Abstract
This work reports on a compact sensor for fast and reagent-free point-of-care determination of glucose, lactate and triglycerides in blood serum based on a tunable (1030–1230 cm−1) external-cavity quantum cascade laser (EC-QCL). For simple and robust operation a single beam set-up was designed and only thermoelectric cooling was used for the employed laser and detector. Full computer control of analysis including liquid handling and data analysis facilitated routine measurements. A high optical pathlength (>100 μm) is a prerequisite for robust measurements in clinical practice. Hence, the optimum optical pathlength for transmission measurements in aqueous solution was considered in theory and experiment. The experimentally determined maximum signal-to-noise ratio (SNR) was around 140 μm for the QCL blood sensor and around 50 μm for a standard FT-IR spectrometer employing a liquid nitrogen cooled mercury cadmium telluride (MCT) detector. A single absorption spectrum was used to calculate the analyte concentrations simultaneously by using a partial-least-squares (PLS) regression analysis. Glucose was determined in blood serum with a prediction error (RMSEP) of 6.9 mg/dl and triglycerides with an error of cross-validation (RMSECV) of 17.5 mg/dl in a set of 42 different patients. In spiked serum samples the lactate concentration could be determined with an RMSECV of 8.9 mg/dl.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Mid-infrared spectroscopy offers direct access to the structure of molecules by measuring their fundamental vibrational states. The method is therefore highly selective as interferences can be minimized by performing proper multivariate data analysis [1–4]. Over decades mid-IR spectroscopy was dominated by Fourier transform infrared (FT-IR) spectrometry using a thermal emitter as source for infrared radiation. Recently small and portable FT-IR spectrometers have been developed for field applications; practical difficulties due to the employed thermal light sources, however, remained. For measuring analytes in strongly absorbing solvents, like the most abundant solvent water, the achievable maximum pathlength for transmission measurements is typically restricted to <80 μm in the carbohydrate region (950–1200 cm−1) and <10 μm in the region of the amide I band of proteins (1600–1700 cm−1) [5]. Such low pathlengths can easily lead to clogging of the measurement cell, therefore substantially limiting the robustness of the method.
With the rise of quantum cascade lasers in the last decade, the applicability of mid-IR spectroscopy in field applications calling for high sensitivity and selectivity has substantially improved. Different types of QCLs with high spectral power density, including single-mode distributed feedback (DFB) and broadly tunable EC-QCLs have been applied to a series of analytical problems in the gaseous and liquid phase [6–10]. The capability of a tunable EC-QCL for multianalyte detection of physiologically relevant parameters in the aqueous phase was shown in [11, 12]. There, glucose and lactate were measured under the presence of interferents with similar spectral appearance, such as xylose and maltose. The latter in particular can result in false diagnosis in standard detection methods, potentially causing serious consequences [13–15].
In this paper we demonstrate the feasibility of a simple single channel-based (i.e. no balanced detection) transmission set-up with a tunable QCL to be used in routine laboratory analysis of human blood serum. Several in-depth studies based on FT-IR spectroscopy have been performed showing the potential of the mid-IR spectral range to be suitable for multianalyte detection in biofluids, such as blood or urine [3, 16–18]. However, various drawbacks have hindered this method to be implemented as a routine analysis in clinical laboratory and point of care analysis respectively. These drawbacks comprise the rather bulky set-up due to the interferometric approach, the sensitivity to mechanical vibrations and—probably the most important characteristic of FT-IR spectrometers—the low power broadband IR emitters which limit the achievable optical pathlength in transmission measurements. Compared to earlier studies using FT-IR spectrometers, the use of QCLs enables increased optical pathlengths of >100 μm. This clears the way to practical application for point-of-care and routine analysis as the robustness is significantly improved by the high optical pathlength.
The analytes under investigation were glucose, lactate and triglycerides. All three substances are frequently determined in medicine. The blood glucose concentration, e.g., is routinely measured using enzymatic assays and can indicate metabolic disorders such as diabetes mellitus. Diabetes mellitus is a widespread metabolic disease with elevated blood glucose levels. According to the World Health Organization (WHO) 346 million people worldwide suffer from diabetes [19]. It fosters vascular diseases like cardiovascular disease (heart disease and stroke) or other circulatory disorders that may affect the legs, eyes or kidneys [20]. Elevated levels of triglycerides may also cause vascular disease which is associated with a higher risk for heart disease and stroke [21]. The triglyceride level in blood can hence be used to assess the risk of coronary heart diseases. The standard laboratory test for triglycerides involves hydrolysis of the triglycerides and photometric determination of the resulting free glycerol. Lactate is a crucial physiological parameter mainly used for assessment of the metabolism in critically ill patients as well as in endurance sports medicine. It assists the diagnosis of hypoxia and pathological metabolic conditions in general. Furthermore, it was shown in [22] that the lactate concentration can be used to predict the risk of mortality of critically ill patients. In clinical chemistry lactate is usually measured by enzymatic or chemical oxidation. The normal concentration ranges of the analytes are summarized in Table 1.
The focus of the paper will be on the optimum optical pathlength in terms of maximum SNR and how the feasible pathlength range can be extended by the use of a pulsed EC-QCL. With the knowledge of the optimum pathlength range the application of the EC-QCL sensor for the determination of glucose, lactate and triglycerides will then be presented.
2 Experimental
2.1 Automated instrumental setup
The experimental set-up (Fig. 1) contained a broadly tunable pulsed EC-QCL (tunable between 1030 and 1230 cm−1, Daylight Solutions Inc., USA) as the core element in the spectroscopic unit as well as devices for automated liquid handling and signal processing. A detailed characterization of the employed EC-QCL’s properties was given in [11, 12]. A spectral resolution better than 2 cm−1 was achieved and the data acquisition time for a single sample was approximately 40 s. The optical path was realized as a single channel transmission configuration with a stainless steel flow cell with CaF2 windows (2 mm, Korth Kristalle GmbH, Germany) and PTFE spacers (135 μm optical pathlength). An off-axis parabolic mirror (63 mm focal length) was used to focus the IR beam on a thermoelectrically cooled MCT detector (detectivity D ∗=4×109 cm Hz0.5 W−1 at 9.2 μm, Infrared Associates Inc., USA).
For automated liquid handling a flow injection system, consisting of a 14-way selection valve (VICI Valco Instruments, Switzerland), a 2.5 ml syringe pump (Cavro XP3000, Tecan Group Ltd., Switzerland) and PTFE tubings in various dimensions was employed. The whole set-up was controlled by a LabVIEW-based GUI. For background, single beam spectra were measured of Ringer solution (for injection purposes, Fresenius Kabi, Austria). The flow cell was cleaned by rinsing it with small amounts (∼100 μl) of ethanol. With the intended application of the sensor in a point-of-care environment in mind, the set-up was designed to reduce the required user interaction to a minimum. Attachment of the blood serum samples to the ports of the selection valve and execution of the measurement routine allowed for automated analysis of up to 8 blood serum samples. Experiments concerning the optimum optical pathlength in a FT-IR set-up were performed on a Vertex 80v spectrometer (Bruker Optics, Germany) at maximum aperture (8 mm) and a spectral resolution of 2 cm−1. The acquisition time was approximately 40 s and therefore similar to the EC-QCL set-up. Noise levels were calculated as the RMS noise of the 100 %-line in the spectral region 1050–1110 cm−1. The same flow cell as for the EC-QCL sensor was used. Different optical pathlengths were achieved by using appropriate PTFE spacers.
2.2 Sample preparation
Human blood serum samples were obtained from the blood donor centre of the Austrian Red Cross in Vienna. Aliquotes of 10 mL whole blood were taken from the diagnostic samples of 42 different blood donors after completion of the routine diagnostics which follows blood donation. Sample preparation was limited to centrifugation of the blood samples for 15 minutes at 2129 g and separation of the supernatant serum within four hours after the collection of the blood to minimize degradation of glucose and lactate by glycolysis. Long-time analyte stability was maintained by deep-freezing the samples at −30 °C within six hours after the separation of the serum. Due to the direct and molecular specific nature of the mid-IR absorption measurement no reagents had to be added and no enrichment or separation step of the analyte prior to measurement was necessary. Spiked serum samples used for lactate determination were prepared by weighing in the desired amounts of sodium-L-lactate puriss. (Sigma-Aldrich, Germany).
2.3 Chemometrics and reference analysis
Given the fact that the blood samples under investigation contained multiple analytes, absorption spectra were evaluated by multivariate data analysis. The reference concentrations for establishing a calibration model can either be provided by spiking serum samples with different concentrations of the target analyte or by using standard clinical reference analysis. The latter has to be preferred as it also covers the natural variations of the blood matrix, however, this requires reliable reference analysis methods for each blood component to be detected. For glucose and triglycerides this was the case as these parameters were routinely determined by the reference laboratory of the blood donor centre. Lactate was not part of the standard test protocol and therefore not available during the study. The accuracy of the employed reference methods (Table 1) according to the Clinical and Laboratory Standards Institute (CLSI) recommendations could be expected to have a run precision of less than 5 % coefficient of variation (CV) and a total precision of less than 5 % CV.
Multivariate data analysis of the absorption spectra was performed using the partial-least-squares (PLS) regression module of the PLS Toolbox (Eigenvector Research Inc., USA) for MATLAB (The MathWorks Inc., USA). Data pre-processing of the measured raw data comprised Fourier-based filtering, mean centring and calculation of Savitzky–Golay first or second derivative. In all cases calibration data have been checked for homoscedasticity and residues for normal distribution. Validation of the calibration models was maintained either by cross-validation using random subsets or external validation by applying the calibration model on a different sample set.
3 Optimum optical pathlength for the EC-QCL blood sensor
3.1 Theory
The envisaged application of a tunable QCL for multianalyte detection in a transmission configuration is in direct competition with the gold standard Fourier-Transform Infrared (FT-IR) spectroscopy. Despite the advantages FT-IR spectrometers bring into the play, such as the multiplex or Fellget advantage and the high spectral precision, they face a lack of applicability in the field of transmission measurements of strong absorbers, such as water at reasonable optical pathlengths (>50 μm). The several magnitudes higher spectral power density of commercially available QCLs creates a new situation, raising the question of the optimum operation range in terms of optical pathlength.
It is well known that for a given solvent there is an optimum optical pathlength L opt for each wavelength which is defined by the solvent’s molar absorption coefficient, where the SNR reaches its maximum value in a transmission spectroscopic set-up [23–25]. Snoer-Jensen et al. [26] showed that this is the case at the pathlength L where the absorbance A Solvent reaches the value of 0.434 AU, according to Eq. (1).
This number is mainly determined by the fact that the background and sample single beam intensity are assumed to stay constant (I≈I 0) if the solvent is a strong absorber and that the analyte absorption can be neglected. It should be noted that the absolute levels of the background (I 0) and sample single beam intensity (I) do not enter this equation. According to this, the optimum optical pathlength for measuring glucose in water can be calculated to be slightly below 20 μm, assuming a molar absorption coefficient α of 4.449 L mol−1 cm−1 at 1082 cm−1 [27]. In this theoretical model the intensity I does not influence the absolute value of the optimum pathlength, however it accounts for the decrease of the SNR when the optimum pathlength range is exceeded (Eq. (5) in [26]).
Equation (1) is based on the assumption that the measurement system is detector-noise limited, which is the case when using an FT-IR spectrometer as here the noise of the infrared light source is typically assumed to be negligible. However, it is a reasonable source of noise in the QCL set-up. The actual behaviour of the SNR of the QCL set-up will therefore possibly deviate from this standard theory.
The spectral power density in the sample compartment of an FT-IR spectrometer (typically in the range of μW/cm−1) is much lower than the power density provided by QCLs (up to hundreds of mW/cm−1). In the present work a maximum power density of 350 mW/cm−1 was available in pulsed mode. Considering this and the fact that a MCT detector only has a limited dynamic range, each system will have an optimum operation range with maximum SNR. It is obvious that the QCL will saturate the detector if the pathlength is set too low. At the same pathlength, however, an FT-IR spectrometer with a thermal light source will deliver reasonable results. Consequently the optimum pathlength will most certainly depend on the source strength of the system under investigation as well.
3.2 Experimental verification
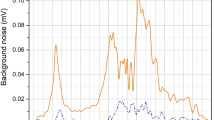
Based on the high spectral power density of the EC-QCL blood sensor we expected an optimum pathlength significantely greater than in an FT-IR set-up. The experimental proof for the theoretical considerations is given by the results of absorption measurements performed on water samples containing 100 mg/dl glucose. This configuration can be seen as a model system for physiological samples where water typically is the predominant absorber. The QCL sensor was compared to a state-of-the-art laboratory FT-IR spectrometer (Bruker Vertex 80v). Figure 2 shows the results. The measured absorbance at the 1082 cm−1 absorption line of glucose served as signal S and the noise N was calculated as the root-mean-square (RMS) noise of the spectrum between 1050 cm−1 and 1110 cm−1 (Fig. 2a). With the desired application to real-world samples (e.g. blood serum) in mind both systems were operated at maximum optical power to achieve the highest possible pathlength which minimizes the probability of cell clogging.
(a) Signal and noise levels of the FT-IR and EC-QCL set-up at different optical pathlengths. The measurements were performed at maximum aperture (8 mm). The measurement time was approximately 40 seconds (128 scans) at a resolution of 2 cm−1 for both set-ups. (b) SNR levels at 1082 cm−1 for the FT-IR set-up using a thermal emitter (left) and the high power EC-QCL (right)
A comparison of the measured SNR curves in Fig. 2b mainly shows two things: As expected, the EC-QCL configuration does not follow the simple theory for detector-noise limited systems. The actual maximum SNR is located around 140 μm and therefore approximately three times higher than for the FT-IR spectrometer. Furthermore, the actual optimum pathlength of the FT-IR spectrometer is slightly higher than it would be expected from Eq. (1). The deviation from theory can be explained by the fact that in the real experimental set-up the noise level, which is the dominating variable in the SNR, is governed by the detector noise, along with other noise sources in the system and the optical power density reaching the detector element. Two extrema can be observed in this regard: In the high pathlength range the noise will rise substantially until all light is absorbed by the liquid. In the low pathlength range the detector will at some point be saturated making meaningful measurements impossible.
The absolute value of the SNR was similar in both set-ups, despite the fact that in the FT-IR spectrometer a liquid nitrogen cooled detector was used, which had a factor 10 higher detectivity than the TE-cooled MCT in the QCL sensor set-up. This advantage of the FT-IR spectrometer could partially be covered by the higher signal in the QCL set-up due to the increased pathlength.
Based on the experimental results it can be concluded that the absolute value of the optimum pathlength does not only depend on the molar absorption coefficients of the involved substances as suggested by [26], but also strongly on the characteristics of the infrared source and the detectivity D* of the detector. It has to be considered, however, that all values will of course vary with changing wavenumber. With regard to the intended application of the QCL set-up to blood serum it should be noted that water is still the predominant absorber there. The additional absorption when changing the matrix from water to blood serum (∼30 mAU) would be the same as if the pathlength was increased by 1.2 μm. The results obtained for the optimum pathlength when measuring glucose in water are therefore considered to be valid for blood serum as well.
4 Multianalyte detection in human blood serum
4.1 Glucose and triglycerides
The accessible spectral range was determined by the tuning range of the EC-QCL, which was ∼200 wavenumbers centred at 1130 cm−1 (8.85 μm)—the wavelength range of the typical absorption bands of carbohydrates. It has already been shown that QCLs with a fixed emission wavelength can be applied for measurements in blood serum which was spiked with a single analyte [28]. However, blood serum represents an elaborate multi-component solution. Therefore, data analysis based on single, analyte specific absorption bands only will not be sufficient when a sample set with blood from different patients is under investigation. This is illustrated in Fig. 3, where samples from four patients with similar blood glucose concentrations are compared, however, no correlation between the typical glucose absorption band at 1082 cm−1 and the actual glucose concentration can be observed there.
Absorption spectra like the examples shown in Fig. 3 were measured from the blood serum of 45 different persons (regular blood donors). Three samples have been identified as outliers and were therefore not considered in the calibration model. Based on the measurement of a single serum sample of each donor, calibration models for both glucose and triglycerides could be established. The results of the internal validation (cross-validation) are shown in Fig. 4 and Fig. 5, where the results of the EC-QCL sensor are plotted against the concentrations provided by the reference analysis. For glucose, an external validation consisting of 11 serum samples was carried out as well. This led to an even better error-level than it was the case for the internal validation (RMSEP Glucose=6.9 mg/dl, Fig. 4).
Linearity of the EC-QCL sensor was given over the whole sample concentration ranges, including the physiological reference ranges as well as ranges already considered pathological. The validation and prediction errors that could be achieved for glucose and triglycerides were all within the accepted variation as specified by [29]. Since average blood donors can be assumed to be healthy persons the glucose levels in the available sample set were basically located close to the normal range.
4.2 Glucose and lactate in spiked blood serum
For lactate determination blood samples were spiked with randomly distributed amounts of lactate (c Lactate=9.8–100 mg/dl). In order to maintain realistic conditions, glucose was added in random concentrations as well, acting as an interferent. Additional information compared to the measurements presented in the previous section was expected by the extended concentration range, also covering pathological glucose levels (c Glucose=45–370 mg/dl). Absorption spectra of 18 samples were recorded. The respective multivariate calibration data are shown in Fig. 6. The internal validation for glucose showed error values well below 10 mg/dl (RMSECV Glucose=9.2 mg/dl, Fig. 6, bottom). The extension of the concentration range was obviously not affecting the sensor performance to any significant degree. Lactate could be measured with an accuracy similar to glucose (RMSECV Lactate=8.9 mg/dl, Fig. 6, top), proving the capability of the EC-QCL sensor to simultaneously detect both analytes in a blood serum matrix.
During the course of the measurements no clogging was observed in the transmission flow cell. This was attributed to the high optical pathlength of 135 μm.
5 Conclusion
It could be shown that the EC-QCL sensor set-up makes the simultaneous detection of multiple analytes in a blood serum matrix feasible. Given the importance of a reasonably high optical pathlength for clinical application (>100 μm), an experimental investigation on the optimum pathlength in terms of SNR was conducted. Based on the results of the comparison between the EC-QCL sensor and an FT-IR spectrometer it could be concluded that the absolute position of the optimum pathlength is influenced by the power of the infrared source. Furthermore, it could be shown that the optimum pathlength does not necessarily coincide with the theoretically suggested value, since current theory only accounts for the molar absorptivities of solvent and analyte and not for the available spectral power density.
By using a 135 μm pathlength transmission flow cell glucose and triglycerides were then determined in a set of blood serum samples of 42 different healthy donors. Furthermore, blood serum was spiked with different concentrations of glucose and lactate and subsequently analyzed with the EC-QCL blood sensor. Again, a successful determination of glucose levels, as well as lactate levels could be shown. In all cases multivariate data analysis was performed using a PLS regression algorithm. The achieved validation and prediction errors for glucose were well below the demanded value of 10 mg/dl. Triglycerides could be measured with an accuracy comparable to standard test methods. Altogether, the obtained RMSEP and RMSECV values are considered highly satisfactorily considering the changes in the concentration of other matrix components which do absorb in the same spectral range, as well as considering the uncertainty associated with the reference values.
Given the fact that the approach was reagent-free and that further miniaturization of the sensor set-up is possible, a point-of-care application seems within reach. Furthermore, the small sample volume (200 μl) and short measurement time (∼40 s) allow smaller measurement intervals enabling a tighter monitoring of the progression of a disease [30].
References
H.M. Heise, R. Marbach, G. Janatsch, Anal. Chem., 2009 (1989)
P. Bhandare, Y. Mendelson, E. Stohr, R.A. Peura, Vib. Spectrosc. 6, 363 (1994)
G. Hoşafçi, O. Klein, G. Oremek, W. Mäntele, Anal. Bioanal. Chem. 387, 1815 (2007)
R. Vonach, J. Buschmann, R. Falkowski, R. Schindler, B. Lendl, R. Kellner, Appl. Spectrosc. 52, 820 (1998)
A. Barth, Biochim. Biophys. Acta 1767, 1073 (2007)
R.F. Curl, F. Capasso, C. Gmachl, A.A. Kosterev, B. McManus, R. Lewicki, M. Pusharsky, G. Wysocki, F.K. Tittel, Chem. Phys. Lett. 487, 1 (2010)
A. Kosterev, G. Wysocki, Y. Bakhirkin, S. So, R. Lewicki, M. Fraser, F. Tittel, R.F. Curl, Appl. Phys. B, Lasers Opt. 90, 165 (2007)
G. Wysocki, R.F. Curl, F.K. Tittel, R. Maulini, J.M. Bulliard, J. Faist, Appl. Phys. B, Lasers Opt. 81, 769 (2005)
S. Schaden, A. Domínguez-Vidal, B. Lendl, Appl. Phys. B, Lasers Opt. 83, 135 (2006)
J. Kuligowski, G. Quintás, B. Lendl, Appl. Phys. B, Lasers Opt. 99, 833 (2007)
M. Brandstetter, A. Genner, K. Anic, B. Lendl, Analyst 135, 3260 (2010)
M. Brandstetter, B. Lendl, Sensor Actuat. B, Chem. (2011). doi:10.1016/j.snb.2011.06.081
S. Mehmet, G. Quan, S. Thomas, D. Goldsmith, Diabet. Med. 18, 679 (2001)
T.G. Schleis, Pharmacotherapy 27, 1313 (2007)
K.M.J. Flore, J.R. Delanghe, Perit. Dial. Int. 29, 377 (2009)
G. Deleris, Vib. Spectrosc. 32, 129 (2003)
C. Petibois, A.M. Melin, A. Perromat, G. Cazorla, G. Déléris, J. Lab. Clin. Med. 135, 210 (2000)
W. Petrich, Appl. Spectrosc. Rev. 36, 181 (2001)
C. de Bode, Diabetes programme, World Health Organization, http://www.who.int/diabetes/en/
N. Sarwar, P. Gao, S.R.K. Seshasai, R. Gobin, S. Kaptoge, E. Di Angelantonio, E. Ingelsson, D.A. Lawlor, E. Selvin, M. Stampfer, C.D.A. Stehouwer, S. Lewington, L. Pennells, A. Thompson, N. Sattar, I.R. White, K.K. Ray, J. Danesh, Lancet 375, 2215 (2010)
J.D. Brunzell, N. Engl. J. Med. 357, 1009 (2007)
T.J. Iberti, A.B. Leibowitz, P.J. Papadakos, E.P. Fischer, Crit. Care Med. 18, 275 (1990)
R. Cole, J. Opt. Soc. Am. 41, 38 (1951)
G.W. Ewing, Instrumental Methods of Chemical Analysis (McGraw-Hill Book Company, New York, 1985)
H.L. Mark, P.R. Griffiths, Appl. Spectrosc. 56, 633 (2002)
P.S. Jensen, J. Bak, Appl. Spectrosc. 56, 1600 (2002)
J. Bertie, Z. Lan, Appl. Spectrosc. 50, 1047 (1996)
W.B. Martin, S. Mirov, R. Venugopalan, Appl. Spectrosc. 59, 881 (2005)
German Medical Association, Guidel. Qual. Assur. Med. Lab. Exam. 105, 341 (2008)
S.J. Finney, C. Zekveld, A. Elia, T.W. Evans, J. Am. Med. Assoc. 290, 2041 (2003)
Acknowledgement
The authors gratefully acknowledge the funding within the RSA (Research Studios Austria) programme of the FFG (Austrian Research Promotion Agency).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brandstetter, M., Volgger, L., Genner, A. et al. Direct determination of glucose, lactate and triglycerides in blood serum by a tunable quantum cascade laser-based mid-IR sensor. Appl. Phys. B 110, 233–239 (2013). https://doi.org/10.1007/s00340-012-5080-z
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-012-5080-z