Abstract
Two miniaturized Raman measurement heads containing microsystem diode lasers emitting at 783 and 671 nm suitable for shifted excitation Raman difference spectroscopy (SERDS) were applied for the non-invasive in situ differentiation of selected meat species. This allows using the fingerprint characteristics of Raman spectra without a disturbing fluorescence background. At 783 nm, two emission lines with a spectral shift of 0.5 nm (7 cm−1) and optical powers of up to 110 mW were realized. For 671 nm excitation, the spectral shift amounts to 0.6 nm (12 cm−1) and optical powers of up to 40 mW were obtained. In both cases, meat Raman spectra could be recorded with integration times of 10 s. The investigations were carried out using selected cuts from the most commonly consumed meat species in the US and Europe, i.e. beef, pork, chicken, and turkey. A principal components analysis of the SERDS spectra revealed a clear separation of the meat species into four distinct groups for both excitation wavelengths. This classification is based on the myoglobin content and gradual differences of protein Raman band intensities and positions. The results demonstrate the potential of SERDS as rapid and non-destructive screening method for the discrimination of selected meat species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The identification of meat species to detect potential adulteration is a recent issue in the field of meat authentication [1]. Here, common problems are the substitution of high-value meat cuts by cheaper material as well as incorrect labeling of meat products [2]. Beside commercial consequences, this also implies the occurrence of ethical, religious (e.g. the prohibition of pork meat consumption [3]), and health [4] conflicts to consumers. Hence, there is a requirement for analytical methods suitable for animal species identification to ensure quality control and to protect the interests of consumers. Traditional techniques for the discrimination of selected animal species can be classified into chromatographic [5–7], electrophoretical [8–10], and enzymatic [11–15] procedures. In the last years, genetic methods based on conventional [16–18] and real-time polymerase chain reaction (PCR) [19, 20] have acquired increasing importance. However, despite their reliability and sensitivity, these laboratory-based techniques are expensive and time consuming as well as require a more or less complicated sample pretreatment and skilled technical staff for operation.
To overcome this drawback, fast and non-destructive optical methods offer a promising alternative for rapid screening purposes. In that way, visible (VIS) and near-infrared (NIR) reflectance spectroscopy were applied for the differentiation between beef and kangaroo meat [21] as well as the identification of beef, lamb, pork, and chicken [22]. Furthermore, spectroscopic studies concerning the authentication of pork, chicken, and turkey are reported in the mid-infrared (MIR) range [23] and using a combined approach in the VIS, NIR, and MIR spectral region for animal species discrimination [24]. For the identification of beef, llama, and horse meat, Mamani-Linares et al. [25] applied NIR reflectance and transflectance spectroscopy for the investigation of meat and meat juice.
Raman spectroscopy providing a fingerprint on molecular level of the probed sample is a further optical technique with a great potential for identification purposes. In this context, Ellis et al. [26] demonstrated the feasibility of the Raman technique to discriminate between leg and breast muscles of chicken and turkey. However, in this study fluorescence interference occurred in the meat Raman spectra despite the applied 785 nm near infrared excitation. Hence, this paper presents an approach using shifted excitation Raman difference spectroscopy (SERDS) [27, 28] for effective rejection of the disturbing fluorescence background. In that way, SERDS also allows for application of shorter excitation wavelengths in the visible range leading to increased Raman scattering intensities according to the ν4-law since the increased fluorescence contribution in this spectral region is efficiently removed.
To apply SERDS, two Raman spectra are recorded using two narrow laser emission lines with a spectral separation in the order of the full width at half maximum of the Raman signals under investigation [28]. In that way, the characteristic Raman bands are slightly shifted whereas the broadband fluorescence background remains essentially unchanged. These two spectra are then subtracted to remove the fluorescence interference and the detector fixed pattern noise while the Raman spectral signature is preserved. A subsequent reconstruction of the difference spectrum results in a nearly background-free SERDS spectrum allowing for determination also of weak Raman bands formerly obscured by fluorescence.
With respect to integration into portable sensor systems [29], compact microsystem diode lasers with low power consumption and adjustable spectral parameters according to the measurement task are well suited as excitation light sources. Here, we present two compact Raman measurement heads including lasers and optics, coupled to a laboratory spectrometer and a miniature spectrometer suitable for SERDS application at 783 and 671 nm, respectively. To demonstrate the potential of these systems for the rapid and non-destructive discrimination of frequently consumed animal species, cuts from beef and pork as red meat as well as from chicken and turkey as white meat were selected.
2 Materials and methods
2.1 SERDS setup for 783 nm excitation
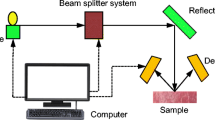
Figure 1 presents the Raman experimental setup used for 783 nm SERDS investigations. To realize the Raman measurement head, an in-house developed and validated Raman optical bench [30] (1) was combined with a laser module (2) including a 783 nm distributed feedback (DFB) microsystem diode laser [31, 32]. For SERDS operation, the emission wavelength of the laser can be switched between the operation points at λ1 = 782.65 nm and λ2 = 783.15 nm resulting in a spectral shift of 0.5 nm (7 cm−1) by variation of the injection current from 110 to 260 mA. The injection current and the laser diode temperature of 25 °C were controlled by a diode laser controller DC 100 (Toptica Photonics) (3). Inside the Raman optical bench the collimated beam of the DFB laser is spectrally cleaned by a bandpass filter (AHF analysentechnik AG) (5) and then guided by dielectric mirrors (4) and a Raman edge filter (Semrock, Inc.) (6) to a lens (7) with a focal length of 10 mm. This lens focuses the excitation radiation through a quartz glass window (8) onto the meat sample (9) and collects the backscattered radiation from the specimen. The Raman edge filter only transmits the Stokes shifted radiation while rejecting the Rayleigh as well as the anti-Stokes scattering. A second lens (10) with a focal length of 16 mm then focuses the Stokes component into an optical fiber (11) with a core diameter of 100 μm. All lenses, mirrors, and the quartz glass window were purchased from Thorlabs GmbH. By means of the optical fiber, the Raman measurement head is connected to a PI 320 spectrometer (Princeton instruments) (12) equipped with a grating with 600 lines/mm realizing an optical resolution of 10 cm−1. The attached back-illuminated deep-depletion CCD detector with 1,024 × 256 pixel (DU420-BR-DD, Andor Technology) (13) is thermo-electrically cooled down to −60 °C and serves for detection of the spectral data which are then recorded using a computer (14) running Andor Solis software (Andor Technology). For the investigation of the meat samples, Raman spectra were measured with an integration time of 10 s and the laser power at sample position was 50 mW (782.65 nm) and 110 mW (783.15 nm).
Experimental setup for shifted excitation Raman difference spectroscopy (SERDS) at 783 nm including (1) Raman measurement head with (2) SERDS laser module, (3) laser driver (4) mirror, (5) bandpass filter, (6) Raman edge filter, (7) lens, (8) quartz window, (9) sample, (10) lens, (11) optical fiber, (12) spectrometer, (13) CCD, (14) computer
2.2 SERDS setup for 671 nm excitation
To investigate the meat samples with an excitation wavelength of 671 nm, a similar setup as displayed in Fig. 1 was applied. Here, the laser module attached to the Raman optical bench includes a microsystem diode laser with two separate laser cavities each containing a frequency selective volume Bragg grating [33]. In that way, two discrete laser emission lines at λ1 = 671.0 nm and λ2 = 671.6 nm with a spectral shift of 0.6 nm (12 cm−1) are realized. The injection current of 400 mA for both laser cavities was controlled by two LDC 210C benchtop LD current controllers (Thorlabs GmbH). The operating temperature of the heat sink was set to 25 °C and controlled by a DTC 100 temperature controller (Toptica Photonics). For Raman signal excitation as well as collection, the same lenses as described in the previous section were used. An optical fiber with a core diameter of 100 μm connects the Raman measurement head to a custom-designed miniature spectrometer (Horiba Scientific) with an optical resolution of 8 cm−1. Detection and recording of spectra was performed using a back-thinned CCD camera (S7031-1006, Hamamatsu) with 1,024 × 58 pixel, thermoelectrically cooled down to −10 °C and a netbook running Versaspec software (Horiba Scientific). With applied laser powers of 30 mW (671.0 nm) and 40 mW (671.6 nm) at the meat sample, Raman spectra were measured with integration times of 10 s.
2.3 Sample preparation and data analysis
For our investigations, we selected cuts from the most commonly consumed meat species in the US and Europe, i.e. beef (rump steak), pork (loin chops), chicken (breast), and turkey (breast). For each animal species, 12 randomly chosen slices of fresh meat were bought in a local supermarket at different days within a period of 8 weeks and measured at the day of purchase. A cylindrical cutting tool was used to cut out three subsamples with a diameter of 2 cm from each meat slice. These meat cylinders were then put in PVC tubes, transferred into Petri dishes to prevent them from drying, and stored at 5 °C in a laboratory refrigerator (Spezial-468, Philipp Kirsch, Germany) until use. Each cylindrical sample was probed at five different positions with the two slightly shifted excitation wavelengths required for SERDS and 10 spectra were recorded and averaged for each measurement spot. In that way, 150 single Raman spectra from 15 sampled positions per meat slice were obtained.
To reject the disturbing fluorescence background from the Raman data, SERDS spectra were obtained from the two slightly shifted spectra from each sample position using an in-house developed and patented reconstruction method [34]. Prior to further data analysis, the SERDS spectra from 15 measurement spots per meat slice were averaged leading to 12 mean spectra for each species. The complete dataset was normalized to the height of the prominent CH bending Raman band at 1,445 cm−1 followed by mean centering. To determine spectral characteristics suitable for meat species differentiation, the statistical tool principal components analysis (PCA) was applied to the data using the program MatLab (MathWorks Inc., Natick, MA) combined with PLS-Toolbox (Eigenvector Research Inc., Wenatchee, WA).
3 Results
3.1 783 nm SERDS investigations
Figure 2 displays a comparative overview of Raman and SERDS spectra obtained for pork, beef, chicken, and turkey impressively illustrating the potential of the SERDS technique for effective fluorescence background rejection. The Raman spectra (Fig. 2a) of the investigated animal species show a variation of the signal background caused by their different optical properties. Here, pork and the poultry species have a lower fluorescence level compared to beef where a significantly increased background level can be recognized. However, all Raman spectra exhibit the characteristic meat protein structure comprising signals of the aromatic amino acids phenylalanine (Phe), tyrosine (Tyr), and tryptophan (Trp) as well as vibrations of the polypeptide backbone (amide, CH bending, and CC stretch bands) [35–38].
For an examination of spectral differences between the animal species suitable for classification it is preferable to remove the disturbing fluorescence-based signal background. Therefore, the SERDS technique was applied to the Raman data enabling an effective background removal resulting in a straight horizontal baseline (Fig. 2b). This fact is also advantageous for performing normalizing procedures, e.g. on the height of selected Raman bands, which can be done much easier as with the Raman spectra containing a fluorescence contribution. The SERDS spectra display essentially the same protein spectral signature with only gradual differences for all investigated meat species. Hence, the application of statistical tools such as PCA to the SERDS dataset is indispensable to reveal animal-specific spectral characteristics suitable for differentiation purposes.
Figure 3a presents a three-dimensional plot of the scored SERDS data for the principal components (PCs) 2, 3, and 6 revealing an entire distinction of the probed meat species into four clearly separated groups. The differentiation between red (beef and pork) and white meat (chicken and turkey) is made according to PC 2 with high positive values for beef, smaller but with the exception of two samples still positive values for pork while the poultry species have essentially the same negative values. The separation between beef and pork becomes even more pronounced by additionally considering PC 3 exhibiting a positive sign for pork and a negative sign for 10 out of 12 beef samples. Therefore, the combination of PC 2 and PC 3 enables for a species classification into pork, beef, and a third group comprising chicken and turkey as displayed in Fig. 3b. Due to the similarity of the poultry species, higher principal components have to be incorporated to obtain a discrimination of chicken and turkey. In that way, PC 6 serves for a separation exhibiting negative values for chicken and positive values for turkey. Consequently, the incorporation of three PCs allowed for a complete distinction of the SERDS spectra of the meat species under investigation.
The loadings of PC 2, PC 3, and PC 6 are presented in Fig. 4 to enable a more detailed examination of spectral characteristics which are responsible for the obtained animal-specific distinction. The major contribution to principal component 2 arises from the characteristic signals of myoglobin [39–41] which are summarized in Table 1. Additionally, the conformation-sensitive amide I band at 1,646 cm−1 becomes obvious. Principal component 3 which was responsible for the discrimination of pork meat revealed main contributions from Phe around 1,000 cm−1, the tyrosine doublet around 825 and 850 cm−1, and signals ascribed to Trp, Phe, and Tyr in the region from 1,600–1,620 cm−1. Furthermore, vibrations of the polypeptide backbone in the range between 1,100 and 1,130 cm−1, a CH bending vibration around 1,450 cm−1, and the amide I band at 1,660 cm−1 can be found. Minor contributions came from the Trp signals around 755 and 1,550 cm−1, the CC stretching vibration at 939 cm−1, and a band from Phe and Tyr around 1,210 cm−1. The separation of chicken and turkey according to PC 6 is mainly based on bands from Phe around 1,000 cm−1, the tyrosine doublet around 825 and 855 cm−1, a band from Phe and Tyr around 1,205 cm−1, and Trp around 1,550 cm−1. Further contributing signals are ascribed to the amide III band around 1,310 cm−1, a CH bending vibration around 1,450 cm−1, and the amide I band around 1,650 cm−1. An overview of the prominent bands present in the loadings is displayed in Table 1. As can be seen from this analysis, the primary species distinction made by loading 2 is related to the differences in myoglobin content which is highest in beef and lowest in poultry meat. Furthermore, gradual variations in signal heights as well as slight Raman band shifts of the meat protein spectrum have contributions to the achieved meat species classification.
3.2 671 nm SERDS investigations
The measurement series was repeated using a 671-nm microsystem diode laser to investigate a possible influence of the excitation wavelength on the spectral data of the selected meat species. An overview of the corresponding Raman and SERDS spectra of pork, beef, chicken, and turkey is presented in Fig. 5. Similar to 783 nm excitation, the Raman spectra of pork and both poultry species exhibit a moderate signal background (left-hand scale in Fig. 5a) whereas strong fluorescence interference becomes obvious in the case of beef (right-hand scale in Fig. 5a). However, application of the SERDS technique enables an efficient rejection of the fluorescence contribution from the spectra (see Fig. 5b) leading to straight horizontal baseline. Here, all probed meat species show essentially the same protein signature demanding for analysis of the SERDS dataset using PCA.
In that way, Fig. 6a displays the scored SERDS data for the principal components 2, 7, and 10 exhibiting a clear separation of the animal species into four distinct groups. PC 2 is responsible for the separation of beef with positive values from all other animal species having negative values except one pork sample showing a small positive value. The pork samples are distinguished from the poultry species not only by means of PC 7 but also with a contribution of PC 2. Here, pork reveals a positive sign according to PC 7 whereas the majority of chicken and turkey samples possess a negative sign. Thus, considering PC 2 and PC 7 allows discriminating between beef, pork, and a third group containing chicken and turkey as presented in Fig. 6b. To achieve a distinction of the poultry species, again the incorporation of higher PCs is necessary. With the exception of one misclassified turkey sample, PC 10 allows for a separation with chicken having positive values whereas turkey possesses negative values.
For a more detailed examination of spectral differences which are responsible for the achieved animal-specific classification, the loadings of PC 2, PC 7, and PC 10 are displayed in Fig. 7. Similar to 783 nm excitation, major contributions to principal component 2 come from signals ascribed to myoglobin (see Table 1). Further bands can be recognized from the tyrosine doublet around 825 and 855 cm−1, signals ascribed to Trp, Phe, and Tyr in the region from 1,600–1,620 cm−1, and the amide I band located around 1,650 cm−1. The strongest signal in PC 7 arises from a band from Phe and Tyr around 1,205 cm−1. Minor contributions are present from the tyrosine doublet around 825 and 850 cm−1, Phe around 1,000 cm−1, vibrations of the polypeptide backbone in the range between 1,080 and 1,130 cm−1, and signals ascribed to Trp, Phe, and Tyr in the region from 1,600–1,620 cm−1. Additionally, conformation-sensitive bands around 937 cm−1 (CC stretch), 1,310 cm−1 (amide III), and 1,655 cm−1 (amide I) can be recognized. In the loading of PC 10 which was responsible for the separation of chicken and turkey, signals from Trp around 755 cm−1, Tyr around 825 cm−1, and 855 cm−1, Phe around 1,000 cm−1, Phe and Tyr at 1,208 cm−1, and Trp, Phe, and Tyr at 1,606 cm−1 become obvious. Furthermore, a vibration of the polypeptide backbone at 1,126 cm−1, a CH bending vibration around 1,340 cm−1 and conformation-sensitive CC stretching vibrations at 899 and 932 cm−1, as well as the amide I band around 1,650 cm−1 are present. As in the case for an excitation wavelength of 783 nm, the myoglobin content of the meat had a major contribution to the obtained classification. However, the obtained animal-specific distinction also relies on gradual changes in Raman band intensities and positions.
4 Conclusion
In this paper, we present an approach for the non-destructive in situ identification of meat from selected animal species applying shifted excitation Raman difference spectroscopy (SERDS). This technique efficiently rejects the disturbing fluorescence-based signal background enabling the use of fingerprint Raman spectra for classification purposes without fluorescence interference. For our investigations, two compact Raman measurement heads including 783 and 671 nm microsystem diode lasers each emitting at two slightly shifted wavelengths suitable for SERDS operation were applied. Cuts from the most commonly consumed red and white meat species in the US and Europe, i.e. beef, pork, chicken, and turkey were chosen as sample material. Independent of the fluorescence impact in the Raman data which was highest for beef, the SERDS technique allowed for a complete background removal resulting in a straight horizontal baseline for both applied excitation wavelengths. Hence, the SERDS spectra of all probed meat species revealed essentially the same protein signature exhibiting only gradual differences. For a detailed analysis of the dataset, PCA was applied to determine animal-specific spectral characteristics suitable for species identification. In that way, a major contribution from the meat myoglobin content as well as slight differences in Raman band intensities and positions of the aromatic amino acids tryptophan, tyrosine, and phenylalanine as well as of conformation-sensitive CC stretching and amide signals were responsible for the obtained classification. The incorporation of three PCs revealed a clear distinction of the meat species into four distinct groups for both excitation wavelengths with only one turkey outlier in the chicken cluster for 671 nm excitation. These results demonstrate the effective fluorescence rejection using the SERDS technique allowing for a better investigation of closely related spectral signatures without fluorescence background interference. In that way, SERDS has a great potential as screening method suitable for the rapid discrimination of selected meat species.
References
N.Z. Ballin, Meat Sci. 86, 577 (2010)
N.Z. Ballin, F.K. Vogensen, A.H. Karlsson, Meat Sci. 83, 165 (2009)
K. Nakyinsige, Y. Bin Che Man, A. Qurni Sazili: Meat Sci. 91, 207 (2012)
A.J. McAfee, E.M. McSorley, G.J. Cuskelly, B.W. Moss, J.M.W. Wallace, M.P. Bonham, A.M. Fearon, Meat Sci. 84, 1 (2010)
S.H. Ashoor, W.G. Monte, P.G. Stiles, J. Assoc. Off. Anal. Chem. 71, 397 (1988)
R.M. Toorop, S.J. Murch, R.O. Ball, Food Res. Int. 30, 619 (1997)
C.C. Chou, S.P. Lin, K.M. Lee, C.T. Hsu, T.W. Vickroy, J.M. Zen, J. Chromatogr. B 846, 230 (2007)
K.-P. Kaiser, G. Matheis, C. Kmita-Dürrmann, H.-D. Belitz, Z. Lebensm. Unters. Forsch. 170, 334 (1980)
M. Cota-Rivas, B. Vallejo-Cordoba, J. Capillary Electrophor. 4, 195 (1997)
B. Vallejo-Cordoba, A.F. González-Córdova, M.A. Mazorra-Manzano, R. Rodríguez-Ramírez, J. Sep. Sci. 28, 826 (2005)
S.J. Jones, R.L.S. Patterson, J. Sci. Food Agric. 37, 767 (1986)
N.K. Sharma, V.A. Srivatava, J.P.S. Gill, D.V. Joshi, Food Control 5, 219 (1994)
U.V. Mandokhot, S.K. Kotwal, J. Food Sci. Technol. 34, 369 (1997)
Y. Ayaz, N.D. Ayaz, I. Erol, J. Muscle Foods 17, 214 (2006)
L. Asensio, I. González, T. García, R. Martín, Food Control 19, 1 (2008)
T. Matsunaga, K. Chikuni, R. Tanabe, S. Muroya, K. Shibata, J. Yamada, Y. Shinmura, Meat Sci. 51, 143 (1999)
Q. Wang, X. Zhang, H.-Y. Zhang, J. Zhang, G.-Q. Chen, D.-H. Zhao, H.-P. Ma, W.-J. Liao, Meat Sci. 85, 265 (2010)
F.C. Lago, B. Herrero, M. Madriñán, J.M. Vieites, M. Espiñeira, Eur. Food Res. Technol. 232, 509 (2011)
R. Köppel, J. Ruf, F. Zimmerli, A. Breitenmoser, Eur. Food Res. Technol. 227, 1199 (2008)
R. Köppel, F. Zimmerli, A. Breitenmoser, Eur. Food Res. Technol. 230, 125 (2009)
H.B. Ding, R.J. Xu, J. Food Sci. 64, 814 (1999)
D. Cozzolino, I. Murray, LWT - Food Sci. Technol. 37, 447 (2004)
O. Al-Jowder, E.K. Kemsley, R.H. Wilson, Food Chem. 59, 195 (1997)
H. Rannou, G. Downey, Anal. Commun. 34, 401 (1997)
L.W. Mamani-Linares, C. Gallo, D. Alomar, Meat Sci. 90, 378 (2012)
D.I. Ellis, D. Broadhurst, S.J. Clarke, R. Goodacre, Analyst 130, 1648 (2005)
A.P. Shreve, N.J. Cherepy, R.A. Mathies, Appl. Spectrosc. 46, 707 (1992)
J. Zhao, M.M. Carrabba, F.S. Allen, Appl. Spectrosc. 56, 834 (2002)
K. Sowoidnich, H. Schmidt, H.-D. Kronfeldt, F. Schwägele: Vib. Spectrosc. doi:10.1016/j.vibspec.2012.04.002 (2012)
H. Schmidt, K. Sowoidnich, M. Maiwald, B. Sumpf, H.-D. Kronfeldt: Proc. SPIE 7312, 73120H-1 (2009)
M. Maiwald, G. Erbert, A. Klehr, H.-D. Kronfeldt, H. Schmidt, B. Sumpf, G. Tränkle, Appl. Phys. B 85, 509 (2006)
H. Wenzel, A. Klehr, M. Braun, F. Bugge, G. Erbert, J. Fricke, A. Knauer, M. Weyers, G. Tränkle, Electron. Lett. 40, 123 (2004)
M. Maiwald, H. Schmidt, B. Sumpf, G. Erbert, H.-D. Kronfeldt, G. Tränkle, Appl. Opt. 48, 2789 (2009)
H. Schmidt, D. Pérez-Kaiser, M. Maiwald, Method for generating and for detecting a Raman spectrum. International patent, WO 2011/033017 A1 (2011)
E.B. Carew, I.M. Asher, H.E. Stanley, Science 188, 933 (1975)
E.B. Carew, H.E. Stanley, J.C. Seidel, J. Gergeley, Biophys. J. 44, 219 (1983)
A.M. Herrero, Crit. Rev. Food Sci. Nutr. 48, 512 (2008)
A.M. Herrero, Food Chem. 107, 1642 (2008)
C.K. Johnson, R. Rubinovitz, Spectrochim. Acta A 47, 1413 (1991)
V. Palaniappan, D.F. Bocian, Biochemistry 33, 14264 (1994)
K.R. Ward, R.W. Barbee, P.S. Reynolds, I.P. Torres Filho, M.H. Tiba, L. Torres, R.N. Pittman, J. Terner, Anal. Chem. 79, 1514 (2007)
Acknowledgments
We wish to thank Bernd Sumpf and Martin Maiwald, Ferdinand-Braun-Institut, Leibniz-Institut für Höchstfrequenztechnik Berlin, for the development of the microsystem diode laser sources suitable for SERDS emitting at 671 and 783 nm. Furthermore, the kind help of Andreas Braunwarth, AHF analysentechnik AG, for providing the custom-designed 783 nm bandpass filter is appreciated. Kay Sowoidnich is grateful for financial support by the state of Berlin in the frame of an Elsa-Neumann scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sowoidnich, K., Kronfeldt, HD. Shifted excitation Raman difference spectroscopy at multiple wavelengths for in-situ meat species differentiation. Appl. Phys. B 108, 975–982 (2012). https://doi.org/10.1007/s00340-012-5160-0
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-012-5160-0