Abstract
A sensor for sensitive in situ measurements of carbon monoxide and temperature in combustion gases has been developed using absorption transitions in the (v′=1←v″=0) and (v′=2←v″=1) fundamental bands of CO. Recent availability of mid-infrared quantum-cascade (QC) lasers provides convenient access to the CO fundamental band near 4.7 μm, having approximately 104 and 102 times stronger absorption line-strengths compared to the overtone bands near 1.55 μm and 2.3 μm used previously to sense CO in combustion gases. Spectroscopic parameters of the selected transitions were determined via laboratory measurements in a shock tube over the 1100–2000 K range and also at room temperature. A single-laser absorption sensor was developed for accurate CO measurements in shock-heated gases by scanning the line pair v″=0, R(12) and v″=1, R(21) at 2.5 kHz. To capture the rapidly varying CO time-histories in chemical reactions, two different QC lasers were then used to probe the line-center absorbance of transitions v″=0, P(20) and v″=1, R(21) with a bandwidth of 1 MHz using fixed-wavelength direct absorption. The sensor was applied in successful shock tube measurements of temperature and CO time-histories during the pyrolysis and oxidation of methyl formate, illustrating the capability of this sensor for chemical kinetic studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Laser absorption spectroscopy techniques play a large and growing role in the measurement of flow-field parameters such as temperature, gas composition, velocity, and pressure [1–4]. These sensors are highly attractive for combustion and propulsion applications due to their nonintrusive nature, fast time response, and in situ measurement capability. Carbon monoxide (CO) is a particularly significant target for hydrocarbon-fueled systems, since it is a toxic pollutant from combustion devices and a primary product of incomplete combustion, and its concentration can be interpreted to indicate combustion efficiency.
The absorption spectra of CO, H2O, and CO2 in the near- to mid-infrared region at 1500 K are illustrated in Fig. 1, where the absorption line-strengths (from the HITRAN 2004 database [5]) are plotted as a function of wavelength from 1–6 μm. The fundamental band of CO holds the most promising candidate transitions owing to their much stronger line-strengths and relatively weaker interference from other combustion species. Work has been reported using transitions in three different vibrational bands of CO: the second overtone band (Δv=3) near 1.55 μm [6–8], the first overtone band (Δv=2) near 2.3 μm [9–12], and the fundamental band (Δv=1) near 4.6 μm [13–19]. The absorption strength of the fundamental band is approximately 104 and 102 times stronger compared to the overtone bands near 1.55 μm and 2.3 μm, respectively, making it promising for sensitive detection with relatively low CO concentration and/or short path length.
Absorption line-strengths of CO, H2O, and CO2 at 1500 K (from HITRAN 2004 database [5])
Early studies of CO detection in the fundamental band were carried out by Hanson et al. [13, 14] using lead-salt tunable diode laser (TDL) absorption. However, this sensor has limited practical applications, as the laser source is multimode and requires cryogenic cooling. Barron-Jimenez et al. [17] demonstrated a CO sensor in the 4.4–4.8 μm spectral region using difference-frequency mixing of radiation from a near-IR external-cavity diode laser (ECDL) and a compact Nd:YAG laser in a periodically poled lithium niobate (PPLN) crystal. CO concentration measurements were performed in a room-temperature gas cell, a well-stirred reactor, and a near-adiabatic hydrogen/air CO2-doped flame by probing the fundamental band CO transitions R(23), R(11), and P(19). Although these demonstrations of the diode-laser-based CO sensor were successful, the weak mid-IR power (<1 μW) significantly limited the sensitivity and signal-to-noise ratio of this sensor. Developments in quantum-cascade (QC) laser technology, resulting in room-temperature, relatively high power (mW), narrow line-width, and single-mode QC lasers, have led to broad applications of these sources in high-resolution spectroscopy and high-sensitivity detection of trace gases [20–22]. Kosterev et al. [18] reported the application of a pulsed, distributed-feedback quantum-cascade laser (DFB-QCL) for CO sensing in ambient air by probing the R(3) transition at 2158.30 cm−1. A noise-equivalent detection limit of 12 parts per billion was demonstrated experimentally with a 102 cm path length at a 10 kHz pulsed-laser repetition rate. Compared to lasers employing pulsed operation, the continuous-wave (cw) lasers generally offer relatively simpler operation and narrower laser line-widths. In work related to the current paper, a cw QC laser was used recently to detect CO absorption in the fundamental band and measure temperature in shock-heated nonreactive gases between 950 and 3500 K near 1 atm by Vanderover and Oehlschlaeger [19]. Temperature measurements were made via a two-line ratio technique using the line pairs R(9) and R(17), and R(10) and R(18), by tuning the laser wavelength across the absorption features at 1–2 kHz. This laser scan rate limits the time response of the sensor for time-resolved measurements in many combustion applications and in shock tube experiments.
In this paper, we discuss the extension of cw DFB-QCL-based mid-IR absorption of CO for in situ detection in combustion gases and specifically in shock tube flows. Sensors for temperature and CO concentration measurements using scanned-wavelength direct absorption (DA) with a single room-temperature QC laser and using fixed-wavelength DA with dual QC lasers are both developed to provide fast and flexible diagnostics for different applications. We first examine the fundamental band of CO (4.3–5.8 μm) to find candidate CO transitions suitable for high-temperature (>1000 K) in situ detection that are isolated from interference of other primary combustion products such as H2O and CO2. Two different cw QC lasers are used to access transitions R(12), R(21), R(13) and R(22) in the R-branch of the vibrational band near 4.6 μm, and P(20) and P(14) in the P-branch near 4.8 μm, respectively. For gas-temperature sensing based on the ratio of two-line absorption, the well-separated lower-state internal energies among these transitions provide high temperature sensitivity. Fundamental spectroscopic parameters are verified (line-strength, included in the HITRAN database [5]) and measured (Ar-broadening coefficient) for the selected lines using a shock tube. The accuracy of both the scanned- and fixed-wavelength CO temperature sensors is then validated in shock-heated nonreactive CO–H2–Ar mixtures. Measurements of temperature and CO concentration are subsequently carried out in the pyrolysis and oxidation of methyl formate to demonstrate the applications of this CO sensor in shock tube chemical kinetic studies. These quantitative validation measurements confirm the potential of mid-IR QC-laser-based sensing of CO concentration and temperature in combustion environments.
2 Fundamental spectroscopy
The fundamental theory of direct absorption spectroscopy is reproduced here only briefly to define our notation, since the theory is well understood and has been detailed in the literature [23]. When spectrally narrow radiation at frequency v passes through a uniform gas medium of length L [cm], the transmitted intensity I t is related to the incident intensity I 0 by the Beer–Lambert law:
where S [cm−2 atm−1] is the line-strength of the specific transition, P [atm] the total pressure, x i the mole fraction of the absorbing species i, and ϕ v [cm] the line-shape function. The dimensionless product α v =SPx i ϕ v L is defined as absorbance, with k v =SPx i ϕ v the absorption coefficient. Since the line-shape function ϕ v is normalized to have unit area across the line, the integrated absorbance can be expressed as
The Voigt line-shape function ϕ v combines both temperature and collisional broadening. The collision-broadened full-width at half maximum (FWHM) of the absorbing species i is
where x j is the mole fraction of the collisional partner j, and 2γ ji [cm−1 atm−1] is the broadening coefficient of j with i. From an experimental point of view, it is of practical interest to have a simple model of the variation of the FWHM with temperature, typified by the following commonly-used expression [24]:
where T 0 is the reference temperature (usually 296 K) and n is the temperature coefficient. The line-strength S [cm−2 atm−1] has a temperature dependence:

where Q(T) is the partition function, E″ [cm−1] is the lower-state energy, v 0 [cm−1] is the line-center frequency, and h,c,k are Planck’s constant, speed of light, and Boltzmann’s constant, respectively.
The absorption measurement of temperature is commonly based on a two-line technique [15]. Temperature is inferred from the ratio of the integrated absorbance under the absorption feature or the line-center absorbance of two molecular transitions of the same species.
3 Line selection
Absorption spectra of CO fundamental band between 4.3 and 5.8 μm were computed based on the HITRAN database [5] for typical shock tube combustion flows (1000–2000 K, 1 atm, 0.1 % CO/1 % H2O/1 % CO2) to find suitable CO transitions. A systematic line-selection procedure was used to find lines with sufficient absorption strength, isolation from interfering absorption, temperature sensitivity, and the availability of the commercial laser sources [25].
Two cw, room-temperature, DFB-QC lasers were subsequently acquired from Alpes Lasers SA to access the R-branch near 4.6 μm and the P-branch near 4.8 μm of the fundamental band of CO, respectively. For the laser frequency ranges of 2048.6 to 2061.3 cm−1 and 2185.8 to 2200.3 cm−1, three sets of closely spaced line pairs were selected for single-laser, scanned-wavelength temperature sensing: line pair A (v″=0, R(12) and v″=1, R(21) near 2190 cm−1), line pair B (v″=0, R(13) and v″=1, R(22) near 2194 cm−1), and line pair C (v″=0, P(20) and v″=1, P(14) near 2060 cm−1). Their spectroscopic parameters (for line pairs A, B, and C) from the HITRAN database [5] are summarized in Table 1. A spectral simulation of 0.1 % CO in air (T=1500 K, P=1 atm, L=10 cm) for these three line pairs is illustrated in Fig. 2, along with the interfering absorption of 1 % H2O and CO2. It should be noticed that the interference from H2O and CO2 is mostly negligible at these wavelengths.
These three line pairs, each with CO transitions from two different vibrational levels, have a spectral separation of 0.4–1.5 cm−1, within the typical 2 cm−1 rapid-tuning range of the commercial QC lasers. A representative analysis of the line-strength ratio and temperature sensitivity for the line pair A is plotted as solid lines in Fig. 3. Typically, the line-strength ratio should not be too far from unity. The sensitivity, defined here as the unit change in line-strength ratio for a unit change in temperature should at least be 1 for sensitive temperature measurements. These two curves suggest this line pair can be used for accurate temperature sensing at elevated temperatures, e.g., between 1000 and 3000 K.
Temperature sensitivities (left-hand axis) and line-strength ratios (right-hand axis) for two representative line pairs. Solid line: line pair A (v″=1, R(21) and v″=0, R(12)) for single-laser scanned-wavelength temperature sensing; dashed line: the v″=1, R(21) and v″=0, P(20) lines for dual-laser fixed-wavelength temperature sensing (selected from the six individual lines listed in Table 1)
Two-line thermometry, achieved by scanning two neighboring transitions with a single laser, enables a relatively simpler system with lower cost. However, the tuning rate of the QC lasers limited the sensor bandwidth to several kHz. High-temperature chemical kinetic studies in a shock tube, where chemical reactions happen within milliseconds, require a faster sensor, with 100 kHz bandwidth or greater. Thus, a dual-laser, fixed-wavelength method was pursued to provide highly time-resolved measurements. We selected the v″=0, P(20) and v″=1, R(21) lines from the six individual lines listed in Table 1 as the optimum line pair for temperature measurement using two different QC lasers. The corresponding line-strength ratio and temperature sensitivity for this line pair are shown as dashed lines in Fig. 3.
4 Spectroscopic measurement and verification
The fundamental spectroscopic parameters such as line-strength, self- and air-broadening coefficients of CO can be found in the HITRAN database [5]. However, argon instead of air is usually used as the bath gas in shock tube kinetic studies. Accordingly, there is need to investigate the Ar-broadening coefficient of each line and its temperature dependence. Moreover, the validation of CO line-strength at high temperature is essential for the accurate measurements as the measured absorbance is compared with the simulation to infer gas mole fraction and temperature.
All spectroscopic measurements were performed in a 15.2 cm diameter stainless-steel high-purity shock tube. The incident shock wave propagates through the tube, raising the temperature and pressure of the test gas from (T 1,P 1) to (T 2,P 2). When the shock wave reaches the end-wall of the tube, it is reflected and further elevates the temperature and pressure of the test gas to (T 5,P 5). The gas temperature and pressure immediately behind the shock wave can be calculated accurately using standard normal-shock relations and the measured incident shock speed, with an uncertainty of ∼1 % in temperature over the high-quality test time of 2 ms. Further details about the shock tube can be found in [26]. Research grade gases (argon, helium, and hydrogen >99.999 %; 0.5 % CO/Ar mixture with uncertainty <0.1 %) were supplied by Praxair Inc. Due to the significant time for CO to vibrationally relax behind the reflected shock wave, a small portion of H2 (1 %) is added to the 0.5 % CO/Ar mixture to accelerate the vibrational relaxation; see Fig. 4 for the evaluation. The test mixtures were manometrically prepared in a turbo-pumped stainless-steel mixing tank (14 L) with a magnetically driven stirrer.
Calculated vibrational relaxation time (P=1.5 atm) for CO–Ar, CO–He–Ar, and CO–H2–Ar mixtures (calculations from [27])
A schematic of the experimental setup is demonstrated in Fig. 5. The room-temperature operated QC laser (Alpes Lasers) used for these measurements was thermoelectrically-cooled and housed with collimation optics in a sealed laser housing (Alpes HHL-L module). In addition, a laboratory water-cooled heat sink was installed to the laser housing to achieve more stable laser performance. The laser wavelength is tuned by varying the injection current and base temperature, which are controlled by a combination of commercial temperature and injection current controllers (Alpes Lasers TCU 200 and ILX Lightwave LDX-3232). The laser wavelength is rapidly tuned (1–10 kHz scan rate) over the desired absorption feature with a linear ramp of current from a function generator. A ZnSe beam splitter was used to split the collimated laser beam (20–40 mW) into two arms to be received by a pair of matched TE-cooled IR photovoltaic detectors (Vigo Systems, 1 MHz bandwidth); one beam passes through the test gas of 15.2 cm path length in the shock tube, while the other propagates through a 7.6 cm long solid germanium etalon in the ambient air. The etalon with a free spectral range (FSR) of 0.016 cm−1 enables the conversion of scan time to relative wavelength. A narrow-bandpass IR filter (half power bandwidth 50 nm) was used to filter out emission and unwanted ambient light. Before each shock tube experiment, the laser wavelength was tuned to the desired transition by monitoring the absolute wavelength using a free-space mid-IR wavemeter (Bristol 621).
The laser wavelength was typically tuned over a range of ∼1 cm−1 at a frequency of 2.5 kHz, while the detector signal was sampled at 10 MHz to fully capture the absorption feature. The data acquisition system was triggered by the pressure transducer located at 2 cm from the shock tube end-wall to record pressure and transmission signal (I t ) of the laser during the shock heating. In the present experiments with large fractional absorption and no significant noise problems, only one single scan of I t behind the reflected shock was analyzed to infer spectroscopic parameters.
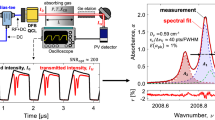
The raw data traces of a typical experiment for high-temperature line-strength and Ar-broadening measurements of CO are plotted in Fig. 6(a). The laser intensity and wavelength were scanned over the R(12) transition at 2190.02 cm−1 and recorded behind the reflected shock at 1450 K and 1.63 atm (vibrational equilibrium) with a mixture of 0.496 % CO/1 % H2/Ar. Prior to each experiment, the shock tube is evacuated by a turbomolecular pump and the baseline reference intensity (I 0) recorded. The spectral absorbance is then determined by the Beer–Lambert law and plotted as a function of wavenumber calibrated using the etalon trace, as demonstrated in Fig. 6(b). The measurement was overlaid with a best-fit Voigt profile in the same figure. The peak-normalized residual values are less than 0.8 % over the entire absorption feature, indicating that the Voigt profile adequately models the absorption line-shape.
Illustration of (a) the measured raw-data traces (pressure, transmission through the shock tube and the etalon) of the R(12) transition at 2190.02 cm−1, taken at 2.5 kHz with 0.496 % CO/1 % H2/Ar mixtures behind the reflected shock (vibrationally equilibrated reflected shock conditions: 1450 K, 1.63 atm); (b) the reduced line-shape of the R(12) transition (solid line, top panel), its best-fit Voigt profile (dashed line, top panel), and the residual (bottom panel)
The line-strength at a selected temperature can be inferred using Eq. (2) by calculating the integrated absorbance of the target line from the best-fit Voigt values. Figure 7 illustrates the measured line-strengths of four representative transitions at 1100–2000 K behind reflected shock waves. The calculated values using Eq. (5) with the line-strength S(296 K) and the lower-state energy E″ from the HITRAN database [5] are also plotted for comparison, illustrating excellent agreement (1−σ deviation between 1.4 % and 1.8 %) with our measurements.
Comparison of the measured line-strengths for the CO transitions at high temperatures with the HITRAN database [5]
Similarly, the collisional full-width at half maximum (FWHM) was inferred from the Voigt fit of the absorption profile as shown in Fig. 6(b). The collisional width is dominantly affected by the Ar-broadening as CO (0.5 %) and H2 (1 %) are both significantly diluted in argon. Thus, at a given temperature, the Ar-broadening coefficient is inferred directly from the measured collisional width with self- and H2- broadening neglected. Figure 8 plots the measured Ar-broadening coefficients (\(2\gamma_{\mathrm{CO}\mbox{\scriptsize-}\mathrm{Ar}}\)) as a function of temperature for CO transitions v″=0, R(12) and R(13) and v″=1, R(21). A two-parameter best fit to the experimental data following Eq. (4) gives \(2\gamma_{\mathrm{CO}\mbox{\scriptsize-}\mathrm{Ar}}\) (296 K) and its temperature coefficient n, as illustrated in Fig. 8 and summarized in Table 2. Note that the errors quoted in the table for the experimental results correspond only to the standard deviations derived by linear least-squared fits of \(\log(2\gamma_{\mathrm{CO}\mbox{\scriptsize-}\mathrm{Ar}})\) versus log(296/T). Experimental results of the line v″=1, R(22) at 2194.46 cm−1 are not included in Table 2, since this line was found to be blended with a neighboring transition v″=2, R(32) at 2194.44 cm−1 especially at higher temperatures, leading to larger uncertainties in the measurement.
Ar-broadening coefficient \(2\gamma_{\mathrm{CO}\mbox{\scriptsize-}\mathrm{Ar}}\) measurements for the CO transitions: R(12), R(13) and R(21). The two-parameter best fit to the high-temperature data, extrapolated to 296 K, gives \(2\gamma_{\mathrm{CO}\mbox{\scriptsize-}\mathrm{Ar}}\) (296 K)=0.079±0.007 cm−1/atm and n=0.581±0.012 for transition R(12), \(2\gamma_{\mathrm{CO}\mbox{\scriptsize-}\mathrm{Ar}}\) (296 K)=0.079±0.009 cm−1/atm and n=0.600±0.016 for transition R(13), and \(2\gamma_{\mathrm{CO}\mbox{\scriptsize-}\mathrm{Ar}}\) (296 K)=0.072±0.007 cm−1/atm and n=0.571±0.012 for transition R(21), respectively
In addition, the room-temperature (296 K) line-strengths and Ar-broadening coefficients of the ground state transitions (v″=0) can be directly determined by examining a frequency scan prior to the passage of the incident shock. Figure 9 illustrates the variation of the measured integrated absorbance and FWHM with pressure at 296 K for the representative transitions v″=0, R(12) and v″=0, P(20). Following Eq. (2) and Eq. (3), the line-strength and Ar-broadening coefficient at 296 K are inferred from the slope of the linear fit to the data as shown in Fig. 9(a) and (b), respectively. These experimental results are also summarized in Table 2. The measured line-strength at 296 K shows excellent agreement with the HITRAN database [5] (within 1.5 %), and the measured Ar-broadening coefficient is also in quite good agreement with the previous room-temperature studies by Bouanich and Haeusler [28], and Varghese and Hanson [29], respectively.
We also compared the \(2\gamma_{\mathrm{CO}\mbox{\scriptsize-}\mathrm{Ar}}\) (296 K) obtained from the direct measurements at room temperature with the extrapolated values (assuming constant n) from the shock tube measurements over the 1100–2000 K range. It should be noted that a 3–10 % difference of \(2\gamma_{\mathrm{CO}\mbox{\scriptsize-}\mathrm{Ar}}\) (296 K) can be found between these two methods. This may be explained by the fact that the temperature coefficient n in Eq. (4) itself is a weak function of temperature over the range from 296 K to 2000 K. Since the Ar-broadening coefficient as a function of temperature on a log-log plot is well-fit by a straight line as illustrated in Fig. 8, n can be treated as a constant over this specific temperature range of 1100–2000 K and utilized in the sensor development for shock tube and combustion applications.
5 Sensor validation in shock tube experiments
CO concentration and temperature sensors using both scanned-wavelength and fixed-wavelength direct absorption strategies are first validated in nonreactive shock-heated gases before being used in combustion kinetics applications. The bandwidth of the fixed-wavelength CO sensor is typically 1 MHz (limited by the detector bandwidth), compared to 2.5 kHz for the scanned-wavelength scheme, which is limited by the scan rate of the laser injection current.
5.1 Scanned-wavelength temperature and CO sensor using a single QC laser
Single-laser sensing has the advantages of simplifying the sensor system and reducing cost. Transitions v″=1, R(21) and v″=0, R(12) with relatively large difference in E″ are close enough to be covered by a single scan of the QC laser. Temperature can be inferred by comparing the measured peak absorbance ratio with the simulation. The simulated peak absorbance ratio for this line pair is plotted in Fig. 10 as a function of temperature. Notice that the pressure effect is also investigated to show that the uncertainty due to pressure variation is negligible in the pressure range of 1–2 atm. At 1500 K, for example, the temperature uncertainty is ∼6 K (0.4 %) with a pressure change from 1 to 2 atm.
Simulated peak absorbance ratio for the line pair R(21)/R(12) and R(21)/P(20) using the spectroscopic parameters listed in Table 2
The experimental setup for the single-laser sensor validation in a shock tube is the same as that shown in Fig. 5. The test gas mixture is known to be 0.49 % CO/ 2 % H2/Ar; similarly, hydrogen is added to accelerate vibrational relaxation. Figure 11 illustrates a representative laser absorption measurement of temperature behind the reflected shock at 1526 K and 1.57 atm (vibrationally relaxed). The laser intensity and wavelength were tuned across these two absorption profiles of interest at 2.5 kHz, as shown in the top panel of Fig. 11, along with the corresponding absorbance profile shown in the bottom panel. Assuming ideal shock conditions, the gas properties were reasonably regarded to be unchanged within each scan of 0.4 ms. During the test time of 2.5 ms, the sensor produced six data points of temperature as illustrated in the bottom panel of Fig. 11, which was in good agreement (1527–1529 K in the first 1 ms, less than 0.2 % difference) with the known value calculated using normal shock equations. Notice that the measured temperature drops significantly by ∼30 K at 2.4 ms, possibly due to a weak interaction of the reflected shock wave with the contact surface (driven and driver gas).
Sample traces of laser transmission and pressure (top panel), as well as absorbance and temperature (bottom panel) measured in nonreactive test gases (0.49 % CO/ 2 % H2/Ar, vibrationally equilibrated reflected shock conditions: 1526 K, 1.57 atm). A single QCL was used to scan over the line pair R(21) and R(12) at 2.5 kHz
With the temperature measured, the CO mole fraction is then inferred from either line of these two transitions. The CO mole fraction is measured to be (0.491±0.003)% using line R(12), again showing good agreement with the known CO concentration of 0.49 %. Moreover, at 2.4 ms when the nonideal shock condition happens, the CO mole fraction is still accurately measured to be 0.489 %.
Experiments were repeated under different shock conditions to measure gas temperature and CO concentration, and the results for a single scan are compared with the known values and plotted in Fig. 12 (solid squares). Good agreement can be seen between measurements and the known values for both the temperature (1−σ deviation 0.8 %) and CO concentration (1−σ deviation 1.6 %).
Shock tube validation measurements for the single-laser scanned-wavelength (measured for a single scan behind the reflected shock, solid squares) and the dual-laser fixed-wavelength (averaged over the first 0.3–1 ms after the shock, solid triangles) direct absorption CO sensors (0.49 % CO/2 % H2/Ar, 1.3–1.7 atm)
5.2 Fixed-wavelength temperature and CO sensor using two QC lasers
The sensor bandwidth of scanned-wavelength direct absorption is limited to several kHz, making it impossible to capture the rapid change of gas properties in chemical reactions. Here a fixed-wavelength CO concentration and temperature sensor with a bandwidth of ∼1 MHz is developed for shock tube experiments using a dual-laser fixed-wavelength diagnostic strategy.
Figure 13 is a schematic of the experimental setup. The light from each laser is collimated and transmitted through a different pair of windows on the shock tube sidewall. The laser wavelengths are fixed at the line-centers of the two selected transitions v″=1, R(21) at 2191.50 cm−1 and v″=0, P(20) at 2059.91 cm−1, respectively. This optical configuration utilizes the fact that the gas properties across the shock tube are uniform.
Figure 14(a) demonstrates a sample time-history of the laser absorbance recorded behind a reflected shock at 1454 K and 1.62 atm with 0.49 % CO/2 % H2/Ar mixture. The laser absorption reaches the plateau level as the CO is fully relaxed at ∼0.2 ms. Note that the sensor essentially measures the vibrational temperature, and hence the absorbance in the v″=1, R(21) line increases with time, after the shock, from zero to its plateau value. Conversely, the v″=0, P(20) absorbance decreases from its elevated initial value to its plateau value as vibrational relaxation takes place. Measured time-histories of pressure and temperature are plotted in Fig. 14(b). The average measured temperature over the time interval 0.2–1.5 ms is 1456 K with a standard deviation of 6 K (0.4 %), showing excellent agreement with the calculated value of 1454 K. Note that the sensor is capable of capturing the slight rise of temperature at later times from about 0.9–1.8, which results from the nonideal shock tube effects of boundary layer growth and incident-shock attenuation. CO mole fraction can be computed using the measured pressure, temperature, and transmission signal of either laser.
The experimental results are summarized and plotted in Fig. 12 (solid triangles). The measured and calculated temperatures are in good agreement (within 1.1 %) over the tested temperature range of 1200–1900 K, and the measured mole fraction agrees with the known values within 1.7 %. These results confirm the sensor accuracy for temperature and CO concentration measurements at combustion temperatures. It is noteworthy that this sensor has the potential to measure both translational/rotational temperatures and vibrational temperatures, when these temperatures differ, by employing line pairs with the same v″ value or with different v″ values, respectively.
6 Temperature and CO concentration measurements in combustion gases
Shock tubes are used to study gas phase combustion reactions by measuring ignition delay times and by monitoring species time-histories over a wide range of temperatures and pressures [4]. Accurate, time-resolved measurements of combustion species in shock tube are therefore critical with laser absorption the most commonly employed method [4, 30]. Here, the fixed-wavelength CO sensor validated in Sect. 5.2 is demonstrated in a kinetic study of the high-temperature pyrolysis and oxidation of methyl formate (MF), a simple biodiesel surrogate.
The shock tube/laser diagnostic experimental setup is shown in Fig. 13. Typical absorbance time-histories for both lasers are plotted in Fig. 15 for a test mixture of 0.5 % MF/Ar shock-heated to 1364 K, 1.63 atm. The absorbance profile for each laser directly represents the CO formation during the high-temperature pyrolysis of methyl formate. Temperature is inferred from the measured absorbance ratio, showing the slight decrease (15 K) that occurs during the decomposition of methyl formate. Our measurement is compared with the chemical kinetic simulation using the Dooley et al. [31] mechanism, performed in a commercial software package CHEMKIN-PRO [32], as illustrated in Fig. 16(a).
Temperature and CO concentration measured during a shock with initial mixture of 0.5 % MF/Ar; simulations using the Dooley et al. [31] mechanism are shown for comparison. Initial reflected shock conditions: T 5=1364 K, P 5=1.63 atm
CO concentration time-history is then inferred from either absorption trace; here, the R(21) transition is used. Figure 16(b) compares the sensor measurement with the simulation using the Dooley et al. [31] mechanism. The model underpredicts the rate of CO formation by about 10 % during MF pyrolysis, revealing the need for minor modifications to the kinetic model.
Similar measurements were performed for high-temperature MF oxidation in the shock tube. The measured temperature and CO mole fraction are plotted in Fig. 17 for a shock with 0.494 % MF and 0.988 % O2 (ϕ=1) in Ar as the initial mixture. The measured temperature time-history shown in Fig. 17(a) reveals that the gas temperature remains almost constant before the ignition happens at ∼1 ms, and then rises significantly by 150 K at 1.2 ms. However, the simulation using the Dooley et al. [31] mechanism significantly overpredicts the temperature rise during the MF oxidation. Since the temperature rises significantly (∼150 K after ignition) during the MF oxidation, it is critical to take into account these temperature and pressure changes in specifying the absorption coefficient when inferring the CO mole fraction. Figure 17(b) illustrates a comparison of the uncorrected CO concentration (assumes unchanged temperature and pressure) with the corrected values using the measured temperature and pressure data. A modest discrepancy (5.3 % difference) is seen after 1 ms when ignition starts in the reaction system. Our measurement is also compared with the model prediction using the Dooley et al. [31] mechanism. The simulation shows good agreement with our measurement at early times (<0.2 ms) and accurately predicts the peak value of CO (8420 ppm in experiment, compared to 8560 ppm in simulation) before starting to decline at 1 ms. Quantitative data sets such as these should greatly aid the validation of existing kinetic mechanisms and the development of more accurate chemical kinetic models.
Temperature and CO concentration measurements during MF oxidation for a mixture of 0.494 % MF, 0.988 % O2 (ϕ=1) and Ar; simulations using the Dooley et al. [31] mechanism are shown for comparison. Initial temperature and pressure behind the reflected shock are T 5=1379 K, P 5=1.67 atm
7 Summary
QC-laser-based absorption measurements of temperature and CO concentration in high-temperature shock-heated gases were reported using the fundamental band of CO near 4.7 μm. The selected transitions, v″=0, R(12), R(13), P(20) and v″=1, R(21), R(22), P(14) were successfully accessed by two different QC lasers. The spectroscopic parameters including line-strengths and broadening coefficients \(2\gamma_{\mathrm{CO}\mbox{\scriptsize-}\mathrm{Ar}}\) were determined at room-temperature (296 K) and high temperatures (1100–2000 K) and compared with literature values. A scanned-wavelength direct absorption CO sensor using a single QC laser was first validated for accurate measurements of temperature and CO concentration in a shock tube. The sensor measured temperature at a scan rate of 2.5 kHz by comparing the measured peak absorbance ratio of the line pair R(21) and R(12) with spectral simulations, showing very good agreement (within 0.8 %) with the calculated temperatures at 1300–2200 K. A fixed-wavelength CO temperature sensor based on transitions R(21) and P(20), accessed by two different lasers centered at 2194.46 cm−1 and 2059.91 cm−1, was then developed to provide in situ detection with faster time response. Sensor validation was first demonstrated in a shock tube by measuring temperatures (1200–1900 K) and CO concentrations of CO/H2/Ar mixtures with 1 MHz bandwidth. The sensor was then applied to the shock tube study of the pyrolysis and oxidation of methyl formate by measuring CO concentration and temperature time-histories to illustrate its capability in chemical kinetic studies. The increased absorption strength in this wavelength region provides opportunities for more sensitive and accurate combustion measurements with shorter optical path length and lower CO concentration than was possible using overtone band absorption. Future shock tube experiments are planned to apply this CO sensor, combined with other species-specific laser diagnostics, to chemical kinetic studies of oxygenates such as esters, ketones, and alcohols.
References
M.G. Allen, Meas. Sci. Technol. 9, 545 (1998)
H. Teichert, T. Fernholz, V. Ebert, Appl. Opt. 42, 2043 (2003)
K. Kohse-Höinghaus, R.S. Barlow, M. Aldén, J. Wolfrum, Proc. Combust. Inst. 30, 89 (2005)
R.K. Hanson, Proc. Combust. Inst. 33, 1 (2011)
D.T. Cassidy, L.J. Bonnell, Appl. Opt. 27, 2688 (1988)
R.M. Mihalcea, D.S. Baer, R.K. Hanson, Meas. Sci. Technol. 9, 327 (1998)
B.L. Upschulte, D.M. Sonnenfroh, M.G. Allen, Appl. Opt. 38, 1506 (1999)
M.E. Webber, J. Wang, S.T. Sanders, D.S. Baer, R.K. Hanson, Proc. Combust. Inst. 28, 407 (2000)
J. Wang, M. Maiorov, D.S. Baer, D.Z. Garbuzov, J.C. Connolly, R.K. Hanson, Appl. Opt. 39, 5579 (2000)
V. Ebert, H. Teichert, P. Strauch, T. Kolb, H. Seifert, J. Wolfrum, Proc. Combust. Inst. 30, 1611 (2005)
X. Chao, J.B. Jeffries, R.K. Hanson, Meas. Sci. Technol. 20, 115201 (2009)
R.K. Hanson, P.A. Kuntz, C.H. Kruger, Appl. Opt. 16, 2045 (1977)
R.K. Hanson, P.K. Falcone, Appl. Opt. 17, 2477 (1978)
M. Schoenung, R.K. Hanson, Combust. Sci. Technol. 24, 227 (1981)
J.H. Miller, S. Elreedy, B. Ahvazi, F. Woldu, P. Hassanzadeh, Appl. Opt. 32, 6082 (1993)
R. Barron-Jimenez, J.A. Caton, T.N. Anderson, R.P. Lucht, T. Walther, S. Roy, M.S. Brown, J.R. Gord, Appl. Phys. B 85, 185 (2006)
A.A. Kosterev, F.K. Tittel, R. Köhler, C. Gmachl, F. Capasso, D.L. Sivco, A.Y. Cho, S. Wehe, M.G. Allen, Appl. Opt. 41, 1169 (2002)
J. Vanderover, M.A. Oehlschlaeger, Appl. Phys. B 99, 353 (2010)
F. Capasso, R. Paiella, R. Martini, R. Colombelli, C. Gmachl, T.L. Myers, M.S. Taubman, R.M. Williams, C.G. Bethea, K. Unterrainer, H.Y. Hwang, D.L. Sivco, A.Y. Cho, A.M. Sergent, H.C. Liu, E.A. Whittaker, IEEE J. Quantum Elect 38, 511 (2002)
A. Kosterev, G. Wysocki, Y. Bakhirkin, S. So, R. Lewicki, M. Fraser, F. Tittel, R.F. Curl, Appl. Phys. B 90, 165 (2008)
R.F. Curl, F. Capasso, C. Gmachl, A.A. Kosterev, B. McManus, R. Lewicki, M. Pusharsky, G. Wysocki, F.K. Tittel, Chem. Phys. Lett. 487, 1 (2010)
V. Nagali, S.I. Chou, D.S. Baer, R.K. Hanson, J. Segall, Appl. Opt. 35, 4026 (1996)
G. Birnbaum, in Adv. Chem. Phys, ed. by J.O. Hirschfelder. Intermolecular forces, vol. 12 (Interscience, New York, 1967)
X. Zhou, J.B. Jeffries, R.K. Hanson, Appl. Phys. B 81, 711 (2005)
W. Ren, D.F. Davidson, R.K. Hanson, Int. J. Chem. Kinet. doi:10.1002/kin.20599 (2012)
R.C. Millikan, D.R. White, J. Chem. Phys. 39, 3209 (1963)
J.-P. Bouanich, C. Haeusler, J. Quant. Spectrosc. Radiat. Transfer 12, 695 (1972)
P.L. Varghese, R.K. Hanson, J. Quant. Spectrosc. Radiat. Transfer 24, 479 (1980)
D.F. Davidson, Z. Hong, G.L. Pilla, A. Farooq, R.D. Cook, R.K. Hanson, Proc. Combust. Inst. 33, 151 (2011)
S. Dooley, M.P. Burke, M. Chaos, Y. Stein, F.L. Dryer, V.P. Zhukov, O. Finch, J.M. Simmie, H.J. Curran, Int. J. Chem. Kinet. 42, 527 (2010)
R.J. Kee, F.M. Ruply, J.A. Miller, Chemkin Collection (Reaction Design, Inc., San Diego, 2010)
Acknowledgements
This work was supported by the Combustion Energy Frontier Research Center funded by the US Department of Energy, Office of Science, Office of Basic Energy Sciences under Award Number DE-SC0001198, the Army Research Office (ARO) with Dr. Ralph Anthenien as contract monitor, and the Air Force Office of Scientific Research (AFOSR) with Dr. Julian Tishkoff as technical monitor. The authors thank Dr. Jay Jeffries for his help on the selection and specification of the lasers and acquisition of the needed support electronics.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
High-resolution absorption spectroscopy of the CO transition v″=1, R(22) at 2194.46 cm−1 has been recorded in high temperature shock tube experiments (0.496 % CO/1 % H2/Ar, hydrogen is added to accelerate CO vibrational relaxation). A representative line-shape for the R(22) transition at 2162 K, 1.3 atm is illustrated in Fig. 18. It is found that R(22) is blended with a weak, nearby transition v″=2, R(32) centered at 2194.44 cm−1. Our observation is also proved by fitting the absorption data using the Voigt profile as shown in Fig. 19. The one-line Voigt fit gives a peak-normalized residual of 10 %, compared to 1.8 % using the two-line Voigt fit. This unknown transition is probably coming from an isotope of CO in the mixture.
One-line and two-line best-fit Voigt profiles for the absorption data in Fig.18. The residual of the fits are shown in the lower panels
Rights and permissions
About this article
Cite this article
Ren, W., Farooq, A., Davidson, D.F. et al. CO concentration and temperature sensor for combustion gases using quantum-cascade laser absorption near 4.7 μm. Appl. Phys. B 107, 849–860 (2012). https://doi.org/10.1007/s00340-012-5046-1
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-012-5046-1