Abstract
YNbO4:Bi3+, Yb3+ phosphor was prepared to study the quantum cutting process of converting one ultraviolet photon into two near-infrared photons. An intense near-infrared emission of Yb3+:2F5/2 → 2F7/2 around 1 μm was observed under the ultraviolet excitation belonging to the broadband absorption of the [NbO4]3- group and the Bi3+ ion. The photoluminescence spectra and decay lifetime measurements indicate efficient energy transfer from Bi3+ to Yb3+ ions, which is attributed to be of a cooperative energy transfer mechanism. The YNbO4:Bi3+, Yb3+ phosphor with optimized doping concentration may be applicable in improving the efficiency of silicon-based solar cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
One of the major challenges in photovoltaic research today is to increase solar cell conversion efficiency by making better use of the solar spectrum [1, 2]. Considerable research recently has been focused on better exploitation of the solar cells via a photon conversion process in order to use this environmentally friendly energy source for future energy demands [3–7]. The most popular solar cells are based on crystalline Si (c-Si) and the band gap of c-Si is about 1.12 eV (∼1100 nm). The process of quantum cutting (QC) can convert one photon of high energy into two photons of lower energy [8]. If conversion of one incident ultraviolet (UV) or visible photon into two near-infrared (NIR) photons is realized, the main energy loss in solar cells due to the thermalization of charge carriers generated by the absorption of high-energy photons will be minimized. Fortunately, rare-earth (RE) ions with abundant energy levels are good candidates for the QC process. The research on QC systems started from single ions capable of cascade emission such as Pr3+ [9, 10], Tm3+ [11], and Gd3+ [12]. Then, the combination of two ions like the Gd3+–Eu3+ couple [8] through stepwise energy transfer aroused extensive attention of researchers. A QC process based on cooperative energy transfer (CET) was reported in Yb x Tb1-x PO4:Tb3+ powder by Vergeer et al. [13] in 2005. Via CET, two NIR photons at 980 nm from the emission of Yb3+can be obtained by the excitation of one Tb3+ ion with one blue photon. Since then, a similar NIR QC phenomenon has been extensively reported in RE codoped systems such as Tb3+–Yb3+ [14, 15], Tm3+–Yb3+ [16], and Pr3+–Yb3+ [17]. The single emission around 1000 nm of the Yb3+ ion matches well with the band gap of c-Si, so it is a good choice to realize the conversion of incident UV or visible photons into NIR photons via the QC process. However, the NIR emission of Yb3+ in the RE3+/Yb3+ systems mentioned above is very weak due to the inefficient excitation of the donors for their parity-forbidden 4f–4f transitions. Though the Ce3+–Yb3+ [18] system overcomes this shortcoming, the combination of Ce3+–Yb3+ codopant is unstable [19, 20]. Recently, strong Yb3+ NIR emission has been reported utilizing the efficient energy transfer from the host YVO4 to Yb3+ [21]. However, the relatively low quenching concentration and the toxicity of the raw material may restrict its practical application in Si-based solar cells. Another phosphor studied to achieve efficient NIR QC via CET is Gd2O3:Bi3+, Yb3+ [22], where the Bi3+ ion was chosen as an activator. The absorption band in Gd2O3:Bi3+, Yb3+ due to the transition between localized electronic energy levels of Bi3+is neither intense nor broad enough. Thus, other QC materials are still urgently needed to be developed to enhance the energy conversion efficiency via spectral modification.
The host material, YNbO4, is a well-known self-activated phosphor, all the niobate groups of which may be considered as fluorescent centers [23]. The self-luminescent complex [NbO4]3- in YNbO4 shows a broad and strong emission band in the spectral region around 400 nm while the absorption band ranges from about 200 to 300 nm. The luminescent property is ascribed to the ligand–metal charge transfer (CT) transition of tetrahedral [NbO4]3- molecular ions [24, 25]. The emission wavelength and intensity can be modified by introducing dopant ions into the host. The RE ions can easily replace the Y3+ ions for the similar properties between Y3+ and other RE ions. So, it is expected that the doping concentration of the RE ions can be high. The photoluminescence properties of YNbO4 activated by RE ions such as Eu3+, Dy3+, Sm3+, and Er3+ [26, 27] have been reported.
Bi3+ is a typical type of localized activator with strong electric-dipole-allowed s2→sp transition. The main emission band is 3P1→1S0 which is nominally spin forbidden but has nevertheless reasonably high oscillator strength [28]. When a phosphor is doped with Bi3+ ions as activators, the position of the main excitation band varies depending on the nature of the host lattice. RE niobates are efficient hosts for Bi3+ known as self-activated luminescent materials. Introducing Bi3+ into YNbO4 shifts the emission band to the longer wavelength of 440 nm [29], making YNbO4:Bi3+ a suitable and efficient blue phosphor for field emission display (FED) application [30]. Compared with the un-doped YNbO4, the blue photon emitted from YNbO4:Bi3+ matches much better the energies of two Yb3+: 2F7/2 → 2F5/2 transitions, making the CET from one Bi3+ to two Yb3+ theoretically possible and potentially more efficient. Besides, the doping of the Bi3+ ion extends the absorption band due to the superposition of the charge transfer of [NbO4]3- and the Bi3+: 6s2→6s6p electric-dipole-allowed transition [31, 32]. The broader absorption band in the UV part leads to better utilization of the solar spectrum. The present work demonstrates the NIR QC phenomenon in YNbO4:Bi3+, Yb3+ phosphors via the CET process.
2 Experimental
Powder samples of Y0.99-x Yb x Bi0.01NbO4 (x=0, 2 %, 8 %, 16 %, 32 %, and 48 %) were prepared by a conventional solid-state reaction method using Y2O3 (99.99 %), Nb2O5 (99.5 %), Bi2O3 (99.9 %), and Yb2O3 (99.99 %) as starting materials. A stoichiometric mixture of the raw materials was ground and calcined in an alumina crucible at 1200 °C for 4 h in air. The crystalline phases of the synthesized samples were identified via an X-ray diffractometer (XRD, MAC Science Co. Ltd., MXPAHF) using Cu Kα radiation in the range of 2θ=20–80∘; the accelerating voltage was 30.0 kV and the tube current was 160.0 mA. The excitation and emission spectra were obtained with a Jobin–Yvon Fluorolog 3 system. For the lifetime measurements, a Q-switched frequency-quadrupled (266 nm) Nd:YAG laser with a pulse duration of 10 ns was used and the signal was analyzed with a Tektronix TDS2024 digital storage oscilloscope. The visible emission was dispersed by a Jobin-Yvon HRD1 double monochromator and was detected by a Hamamatsu R928 photomultiplier. The NIR emission was dispersed by a Zolix SBP750 monochromator and was detected by an Acton ID-441-C InGaAs NIR detector. The signal was analyzed by an EG&G7265 DSP lock-in amplifier and stored into computer memories. The spectra were recorded under identical experimental conditions so that the emission intensities of the samples with different Yb3+ doping concentrations can be compared. All the measurements were carried out at room temperature.
3 Results and discussion
Figure 1 shows the representative XRD patterns of YNbO4, Y0.99Bi0.01NbO4, and Y0.51Yb0.48Bi0.01NbO4 samples as synthesized. It can be seen that all the diffraction peaks for the samples can be well assigned to the reported data of monoclinic YNbO4 (JCPDS 23-1486). No obvious shifting of peaks or second phase can be detected at the current doping level, indicating that the introduction of Bi3+ and Yb3+ ions does not change the crystal structure of the powder. This may be due to their similar ionic radii (Y3+, r=0.089 nm; Bi3+, r=0.096 nm; Yb3+, r=0.086 nm).
The concentration of Bi3+ is fixed at the optimal doping level of 1 mol %. The photoluminescence excitation (PLE) and photoluminescence (PL) spectra of YNbO4:Bi3+ 1 mol %, Yb3+ 16 mol % are presented in Fig. 2. A broad excitation band ranging from 250 to 350 nm was recorded when 450 nm was monitored (Fig. 2a). The peak centered at about 274 nm is most likely assigned to an electron transition of the molecular orbital of [NbO4]3- while the shoulder around 294 nm is obviously a Bi effect that influences the optical properties of the [NbO4]3- group. Electronic structure calculations of YNbO4 indicated that the conduction band is composed of Nb 4d orbitals and the valence band of O 2p orbitals [29]. The Bi 6s6p is located at a lower energy level than that of Nb 4d. The calculated partial densities of states show that the Bi 6s2 valence band levels lie just above the O 2p valence band levels and the Bi 6s2 levels overlap with the O 2p ones [29, 32]. Thus, the electron in O 2p (Bi 6s2) can be excited to Nb 4d and Bi 6s6p. The broad absorption band is attributed to the combined action of the [NbO4]3- group and the Bi ion. By monitoring the Yb3+: 2F5/2 → 2F7/2 transition at 1003 nm (Fig. 2b), a similar broad excitation band was also recorded. The essential coincidence of the two excitation spectra reveals the identical energy origin of the visible emission and NIR emission. Under the excitation of 274 nm (Fig. 2c), a broad blue emission centered at 450 nm was observed. According to Ref. [31], when Bi3+ is introduced into YNbO4, the excited Nb 4d electron is transferred to Bi 6s6p. Then, the 6s6p→6s2 transition produces the 450-nm emission. Since the Bi 6s6p levels overlap with the O 2p ones, it is expected that the emission is due to transitions inside the Bi electronic energy levels and transitions between Bi and O via CT [33]. Besides the blue emission from Bi3+ ions, there is an intense emission band peaking at 1003 nm along with several small shoulders. Since the Bi3+ ions do not contribute to NIR emission [34], this NIR emission is attributed to the 2F5/2 → 2F7/2 Stark level transitions of Yb3+ ions.
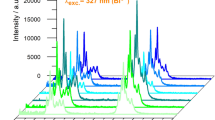
The dependence of Bi3+ and Yb3+ emission intensities on various Yb3+ concentrations in YNbO4:Bi3+, Yb3+ phosphors is shown in Fig. 3. Emission intensities were recorded under 266-nm excitation with identical conditions at room temperature for all the samples. It is noticed that the intensity of Bi3+ emission decreases monotonically with the increase of the Yb3+ concentration, whereas the intensity of Yb3+ emission first reaches a maximum at a concentration of 16 mol % and then gradually decreases with further increase of concentration. This is a common phenomenon called concentration quenching. Many factors lead to the concentration quenching of Yb3+ ions which plays a negative effect on the Yb3+ NIR emission, including an increased amount of impurities and an increased possibility of energy migration to the quenching centers [15, 21]. The decay curves of Yb3+ emission at 1003 nm upon excitation of 266 nm are shown in Fig. 4. The lifetime decreases monotonically from 0.34 to 0.17 ms with Yb3+ concentration increasing from 2 to 48 mol %, verifying the Yb3+ concentration quenching process that results from increased migration. The variation of emission intensities of Bi3+ and Yb3+ emission intensities on Yb3+ doping concentration is further indicative of energy transfer from Bi3+ to Yb3+.
In Fig. 5, the decay curves for Bi3+ emission at 450 nm are plotted for various Yb3+ concentrations. An obvious trend is that the lifetime of the Bi3+ emission decreases with increasing Yb3+ concentration. The lifetime is about 1.85 μs for the sample without Yb3+ while it is reduced to 0.72 μs when the Yb3+ concentration is 48 mol %. Since the only variable for different samples is the Yb3+ concentration, the fast decline of the lifetime is naturally ascribed to the incorporation of Yb3+ ions. Yb3+ doping provides an extra decay pathway: energy transfer from Bi3+ to Yb3+ that accelerates the Bi3+ emission decay rate. The result is considered to be a further proof of the efficient energy transfer from Bi3+ to Yb3+. The non-exponential decay is a character of the decay of donors, which have a fluctuating distribution of surrounding acceptors Yb3+.
Generally, the O2-–Yb3+ CT band is located at the relatively high energy region with wavelength shorter than 250 nm [35]. It has also been pointed out that the CT absorption band is higher in energy for Yb3+ than for Eu3+ in the same host lattice, and the CT absorption band of Eu3+ is located in about the range of 230–260 nm [36, 37]. Hence, there is no influence of O2-–Yb3+ CT on Bi3+ and Yb3+ emissions, and the intense UV-excited NIR emissions can only be attributed to the energy transfer from [NbO4]3- and Bi3+ to Yb3+, since the Yb3+ ion has no levels in the UV region. Due to the absence of the energy overlap between the emission of the Bi3+ ion and a single Yb3+ ion and the existence of the resonance between the emission energy of Bi3+ and twice the photon energy of absorption of Yb3+, we conclude that the Bi3+→Yb3+ CET process is the only possible energy transfer process to achieve the Yb3+ NIR emission. The variation of emission intensities of Bi3+ and Yb3+ on Yb3+ doping concentration and the increased decay rates for Bi3+ emission with increasing Yb3+ concentration support this.
Figure 6 pictures the schematic configuration coordinate diagram for [NbO4]3-, Bi3+, and Yb3+ as well as the CET process for the NIR QC emission. Since the Bi 6s6p levels overlap with the O 2p ones, at the excitation of a UV photon, an electron situated in the O 2p (Bi 6s2) valence band is excited to the excited energy levels consisting of Nb 4d orbitals of [NbO4]3- groups and the lower Bi 6s6p levels. The excitation will reside in the lowest 6s6p levels before relaxing by emission or via CET to two Yb3+ ions, which subsequently emit two NIR photons.
From the luminescence decay curves shown in Fig. 5, the CET efficiency (η CET) and the total theoretical downconversion quantum efficiency (η QE) could be calculated by the following equations [13]:


where I denotes the decay intensity, x % Yb stands for the Yb3+ concentration, and η Bi and η Yb refer to the internal quantum efficiencies of Bi3+ and Yb3+, respectively. With Yb3+ concentration increasing from 0 to 48 mol %, the CET efficiency from Bi3+ to Yb3+ increases monotonically from 0 to 80 % as shown in the inset of Fig. 5. Supposing that the excited Yb3+ decay radiatively (η Yb=1) for the sample YNbO4:Bi3+ 1 mol %, Yb3+ 2 mol % with the lifetime τ 2 % Yb=0.34 ms, the quantum efficiencies of Yb3+ (η Yb) could be estimated from the lifetimes of Yb3+ emission by the following equation:
where A total=1/τ x % Yb, representing the total decay rate for the samples with various Yb3+ doping concentrations, and A R=A total=1/τ 2 % Yb is considered as concentration independent. The calculated η Yb is 1, 0.91, 0.71, 0.59, and 0.50 for the samples with Yb3+ doping concentrations of 2, 8, 16, 32, and 48 mol %, respectively. The decrease of Yb3+ internal quantum efficiency is due to concentration quenching. Meanwhile, assuming that all the excited Bi3+ decay radiatively (η Bi=1), for the samples with the Yb3+ doping concentrations of 2, 8, 16, 32, and 48 mol %, the total quantum efficiency is calculated to be 109 %, 121 %, 120 %, 113 %, and 100 %. However, if the value of η Yb is promoted to approach 1 by reducing the concentration of quenching centers, an upper limit of the theoretical quantum efficiency of 109 %, 125 %, 147 %, 170 %, and 180 % will be reached for these samples with the Yb3+ doping concentrations of 2, 8, 16, 32, and 48 mol %, respectively. A maximum quantum efficiency of 180 % is close to the limit of 200 % for the case of ideal one-to-two QC.
4 Conclusion
Efficient broadband NIR QC YNbO4:Bi3+, Yb3+ phosphor that converts UV photons which are almost useless in the silicon solar cell to NIR photons around 1 μm where the Si solar cell exhibits the most efficient spectral response has been successfully developed. A CET mechanism was proposed to rationalize the process. The PLE and PL spectra and decay lifetime measurements evidence the occurrence of CET from Bi3+ to Yb3+ ions with quantum efficiencies higher than 100 %. The NIR QC YNbO4:Bi3+, Yb3+ phosphor with reduced concentration quenching and optimized doping concentration may reach 180 % in quantum efficiency and serve as a spectral modifier to enhance the photovoltaic conversion efficiency of silicon-based solar cells.
References
A. Luque, A. Martí, A. Bett, V.M. Andreev, C. Jaussaud, J.A.M. Van Roosmalen, J. Alonso, A. Räuber, G. Strobl, W. Stolz, C. Algora, B. Bitnar, A. Gombert, C. Stanley, P. Wahnon, J.C. Conesa, W.G.J.H.M. van Sark, A. Meijerink, G.P.M. van Klink, K. Barnham, R. Danz, T. Meyer, I. Luque-Heredia, R. Kenny, C. Christofides, G. Sala, P. Benítez, Sol. Energy Mater. Sol. Cells 87, 467 (2005)
A. Shalav, B.S. Richards, T. Trupke, K.W. Krämer, H.U. Güdel, Appl. Phys. Lett. 86, 013505 (2005)
W.G.J.H.M. van Sark, Appl. Phys. Lett. 87, 151117 (2005)
E. Klampaftis, D. Ross, K.R. McIntosh, B.S. Richards, Sol. Energy Mater. Sol. Cells 93, 1182 (2009)
A. Shalav, B.S. Richards, T. Trupke, K.W. Krämer, H.U. Güdel, Appl. Phys. Lett. 86, 013505 (2005)
M. Peng, L. Wondraczek, J. Mater. Chem. 19, 627 (2009)
H. Shpaisman, O. Niitsoo, I. Lubomirsky, D. Cahen, Sol. Energy Mater. Sol. Cells 92, 1541 (2008)
R.T. Wegh, H. Donker, K.D. Oskam, A. Meijerink, Science 283, 663 (1999)
W.W. Piper, J.A. DeLuca, F.S. Ham, J. Lumin. 8, 344 (1974)
J.L. Sommerdijk, A. Bril, A.W. de Jager, J. Lumin. 8, 341 (1974)
R. Pappalardo, J. Lumin. 14, 159 (1976)
R.T. Wegh, H. Donker, A. Meijerink, R.J. Lamminmäki, J. Hölsä, Phys. Rev. B 56, 13841 (1997)
P. Vergeer, T.J.H. Vlugt, M.H.F. Kox, M.I. den Hertog, J.P.J.M. van der Eerden, A. Meijerink, Phys. Rev. B 71, 014119 (2005)
Q.Y. Zhang, C.H. Yang, Z.H. Jiang, X.H. Ji, Appl. Phys. Lett. 90, 061914 (2007)
X. Liu, S. Ye, Y. Qiao, G. Dong, B. Zhu, D. Chen, G. Lakshminarayana, J. Qiu, Appl. Phys. B 96, 51 (2009)
L.C. Xie, Y.H. Wang, H.J. Zhang, Appl. Phys. Lett. 94, 061905 (2009)
B.M. van der Ende, L. Aarts, A. Meijerink, Adv. Mater. 21, 3073 (2009)
D. Chen, Y. Wang, Y. Yu, P. Huang, F. Weng, J. Appl. Phys. 104, 116105 (2008)
G. Blasse, B.C. Grabmaier, Luminescent Materials (Springer, Berlin, 1994)
J.W.M. Verweij, C. Pedrini, D. Bouttet, C. Dujardin, H. Lautesse, B. Moine, Opt. Mater. 4, 575 (1995)
X.T. Wei, S. Huang, Y.H. Chen, C.X. Guo, M. Yin, W. Xu, J. Appl. Phys. 107, 103107 (2010)
X.Y. Huang, Q.Y. Zhang, J. Appl. Phys. 107, 063505 (2010)
G. Blasse, Philips Res. Rep. 23, 344 (1968)
D.A. Grisafe, C.W. Fritsch, J. Solid State Chem. 17, 313 (1976)
G. Blasse, K.C. Bleijenberg, R.C. Powell, Luminescence and Energy Transfer (Springer, Heidelberg, 1980)
A.M.G. Massabni, G.J.M. Montandon, M.A. Couto dos Santos, Mater. Res. 1, 1 (1998)
B. Yan, X. Xiao, J. Alloys Compd. 433, 251 (2007)
J.L. Sommerdijk, J.M.P.J. Verstegen, A. Bril, Philips Res. Rep. 29, 517 (1974)
C. Han, H. Kim, H. Chang, S.K. Lee, H.D. Park, J. Electrochem. Soc. 147, 2800 (2000)
A. Vecht, D. Charlesworth, D.W. Smith, in Dig. Tech. Papers SID Int. Symp., Boston (1997), p. 588
S.H. Shin, D.Y. Jeon, K.S. Suh, J. Appl. Phys. 90, 5986 (2001)
S.K. Lee, H. Chang, C.H. Han, H.J. Kim, H.G. Jang, H.D. Park, J. Solid State Chem. 156, 267 (2001)
J.H. Bahng, E.S. Oh, S.H. Seo, J.S. Kim, M. Lee, H.L. Park, C. Lee, G.C. Kim, K.J. Kim, Phys. Status Solidi A 191, 291 (2002)
M. Peng, L. Wondraczek, J. Mater. Chem. 19, 627 (2009)
P. Dorenbos, J. Phys., Condens. Matter 15, 8417 (2003)
L. van Pieterson, M. Heeroma, E. de Heer, A. Meijerink, J. Lumin. 91, 177 (2000)
X. Xiao, B. Yan, Appl. Phys. A 88, 333 (2007)
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant Nos. 10904139, 11011120083, 11111120060, and 11074245), the Knowledge Innovation Project of the Chinese Academy of Sciences (Grant No. KJCX2-YW-M11), the China Postdoctoral Science Foundation (Grant No. 20100480693), and the Special Foundation for Talents of Anhui Province, China (Grant No. 2007Z021).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, R., Kou, Y., Wei, X. et al. Broadband downconversion based near-infrared quantum cutting via cooperative energy transfer in YNbO4:Bi3+, Yb3+ phosphor. Appl. Phys. B 107, 483–487 (2012). https://doi.org/10.1007/s00340-012-4956-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-012-4956-2