Abstract
Optical absorption and emission properties of Ho3+ ions in barium–gallium-germanate glass are investigated. A significant result is that the emission intensity of Ho3+:5I7→5I8 excited by 808 nm through the energy level Tm3+:3H4 is stronger than that of the direct excitation of Ho3+. The emission cross section of Ho3+ is calculated to be 9.02×10−21 cm2 at the wavelength of 1.994 μm. Comparatively, the larger emission cross section and efficient energy transfer process reveal that the prepared glass could be a kind of promising material for laser emission at 2 μm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Solid-state lasers emitting in the 2 μm eye-safe region are promising candidates for various applications in laser medicine surgery, monitoring of atmospheric pollutants, and high-resolution spectroscopy of low pressure gases, etc. [1–4]. Ever since Johnson has reported the first laser action at 2.0 μm from Ho3+ doped CaWO4 crystal in 1962 [5], an intense level of research has been focused on the spectroscopic and lasing properties of Ho3+ doped various crystals and glasses [6, 7]. Among the rare-earth ions, Ho3+ ion is one of the most important active ions applied to the infrared emission, because of its favorable energy level structure. The Ho3+ ion has a relatively long-lived 5I7 level which can act as a good population reservoir for infrared luminescence process. It is reported that Ho3+ doped fibers can be pumped by semiconductor disk laser around 1.16 μm and the passively mode-locked fiber laser has been demonstrated [8, 9]. Ho3+ can also be pumped by sensitizers such as Tm3+, Er3+ or Yb3+ ions. Ho3+ doped fiber laser has been achieved when pumped by an Yb3+ doped fiber laser at 1147 nm [10]. In our previous studies, the spectroscopic properties of Ho3+ sensitized by Yb3+, and co-doped with Er3+/Tm3+ have been studied [11, 12]. The Yb3+ ion has large absorption coefficient and emission cross section at 980 nm, but the energy utilization efficiency is relatively low. These studies have shown that the energy transfer microparameter from Yb3+ to Ho3+ is much lower than that of Tm3+/Er3+ to Ho3+. What’s more, strong upconversion luminescence band be observed in the Yb3+/Ho3+ codoped glasses, so Tm3+/Er3+ ions have become the main choice as sensitizers of Ho3+.
In this research, we would like to analyze the mechanism of Ho3+ fluorescence and Tm3+ singly sensitized Ho3+ in glass host. The ratio of number of photos emitted by Tm3+ ions to the number of photons absorbed, namely, quantum efficiency, can reach as high as 200 % (see Fig. 1). After the ground state absorption (GSA) of one pump photon, two ground level Tm3+ ions can be excited to the upper laser level through a cross relaxation (CR) process 3H4,3H6→3F4,3F4. The pumped energy of the excited level of Tm3+:3F4 transfers to the level of Ho3+:5I7 through a resonant or nonresonant energy transfer process [13]. The nonresonant process can be assisted by local phonons and energy difference must be given to the lattice vibrations. Germanate glass has been chosen as the matrix material for its good thermal stability and high transparency in a wide wavelength range [14, 15]. Compared with silicate and fluorophosphates glass, its low phonon energy and broad transmission range allow the observation of laser emission from rare earth ions in a wide optical range. There are quite few studies on infrared emission around ∼2 μm from Ho3+ singly or Tm3+ codoped of germanate glasses. The present paper mainly aims to report on the studies of ∼2 μm emission characteristics of Ho3+ ions both by direct excitation and by sensitized excitation through energy transfer from Tm3+ ions in the codoped barium gallium germanate glasses. Additionally, a systematic characterization of spectroscopic properties of Ho3+ ions in the present glass by applying the Judd–Ofelt theory has been dealt along with the physical and structural analysis.
2 Experiments
The Ho3+/Tm3+ codoped, Ho3+ singly doped and undoped barium gallium germanate having chemical composition (mol %) 65GeO2-10Ga2O3-20(BaO+BaF2)-5La2O3-xTm2O3-yHo2O3, (x=0, 2 and y=0, 0.5) were prepared by traditional melt-quenching method with high purity reagent as raw materials. Before melting the powders in a SiC-resistance electric furnace, the molar masses were weighted, mixed and dried. The mixtures were stirred 1 h with a platinum rod and then homogenized at 1400 °C in a platinum crucible in furnace. The homogeneous melt was poured onto a preheated brass mould and annealed for several hours around 600 °C in a muffle furnace, and then allowed to cool slowly inside the furnace by turning the power supply off. The two-face polished sample has plate shape with 15×15×1 mm3 for optical measurements.
The refractive index of the host glass was measured by the prism minimum deviation method at four wavelengths, 656, 588, 486, and 1053 nm, and they are 1.7292, 1.7348, 1.7473, and 1.7195, respectively. The resolution of the instrument is ±0.5×10−4. The standard deviation in refractive index at different points of the same glass is around ±1×10−4. The refractive index dispersion curve was calculated by Cauchy’s formula n(λ)=a+b/λ 2+c/λ 4, where a, b and c are found to be 1.7061, 1.0222×104 and 1.1171×108 respectively. The density of the glass was measured by Archimedes method using distilled water as buoyancy liquid at room temperature. The analysis of Ho3+ ions absorption was carried out using a Perkin-Elmer-Lambda 900UV/VIS/NIR spectrophotometer in the range of 350–2200 nm. The near infrared emission spectra and excitation spectra were recorded on a Traix 320 type spectrometer (Jobin-Yvon Co., France). A Xenon flash lamp was employed as an excitation source. All the measurements were performed at room temperature.
3 Results and discussion
The Raman spectra of Ho3+/Tm3+ codoped and Ho3+ singly doped barium gallium germanate are shown in Fig. 2. For both glasses, the spectra comprise identical nature suggesting that the incorporation of Tm3+ in glass network does not bring significant change in the glass structure. The spectra revealed a broad band with superimposition of two reflectance bands centered at 846 and 756 cm−1 which are generally assigned to the stretching mode contributions not only from Ge–O structure units but also from Ga–O units in the environment of bridging and nonbridging oxygen [16]. The frequencies higher than 700 cm−1 are generally assigned to localized vibrational modes within the tetrahedral units. Intermediate frequency region are dominated by a complicated envelope of overlapping peaks e (487 cm−1), f (546 cm−1) and g (598 cm−1), which have been assigned to various vibrational modes involving Ge–O–Ge and Ga–O–Ga bending [17]. The Raman peaks at lower wave number a (126 cm−1), b (156 cm−1), c (204 cm−1) and d (284 cm−1) are due to the vibration of network modifying cations in large interstitial sites or to acoustic modes involving motions of the tetrahedral cations in large clusters.
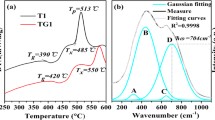
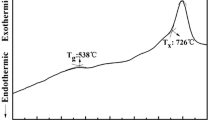
The thermal parameters are based on characteristic temperature including the glass transition T g , onset of devitrification T x , the peak crystallization peak T p temperatures and the melting temperature T m . Dietzel introduced the glass criterion [18], ΔT=T x −T g , which is often an important parameter for evaluating the glass forming ability. Large ΔT means strong inhibition to processes of nucleation and crystallization [19]. The glass formation factor of the materials is given by parameter k gl =(T x −T g )/(T m −T g ) and is more suitable for estimating the glass thermal stability than ΔT. The higher values of the criterion parameters reflect greater thermal stability of the glass. The measured densities of Ho3+/Tm3+ codoped and Ho3+ singly doped barium gallium germanate glasses along with the average molecular weight of the respective glasses have been used to estimate the rare earth ion concentration. Table 1 shows the results of characteristic temperatures, rare earth concentrations and densities of the samples. It is clear from the data that T g , T x , T p , along with the thermal stability factor ΔT and k gl increase with thulium addition. The increase in glass stability with thulium addition is due to its conditional forming ability.
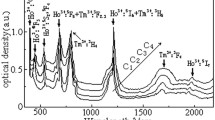
The room temperature absorption spectra of Ho3+/Tm3+ codoped and Ho3+ singly doped barium gallium germanate glasses have been presented in Fig. 3. The Ho3+ doped glass exhibits a number of distinct absorption bands in the Vis–IR range at 361, 418, 448, 486, 537, 642, 1151 and 1950 nm for Ho3+ ions and at 685, 790, 1209 and 1648 nm for Tm3+ ions. It is observed that the intensity of the Ho3+:5I8→5G6 transition is highest among the detected peaks of the Ho3+ singly doped sample. This is due to its hypersensitive nature in 4f 10 electronic configuration of Ho3+ ions with invariably obeying ΔS=0, ΔJ=2 and ΔL=2 spectral rule possessing strong dependence on the ligand field environment surrounding the rare earth ions [20]. In general, magnitude of the hypersensitivity of a transition mainly linked to the ligand covalency, crystal field symmetry and it is correlated strongly with the Ω 2 intensity parameter [5]. It also can be seen from Fig. 3 that the absorption band of Tm3+:3F4 is close to that of Ho3+:5I7 and the energy is slightly higher than that of Ho3+:5I8→5I7 transition. Efficient energy transfer can be expected from Tm3+:3F4 to Ho3+:5I7, as shown in Fig. 1. Meanwhile, Tm3+ has strong absorption band 3H4 around 800 nm which is available using commercial laser diode.
Oscillator strengths obtained from the absorption spectra are used as experimental input for Judd–Ofelt intensity analysis. The Judd–Ofelt model describes the perturbation of the 4f n states by opposite parity f n−1 nl (where nl is mainly 5d) on the basis of intensity parameters Ω λ (λ=2,4,6) [21, 22]. The model assumes the states of the perturbing 4f n−1 nl configurations to be degenerate and the energy difference between these perturbing states and all the states of the 4f n configuration to be equal. The model further assumes an isotropic medium, equal frequencies of the crystal field transitions within a |SLJ〉→|S′L′J′〉 multiplet transition, and equal thermal population of the crystal field levels within the |SLJ〉 initial state. These conditions are typically sufficiently well satisfied for rare earth doped glasses at room temperature. By the absorption spectrum measured above, the electric-dipole-included oscillator strength, f ed, of a transition |SLJ〉→|S′L′J′〉 can be calculated using the expression

where m is the mass of the electron, c is the speed of the light in vacuum, n is the refractive index of the sample and υ is the transition frequency. The term |〈U λ〉|2 is the reduced matrix element of the tensor operators; generally, it can be considered independent on the host materials, and the values used in this work are quoted from Ref. [23]. The magnetic-dipole induced isotropic oscillator strength, f md, which is nonzero for transitions with ΔJ=0,±1, is given by
where e is the electron charge and μ B is the Bohr magneton. The intensity parameters Ω λ (λ=2,4,6) are obtained from a least square fitting of calculated total oscillator strength f theor=f ed+f md (Eqs. (1) and (2)) to experimental oscillator strengths f exp. With the addition of glass modifiers such as rare-earth ions, linkages in the [GeO4] network of germanate glass are broken. Consequently, there are bridging oxygen (BO) atoms and nonbridging oxygen (NBO) ions, the latter having a formal charge of −1 and being the only negatively charged species in the glass. In effect, the germanate glass network becomes a polyanion with net negative charge primarily localized at NBO’s. Charge balance is achieved with the presence of the constituent cations in interstitial sites. Depending on the structure of the network and on the size, charge, and field strengths of these cations, they will form associates with network oxygens with varying degrees of ionic/covalent character.
The Judd–Ofelt parameters Ω λ (λ=2,4,6) are important for the investigation of the local structure and bonding in the vicinity of rare earth ions. The Ω 2 parameter is proposed to be a measure of the degree of covalency of the chemical bonds between rare earth ions and its nearest neighbor atoms [24]. Ω 6 reflects the bulk properties of the host such as rigidity and viscosity [24]. Previous research has also reported that the 2 μm emission transition of Ho3+:5I7→5I8 is mainly affected by Ω 6 intensity parameter due to its larger |〈U 6〉|2 matrix element and as the radiative spontaneous emission probability is proportionately dependent on Ω 6 [25]. The Ω 2,Ω 4 and Ω 6 values are calculated to be 7.83×10−20 cm2,6.37×10−20 cm2 and 2.05×10−20 cm2, respectively. These are larger than those of Ga2S3–La2S3 glass, whose Ω 2,Ω 4 and Ω 6 values are 6.9×10−20 cm2, 5.5×10−20 cm2 and 1.07×10−20 cm2, respectively [26]. These are also larger than those of ZBLAN glass, whose Ω 2,Ω 4 and Ω 6 values are 2.3×10−20 cm2,2.3×10−20 cm2 and 1.7×10−20 cm2, respectively [27]. Large Ω 2 values are probably caused by relatively high covalency of the chemical bond between rare earth and oxygen ions. The Ω 6 parameter is proposed to increase with increasing rigidity of the medium, i.e., the mean force constant of the Ho3+–oxygen/sulfide/fluoride bonds. It can be seen that the Ω 6 values are higher for germanate than for sulfide and fluoride glasses. The Judd–Ofelt analysis therefore indicates that germanate glass has a higher covalency and a higher force constant of Ho3+-oxygen bonds than those of sulfide and fluoride glasses.
Figure 4 shows the near-infrared emission spectra of Ho3+/Tm3+ codoped germanate glass when excited by 808 nm through 3H4 excited state of sensitizer Tm3+ ions and by direct Ho3+ ions excitation through 5F5 level with 644 nm wavelength. These respective excitation wavelength have been chosen from the recorded excitation spectrum of co-doped sample by monitoring 2.0 μm emission of Ho3+ ions as seen inset of Fig. 4. The emission peaks detected at 1.8 and 2.016 μm are assigned to the transition of Tm3+:3F4→3H6 and Ho3+:5I7→5I8, respectively. The intensity of the excitation sources keeps the same, so the results are comparable. The interesting observation is that the emission intensity of Ho3+:5I7→5I8 had becomes stronger when excited by 808 nm through the energy level Tm3+:3H4 than that of direct excitation of Ho3+. This is because of high absorption cross section of Tm3+ accompanied with efficient sensitized energy transfer from Tm3+ to Ho3+ ions.
Figure 5 represents the emission spectra of Ho3+ doped germanate glass when excited by a xenon lamp at the wavelength of 658 nm. The spectra depict a broad emission band due to Ho3+:5I7→5I8 transition with several stark components ranging from 1850 to 2150 nm. The quality of the curve fit is justified by the best superimposition of the fitted curve over the measured emission peak. The deconvolution has displayed four major transitions between energy level stark components as represented in the figure peaking at 1910, 1954, 2008 and 2070 nm. Upon critical observation of emission peak, it can be seen that the relative intensity of 2070 component is higher than that of 2008 component in the glass indicating there is slight energy re-absorption at the considered dopant ion concentration.
The stimulated emission cross section σ e can be calculated by using the Fuchbauer–Ladenbury expression [28, 29]:
where λ p is the fluorescence peak wavelength, Δλ eff is the effective band width, n is refractive index (1.73), c is velocity of light and A rad is the radiative spontaneous emission probability (165 s−1). The emission cross section of Ho3+ is calculated to be 9.02×10−21 cm2 at the wavelength of 1.994 μm. The value is larger than that of fluoride glass (5.3×10−21 cm2) [30] but smaller than that of tellurite glass (10.0×10−21 cm2) [31]. This may properly be due to the refractive index of the host glass, the refractive index of the germanate (1.73) is larger than that of the fluoride glass (∼1.4) [32] while lower than that of tellurite glass (∼2) [31]. It is worthwhile to notice that the value of effective band width is found to be 170 nm, which is highly useful for laser tuning.
Figure 6(a) shows the emission spectra (σ e ) of Tm3+:3F4→3H6 and absorption spectra (σ a ) of Ho3+:5I8→5I6, it can be seen that the overlap between the σ e and σ a is quite large, so efficient energy transfer can be expected from Tm3+ to Ho3+. According to Dexter’s model, the extent of energy transfer depends on the spectral overlap of donor’s emission and acceptor’s absorption [33]. In the case of resonant energy transfer between Tm3+ and Ho3+, the model predicts a linear relationship between the transfer probability (P ET) and the spectral overlap between donor emission and acceptor absorption bands:
where f A (E) and f D (E) are the line-shape functions of the acceptor absorption and donor emission cross sections, respectively. The energy gap between Tm3+ and Ho3+ is about 712 cm−1, as the phonon energy of the germanate glasses is 846 cm−1, about one or less phonon is required to bridge the energy gap. Previous studies have shown that the direct transfer is found to be a quasiresonant process with nonphonon 79.5 %, having a participation of one phonon 20.5 % [12, 34]. The efficient energy transfer requires the participation of one phonon [13], the Dexter model can be generalized to the nonresonant phonon assisted energy transfer case by taking into account the energy of the phonon involved (E PH) as well as the phonon density [35]. Thus, the transfer probability can be estimated from the phonon modified spectral overlap integral I(E PH). If we assume that only one phonon is involved,

where k B and T are Boltzmann constant and absolute temperature, respectively. By using the cross section of Fig. 6(a) we have calculated the I(E PH) function at room temperature for phonon energy up to 1000 cm−1, as shown in Fig. 6(b). It can be seen that the phonon assisted overlap integral increases with an increase in phonon energy and it reaches a maximum for phonons with energy of about 400 cm−1. For further increase in the phonon energy, the energy transfer probability decreases and diminishes. Though the lower phonon energy matrices, such as tellurite and fluoride glass is suitable to promote the energy transfer from Tm3+ to Ho3+ with one phonon, but it also possess higher nonradiative relaxation of 5I7 level of Ho3+ leading to less probability of 2 μm emission.
4 Conclusions
A detailed spectroscopic analysis of Ho3+ ions in barium-gallium-germanate glass has been investigated. The 2 μm emission characteristics of Ho3+ both by direct excitation and through Tm3+ sensitization in barium gallium germanate glass have been reported. The emission intensity of Ho3+:5I7→5I8 is much stronger when excited by 808 nm through the energy level Tm3+:3H4 than that of the direct excitation of Ho3+. The Judd–Ofelt theory and Fuchbauer–Ladenbury formula have been used to calculate the intensity parameters and emission cross sections. The Judd–Ofelt analysis indicates that germanate glass has a higher covalency and a higher force constant of Ho3+-oxygen bonds than those of sulfide and fluoride glasses. The emission cross section of Ho3+ is calculated to be 9.02×10−21 cm2 at the wavelength of 1.994 μm. Dexter’s model and multiphonon relaxation theory have been used to analyze the energy transfer process between Tm3+ and Ho3+ ions on the energy gap ΔE. A comparative assessment of parameters of the emission cross section and energy transfer process suggests that the germanate glass could be identified as a promising optical material at 2 μm.
References
S.D. Jackson, A. Lauto, Lasers Surg. Med. 30, 184 (2002)
A. Serra, M. Pineiro, N. Pereira, A.R. Gonsalves, M. Laranjo, M. Abrantes, F. Botelho, Oncol. Rev. 2, 235 (2008)
Y. Guo, M. Li, Y. Tian, R. Xu, L. Hu, J. Zhang, J. Appl. Phys. 110, 013512 (2011)
B. Richards, A. Jha, Y. Tsang, D. Binks, J. Lousteau, F. Fusari, A. Lagatsky, C. Brown, W. Sibbett, Laser Phys. Lett. 7, 177 (2010)
G.K. Liu, B. Jacquier, Spectroscopic Properties of Rare Earths in Optical Materials (Springer, Beijing, 2005)
K. Tanimura, M.D. Shinn, W.A. Sibley, M.G. Drexhage, R.N. Brown, Phys. Rev. B 30, 2429 (1984)
B. Zhou, E.Y.-B. Pun, H. Lin, D. Yang, L. Huang, J. Appl. Phys. 106, 103105 (2009)
A. Chamorovskiy, A.V. Marakulin, S. Ranta, M. Tavast, J. Rautiainen, T. Leinonen, A.S. Kurkov, O.G. Okhotnikov, Opt. Lett. 37, 1448 (2012)
A.Y. Chamorovskiy, A.V. Marakulin, T. Leinonen, A.S. Kurkov, O.G. Okhotnikov, Quantum Electron. 42, 12 (2012)
A.S. Kurkov, V.V. Dvoyrin, A.V. Marakulin, Opt. Lett. 35, 490 (2010)
R. Xu, J. Pan, L. Hu, J. Zhang, J. Appl. Phys. 108, 043522 (2010)
R. Xu, M. Wang, Y. Tian, L. Hu, J. Zhang, J. Appl. Phys. 109, 053503 (2011)
L.V.G. Tarelho, L. Gomes, I.M. Ranieri, Phys. Rev. B 56, 14344 (1997)
S.S. Bayya, G.D. Chin, J.S. Sanghera, I.D. Aggarwal, Opt. Express 14, 11687 (2006)
J.R. Lincoln, C.J. Mackechnie, J. Wang, W.S. Brocklesby, R.S. Deol, A. Pearson, D.C. Hanna, D.N. Payne, Electron. Lett. 28, 1021 (1992)
F.L. Galeener, G. Lucovsky, Phys. Rev. Lett. 37, 1474 (1976)
P.F. Moulton, G.A. Rines, E.V. Slobodtchikov, K.F. Wall, G. Frith, B. Samson, A.L.G. Carter, IEEE J. Sel. Top. Quantum Electron., 15, 85 (2009)
A. Dietzel, Glastech. Ber. 22, 41 (1968)
N.-H. Chan, R.K. Sharma, D.M. Smyth, J. Am. Ceram. Soc. 65, 167 (1982)
W.T. Carnall, P.R. Fields, B.G. Wybourne, J. Chem. Phys. 42, 3797 (1965)
B.R. Judd, Phys. Rev. 127, 750 (1962)
G.S. Ofelt, J. Chem. Phys. 37, 511 (1962)
M.J. Weber, B.H. Matsinger, V.L. Donlan, G.T. Surratt, J. Chem. Phys. 57, 562 (1972)
C.K. Jorgensen, R. Reisfeld, J. Less-Common Met. 93, 107 (1983)
T. Schweizer, B.N. Samson, J.R. Hector, W.S. Brocklesby, D.W. Hewak, D.N. Payne, J. Opt. Soc. Am. B 16, 308 (1999)
T. Schweizer, B.N. Samson, J.R. Hector, W.S. Brocklesby, D.W. Hewak, D.N. Payne, Infrared Phys. Technol. 40, 329 (1999)
L. Wetenkamp, G.F. West, H. Többen, J. Non-Cryst. Solids 140, 35 (1992)
D.C. Yeh, R.R. Petrin, W.A. Sibley, V. Madigou, J.L. Adam, M.J. Suscavage, Phys. Rev. B 39, 80 (1989)
M.J. Weber, D.C. Ziegler, C.A. Angell, J. Appl. Phys. 53, 4344 (1982)
M. Peng, J. Qiu, D. Chen, X. Meng, I. Yang, X. Jiang, C. Zhu, Opt. Lett. 29, 1998 (2004)
K. Li, G. Wang, J. Zhang, L. Hu, Solid State Commun. 150, 1915 (2010)
N. Rigout, J.L. Adam, J. Lucas, J. Non-Cryst. Solids 184, 319 (1995)
D.L. Dexter, J. Chem. Phys. 21, 836 (1953)
Q. Zhang, J. Ding, Y. Shen, G. Zhang, G. Lin, J. Qiu, D. Chen, J. Opt. Soc. Am. B 27, 975 (2010)
R.L. Orbach, Optical Properties of Ions in Solids: Relaxation and Energy Transfer (Plenum, New York, 1975)
Acknowledgement
This work is financially supported by National Natural Science Foundation of China (No. 51172252).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, R., Tian, Y., Hu, L. et al. Efficient ∼2 μm emission and energy transfer mechanism of Ho3+ doped barium gallium germanate glass sensitized by Tm3+ ions. Appl. Phys. B 108, 597–602 (2012). https://doi.org/10.1007/s00340-012-5113-7
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-012-5113-7