia on the high-harmonic ethylene reference signals are negligible.
In polluted urban areas, ambient ethylene concentration may reach up to 30 ppbv [33, 34], which gives a signal about 0.01 % of the low-pressure ethylene reference signal at high harmonics. Hence the interferences from ambient ethylene are also negligible to the ethylene reference signal. Ambient ethylene may cause interferences to ambient ammonia signals at 2f when the ethylene concentration is >100 times higher than ammonia. However, these conditions are unlikely to happen and the signals can still be separated by spectral fitting.
5 Field demonstration
This inline calibration technique has been used in a prototype ammonia sensor and tested in Baltimore Ecosystem Study (BES) in October 2011. An ethylene reference cell (L = 5 cm; P = 50 hPa; filled with 2 % ethylene in nitrogen) was fixed in series with an open-path cylindrical multi-pass cell with a path length of 40 m. Instead of the digital lock-in amplifier, a software-based virtual lock-in amplifier was used to extract the WMS signals. Due to the constraint of the AD sampling rate (1 MHz) and optical fringing caused by the multi-pass cell, only 8f was used as the reference signal. The ammonia absorption used in Fig. 9 is equivalent to 750 ppbv ammonia in a path length of 40 m. However, ambient ammonia concentration is rarely higher than 100 ppbv even under polluted conditions [35]. Therefore the ambient level ammonia signal does not cause any significant interference on the reference signal with the sensor configuration.
Figure 10 shows a time series of retrieved ammonia concentrations and amplitudes of the ethylene 8f signal. The Allan deviation of the ethylene 8f signal shows a precision of 3.6 % at an integrating time of 10 s, and 2 % at 700 s, with respect to ammonia concentration change for 7–24 ppbv. The larger variation of the reference signal compared to Fig. 9 can be caused by the following reasons:
a Time series of ethylene 8f reference signal (red) and retrieved ammonia mixing ratio using 2f signal (blue) measured by open-path ammonia sensor under field condition for 7 h. b Allan deviation plot for the ethylene reference signal. The precision reaches its minimum at an integration time of about 700 s
-
1.
The base length of the open-path multi-pass cell is 50 cm, leading to optical fringing with free spectral range (FSR) comparable to the Voigt line width of ethylene absorption line at 50 hPa. When the FSR is comparable to the line width, it has the largest interference with the absorption signal. Since this FSR is much smaller than ammonia Voigt line width, its influence on ammonia signal is very small.
-
2.
Significant thermal drifting of laser wavelength was observed under the field conditions. The wavelength drifting was prevented by actively changing the laser current offset to lock the peak position of the ethylene 8f signal. However, the changes of intensity and tuning rate, which are potentially affected by wavelength drifting, cannot be fully quantified.
6 Conclusion
We demonstrate a real-time inline calibration of ambient ammonia measurements with an ethylene reference cell. Simultaneous multi-harmonic detection is used to resolve and optimize the overlapping ammonia and ethylene reference signals. We detect ammonia at 2f and ethylene at higher harmonics, up to 12f. By controlling the pressure in the reference cell and the modulation depth, we optimize ammonia and ethylene signals, respectively, and simultaneously. The interferences from ambient ammonia absorption to ethylene reference signals are less than 2 % even for extremely large ammonia signals. The laboratory precision of high-harmonic ethylene reference signal is within 1 %. Initial tests of this calibration method have been performed on the prototype open-path ammonia sensor in the Baltimore Ecosystem Study. Future improvements include using the inline approach with a smaller concentration reference cell to calibrate at cleaner ammonia conditions in the atmosphere (sub-ppbv).
References
Intergovernmental Panel on Climate Change. (Cambridge University Press, Cambridge, 2007), http://www.ipcc.ch
V.P. Aneja, W.H. Schlesinger, J.W. Erisman, Nat. Geosci. 1, 7 (2008)
R.W. Pinder, A.B. Gilliland, R.L. Dennis, Geophys. Res. Lett. 35, 12 (2008)
R. Bobbink, K. Hicks, J. Galloway, T. Spranger, R. Alkemade, M. Ashmore, M. Bustamante, S. Cinderby, E. Davidson, F. Dentener, B. Emmett, J.W. Erisman, M. Fenn, F. Gilliam, A. Nordin, L. Pardo, W. De Vries, Ecol. Appl. 20, 1 (2010)
K. Von Bobrutzki, C.F. Braban, D. Famulari, S.K. Jones, T. Blackall, T.E.L. Smith, M. Blom, H. Coe, M. Gallagher, M. Ghalaieny, M.R. Mcgillen, C.J. Percival, J.D. Whitehead, R. Ellis, J. Murphy, A. Mohacsi, A. Pogany, H. Junninen, S. Rantanen, M.A. Sutton, E. Nemitz, Atmos. Meas. Tech. 3, 1 (2010)
H. Volten, J.B. Bergwerff, M. Haaima, D.E. Lolkema, A.J.C. Berkhout, G.R. Van Der Hoff, C.J.M. Potma, R.J.W. Kruit, W.A.J. Van Pul, D.P.J. Swart, Atmos. Meas. Tech. 5, 2 (2012)
J.B. Nowak, J.A. Neuman, R. Bahreini, C.A. Brock, A.M. Middlebrook, A.G. Wollny, J.S. Holloway, J. Peischl, T.B. Ryerson, F.C. Fehsenfeld, J. Geophys. Res.-Atmos. 115 (2010)
C. Spirig, J. Sintermann, A. Jordan, U. Kuhn, C. Ammann, A. Neftel, Atmos. Meas. Tech. 4, 3 (2011)
D.R. Benson, A. Markovich, M. Al-Refai, S.H. Lee, Atmos. Meas. Tech. 3, 4 (2010)
R.A. Ellis, J.G. Murphy, E. Pattey, R. Van Haarlem, J.M. O’brien, S.C. Herndon, Atmos. Meas. Tech. 3, 2 (2010)
J.B. McManus, J.H. Shorter, D.D. Nelson, M.S. Zahniser, D.E. Glenn, R.M. McGovern, Appl. Phys. B-Lasers O. 92, 3 (2008)
Y.Q. Li, J.J. Schwab, K.L. Demerjian, J. Geophys. Res.-Atmos. 111, D10 (2006)
L. Gong, R. Lewicki, R.J. Griffin, J.H. Flynn, B.L. Lefer, F.K. Tittel, Atmos. Chem. Phys. 11, 18 (2011)
J. Manne, O. Sukhorukov, W. Jager, J. Tulip, Appl. Opt. 45, 36 (2006)
J.D. Whitehead, I.D. Longley, M.W. Gallagher, Water Air Soil Pollut. 183, 1–4 (2007)
D.J. Miller, M.A. Zondlo. Open-Path High Sensitivity Atmospheric Ammonia Sensing with a 9 um Quantum Cascade Laser. in Conference on Lasers and Electro-Optics. Optical Society of America, paper JThJ4, 2010
W.C. Kuster, F.J.M. Harren, J.A. De Gouw, Environ. Sci. Technol. 39, 12 (2005)
L. Tao, K. Sun, D.J. Miller, M.A. Khan and M.A. Zondlo, Optics Letters (2011 in press)
J.A. Silver, Appl. Opt. 44, 31 (2005)
A.N. Dharamsi, J. Phys. D-Appl. Phys. 29, 3 (1996)
O. Axner, P. Kluczynski, J. Gustafsson, A.M. Lindberg, Spectrochim Acta B 56, 8 (2001)
S. Schilt, L. Thevenaz, P. Robert, Appl. Opt. 42, 33 (2003)
G.V.H. Wilson, J. Appl. Phys. 34, 11 (1963)
F. Schreier, J. Quant. Spectrosc. Ra. 48, 5–6 (1992)
G.B. Rieker, J.B. Jeffries, R.K. Hanson, Appl. Opt. 48, 29 (2009)
L.S. Rothman, I.E. Gordon, A. Barbe, D.C. Benner, P.E. Bernath, M. Birk, V. Boudon, L.R. Brown, A. Campargue, J.P. Champion, K. Chance, L.H. Coudert, V. Dana, V.M. Devi, S. Fally, J.M. Flaud, R.R. Gamache, A. Goldman, D. Jacquemart, I. Kleiner, N. Lacome, W.J. Lafferty, J.Y. Mandin, S.T. Massie, S.N. Mikhailenko, C.E. Miller, N. Moazzen-Ahmadi, O.V. Naumenko, A.V. Nikitin, J. Orphal, V.I. Perevalov, A. Perrin, A. Predoi-Cross, C.P. Rinsland, M. Rotger, M. Simeckova, M.A.H. Smith, K. Sung, S.A. Tashkun, J. Tennyson, R.A. Toth, A.C. Vandaele, J. Vander Auwera, J. Quant. Spectrosc. Ra. 110, 9–10 (2009)
V. Zeninari, B. Parvitte, L. Joly, T. Le Barbu, N. Amarouche, G. Durry, Appl. Phys. B-Lasers O. 85, 2–3 (2006)
I. Kleiner, G. Tarrago, C. Cottaz, L. Sagui, L.R. Brown, R.L. Poynter, H.M. Pickett, P. Chen, J.C. Pearson, R.L. Sams, G.A. Blake, S. Matsuura, V. Nemtchinov, P. Varanasi, L. Fusina, G. Di Lonardo, J. Quant. Spectrosc. Ra. 82, 1–4 (2003)
M. Guinet, P. Jeseck, D. Mondelain, I. Pepin, C. Janssen, C. Camy-Peyret, J.Y. Mandin, J. Quant. Spectrosc. Radiat. Transf. 112, 12 (2011)
A.N. Dharamsi, A.M. Bullock, Appl. Phys. B-Lasers O. 63, 3 (1996)
K. Mohan, M.A. Khan, A.N. Dharamsi, Appl. Phys. B-Lasers O. 102, 3 (2011)
J. Chen, A. Hangauer, R. Strzoda, M.C. Amann, Appl. Phys. B-Lasers O. 102, 2 (2011)
J.A. De Gouw, S.T.L. Hekkert, J. Mellqvist, C. Warneke, E.L. Atlas, F.C. Fehsenfeld, A. Fried, G.J. Frost, F.J.M. Harren, J.S. Holloway, B. Lefer, R. Lueb, J.F. Meagher, D.D. Parrish, M. Patel, L. Pope, D. Richter, C. Rivera, T.B. Ryerson, J. Samuelsson, J. Walega, R.A. Washenfelder, P. Weibring, X. Zhu, Environ. Sci. Technol. 43, 7 (2009)
Y. Liu, M. Shao, S.H. Lu, C.C. Liao, J.L. Wang, G. Chen, Atmos. Chem. Phys. 8, 6 (2008)
Z.Y. Meng, W.L. Lin, X.M. Jiang, P. Yan, Y. Wang, Y.M. Zhang, X.F. Jia, X.L. Yu, Atmos. Chem. Phys. 11, 12 (2011)
Acknowledgments
We acknowledge support from NSF-ERC-0540832 and a private gift from Thomas and Lynn Ou. David J. Miller is supported by a Graduate Research Fellowship from the National Science Foundation. We thank W. Wang for helpful discussion on spectral fitting.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, K., Tao, L., Miller, D.J. et al. Inline multi-harmonic calibration method for open-path atmospheric ammonia measurements. Appl. Phys. B 110, 213–222 (2013). https://doi.org/10.1007/s00340-012-5231-2
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-012-5231-2