Abstract
The photoluminescence properties of alkali-earth magnesium silicates (M2MgSi2O7, M=Ca, Sr, and Ba) doped with Eu2+ were investigated. Solid solutions of Ba x Sr2−x Si2O7, Ca2MgSi2O7, and Sr2MgSi2O7 were prepared. Ba x Sr2−x Si2O7 retained a tetragonal crystal structure similar to the structure of the other compounds up to a stoichiometry of x=1.6, which enabled a systematic study of the common structure. Monoclinic Ba2MgSi2O7 was prepared, and the luminescence properties were compared with those of other samples. The emission and excitation spectra of tetragonal M2MgSi2O7 (M=Ca, Sr, and Ba) changed as a function of the covalency, site symmetry, and crystal field strength. The luminescence properties showed excellent agreement with theoretical predictions based on these factors. The Stokes shift differentiated the emission behaviors of the tetragonal and monoclinic structures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Solid-state illumination approaches that rely on a combination of phosphors and LED chips require the development of novel phosphors that can be excited by relatively long wavelengths. The excitation spectrum should match the emission spectrum of a UV-LED (400 nm) or a blue LED (450 nm). A variety of materials have been investigated and described as new phosphors for use with white LED applications [1, 2].
The fluorescence of Eu2+ yields a variety of colors, depending on the nature of the Eu2+ ligand environment in the host matrix. The 5d electrons in the excited state are sensitive to the environment because they are not shielded as effectively as 4f electrons in the ground state. It is important to investigate luminescence properties in relation to the relevant properties of the host material. The major factors that determine luminescent properties are covalency, site symmetry, and crystal field strength [2–4]; however, it is difficult to observe their effects in the context of a crystal structure because of the high sensitivity of crystal structures to these properties.
The luminescence properties of Eu2+-doped M2MgSi2O7 (M=Ca, Sr, and Ba) phosphors in the context of designed LEDs have been reported previously [5–7], although the luminescence properties have not been fully investigated in terms of the properties of the host crystal, such as the covalency, crystal field strength, and Stokes shift. It is therefore necessary to investigate the relationship between the luminescence character and the crystal structure. M2MgSi2O7 (M=Ca, Sr, and Ba), Ca2MgSi2O7, and Sr2MgSi2O7 have the same akermanite structure, but Ba2MgSi2O7 assumes a distinct monoclinic structure. In the present study, we investigated the luminescence properties of phosphors belonging to the M2MgSi2O7 system, with a focus on the effects of crystal structure on luminescence properties.
2 Experimental
Powdered samples with the general formula M1.95Eu0.05MgSi2O7 (M=Ca, Sr, and Ba) were prepared via a high-temperature solid-state reaction. The starting materials were CaCO3, SrCO3, BaCO3, MgCO3, SiO2, and Eu2O3 (from Sigma–Aldrich, >99.9% purity). Stoichiometric amounts of the raw materials were mixed homogeneously in an agate mortar and fired at 1000°C for 1 h in an air atmosphere. Final powders were obtained after a second heat treatment at 1300°C for 8 h in a reducing 5%H2+95%N2 atmosphere. XRD powder patterns were obtained using a diffractometer (Rigaku D-max 2500, Japan) equipped with a Cu-Kα (λ=1.54056 Å) radiation source and a rotating anode. Excitation and emission spectra were measured using a JASCO FP-5600 spectrofluorometer. All spectra were recorded at room temperature.
3 Results and discussion
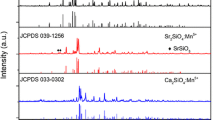
The XRD patterns of the synthesized 5 mol% Eu2+-doped Ca2MgSi2O7 (CMSO), Sr2MgSi2O7 (SMSO), and Ba2MgSi2O7 (BMSO) powders are shown in Fig. 1 together with the reference patterns. CMSO and SMSO displayed the same crystal structure, whereas BMSO displayed a different structure. According to the references, CMSO and SMSO have orthorhombic akermanite structures and BMSO has a monoclinic structure (mBMSO). A calculated diffraction pattern was used as a reference for the mBMSO structure, as reported recently [7].
Observing the systematic luminescence behavior of these alkali earth magnesium silicates was difficult because mBMSO assumed a monoclinic crystal structure whereas the other compounds were tetragonal. Therefore, experiments were performed to reveal the luminescence properties of tetragonal BMSO (tBMSO). To prepare tetragonal BMSO, various amounts of Ba were added to the tetragonal SMSO, and the crystal structure and luminescence property changes were observed. Figure 2 shows the XRD pattern of samples prepared with different amounts of Ba. The SMSO and mBMSO samples are shown together for comparison. As can be seen in the figure, the main peaks shifted to lower angles as the Ba concentration increased, suggesting that Ba incorporation into the tetragonal structure increased the lattice parameters. The tetragonal structure of Sr2−x Ba x MgSi2O7 was retained up to x=1.6, but the monoclinic phase was favored at x=1.8. Therefore, the crystal structure transitioned over the interval 1.6<x<1.8.
Figures 3 and 4 show the emission and excitation spectra of CMSO, SMSO, and mBMSO. The emission spectra were gathered using an excitation wavelength of 396 nm, and the excitation spectra were obtained by monitoring each emission peak. Upon excitation, CMSO and SMSO showed green emission, whereas mBMSO showed blue emission. The full width at half maximum (FWHM) of each emission spectrum corresponded to a large spectral width (>50 nm), which was desirable for achieving a high color rendering index (CRI). In addition, the excitation spectra met the general requirement for use in UV-LEDs: they absorbed the excitation energy over a broad range from 254 nm to 400 nm. These results agreed with previous reports, indicating that the phosphors are potential candidates for UV-LED phosphors [5, 6].
Figures 5 and 6 show the emission and excitation spectra of materials prepared with different Ba concentrations. The wavelength of the main emission peak decreased relative to that of SMSO, 470 nm, as the Ba concentration increased. The wavelength of the main peak gradually shifted to 443 nm over the composition range 1.0<x<1.6. However, in the Ba-rich composition (x>1), the intensity in the long-wavelength region (500 nm), which corresponded to the emission spectrum of mBMSO, gradually increased. The emission spectral changes shown in Fig. 5 followed the same trend as that observed in the XRD results, shown in Fig. 2. The abrupt change in the emission spectrum at x=1.8 was related to a crystal structure transition from tetragonal to monoclinic. A comparison of the XRD results with the corresponding emission spectra revealed that the maximum blue-shift of the main peak position (443 nm) resulted from the tetragonal tBMSO, because the tetragonal structure persisted, as observed in the XRD results, for x<1.8.
Many factors determine the luminescence properties of Eu2+, including the covalency, the site symmetry, the crystal field, and the Stokes shift [2, 3, 8–11]. Eu2+ emission is generated by a d–f transition, not by a d–d transition, as in transition metal ions. In such ions, d orbitals are split by a crystal field, and transitions occur between the split d orbitals. In the case of Eu2+, the ground 4f state is insensitive to the crystal field strength because the 4f orbitals are effectively shielded by the outer orbitals. Emission occurs between the lowest level of the split 5d levels and the 4f ground level.
Thus, the most important information required to explain the luminescence properties of Eu2+ are the position and the energy splitting of the 5d levels. It is required to determine representative energy level of 5d from broad excitation spectrum for the comparison of the position of excitation spectra. The location of the 5d level before splitting may be used as a standard of the position. In general, the central wavelength (center of gravity) is considered as the 5d energy level before splitting [12, 13].
Covalency is related to the position of the 5d levels relative to the 4f levels. In the absence of covalent bonding character, the 5d level of the Eu2+ ion is higher in energy than the 4f level by 4.3 eV (290 nm) [2]. When Eu2+ is embedded in a host material, the covalency increases and the 5d energy level decreases. This is related to the relatively large spread out of the 5d orbitals compared with the 4f ones. As the covalency increases, more electrons exist in the spaces between atoms, which facilitated the transition to the 5d orbitals. Therefore, an energy gap between the 4f and 5d energy levels decreases, and the PL spectrum shifts to longer wavelengths. However, it should be noted that only the excitation spectrum, not the emission spectrum, is directly related to covalency.
Within a given crystal structure, the luminescence properties of Eu2+ should be governed mainly by covalency. Among the materials CMSO, SMSO, and tBMSO, tBMSO has the lowest covalency corresponding to the M–O bond (M=Ca, Sr, and Ba), and covalency increased in the order of tBMSO<SMSO<CMSO. The covalency is determined by the electronegativity of the alkali earth ions substituted by the Eu2+ ions. Ba has the lowest electronegativity among Ca, Sr, and Ba (1.00, 0.95, and 0.89, respectively, in the Pauling scale), which leads to the largest electronegativity differences between Ba and oxygen and the lowest covalency. A comparison of the excitation spectra indicated that the central wavelength decreased in the order of CMSO>SMSO>mBMSO, which is consistent with the order of the covalency. The emission wavelength of a perfect tetragonal BMSO material (if it could be synthesized) was expected to be shorter than the emission wavelength of the other materials due to a higher covalency. In conclusion, the excitation and emission wavelengths of tBMSO (in this study, the Ba–Sr solid solution) were the shortest, followed by SMSO and CMSO. This result agreed well with the general relationship between covalency and the photoluminescence wavelength.
The excitation spectrum of mBMSO also shifted to shorter wavelengths compared with the excitation spectra of both CMSO and SMSO. However, the emission spectrum was situated between CMSO and SMSO, which was difficult to explain solely in terms of covalency. These results suggested that the excitation spectrum of mBMSO was mainly affected by the covalency, whereas other factors affected the position of the emission spectrum, including the Stokes shift, as discussed below.
Another important factor that characterizes Eu2+ emission spectra is site symmetry. Ligand field theory predicts that the five d orbitals of free Eu2+ ions are degenerate, but they are split within a crystal due to the spatial distribution of the ligands [2–4]. The number of split levels is determined by the degree of symmetry. Eu2+ in an akermanite structure is classified as a C1v point group, which corresponds to very low symmetry. All d orbitals are expected to be split differently to yield 5 different levels. Figure 4 shows that all excitation spectra were broad and composed of many peaks.
In principle, the excitation spectrum can be deconvoluted into five distinct peaks; however, we used the shape of the excitation spectrum only as a fingerprint for the symmetry at the luminescence site. In CMSO and SMSO, the shapes of the excitation spectra were similar, indicating that the site symmetries, in addition to the crystal structure, are similar. In contrast, mBMSO had a totally different shape, which confirmed that it has different crystal structure and site symmetry. In fact, Eu2+ in the monoclinic mBMSO occupies the C1 point group, indicating a lack of symmetry. Thus, the shape of the excitation spectra in this study reflected the symmetric character of the Eu2+ sites.
According to crystal field theory, the energy levels of central ions are determined by the strength of the crystal field. In general, the crystal field strength is expressed according to the following equation [2, 6]:
where R is the distance between the central ion and the ligands, and q is the charge on the ions. This equation is based on the coulombic interaction energy, which is inversely proportional to the separation distance and directly proportional to the magnitude of the charges. In this experiment, the charges were identical for all compositions, and only the Eu–O bond length changed. Eu2+ ions had different bond lengths, depending on the size of the host alkali earth metal ion. Thus, the strength of the crystal field was expected to increase as follows: tBMSO<SMSO<CMSO. The width of the excitation spectrum is proportional to the crystal field strength because strong crystal fields increase the energy gap between the lowest and highest energy levels of the 5d orbitals, which increases the width of the excitation spectrum.
This was confirmed in Figs. 4 and 6. CMSO showed the largest excitation spectral width, and the width decreased in the order of CMSO>SMSO>BSMSOs (solid solution samples). Although mBMSO showed a width similar to that of SMSO in Fig. 3, the spectral width of the tetragonal SBMSO in Fig. 6 decreased as the Ba concentration increased. Therefore, the excitation spectral width changed according to the strength of the crystal field within a given crystal structure. The relatively large spectral width of mBMSO seems to be related to the low site symmetry (C1) of mBMSO.
The emission spectrum of mBMSO did not follow the covalency rule. The emission spectrum of mBMSO fell between CMSO and SMSO in Fig. 3. As mentioned above, covalency is determined by the electronegativities of the individual atoms and does not change significantly with the crystal structure. Therefore, abnormally long emission wavelengths for mBMSO must arise from another factor; namely, the Stokes shift.
The emission wavelength of Eu2+ is much longer than that of the excitation spectrum due to a large Stokes shift. The Stokes shift is generally explained in terms of a configurational coordinate diagram, and electron-lattice coupling provides the underlying mechanism [3]. The emission wavelength could be estimated simply from the factors that affected the 5d energy levels if the difference in the Stokes shift is negligible. If not, the effects of the Stokes shift should be considered seriously, as observed in the case of mBMSO.
Monoclinic mBMSO has a different crystal structure, but its excitation spectrum did not deviate significantly from the expectations based on the covalency and crystal field strength. The spectrum was slightly wider than that of tBMSO, but was situated at shorter wavelengths than the spectra of CMSO and SMSO. However, the emission wavelength was longer than that of SMSO, indicating that the Stokes shift of mBMSO was far larger. The relatively long emission wavelength of mBMSO was attributed to a large Stokes shift in the mBMSO structure, indicating that the strength of the electron-lattice coupling is higher in monoclinic BMSO than in tetragonal BMSO.
4 Conclusions
We investigated the luminescence properties of M2MgSi2O7:Eu2+ (M=Ca, Sr, and Ba) as a function of crystal structure. Ca2MgSi2O7 and Sr2MgSi2O7 assumed identical tetragonal structures, whereas Ba2MgSi2O7 assumed a monoclinic structure. We prepared solid solutions of Sr2MgSi2O7 and Ba2MgSi2O7, which enabled us to estimate the properties of tetragonal Ba2MgSi2O7 by maintaining a tetragonal structure up to a composition of Ba1.6Sr0.4MgSi2O7.
The excitation and emission spectra of the samples showed systematic changes with respect to the alkali earth metals used and the Ba concentration in Ba x Sr2−x MgSi2O7. These changes agreed with predictions based on the covalency and crystal field strength. The trend was determined by the electronegativities and radii of the metal ions, as long as the tetragonal structure was maintained.
Even when the crystal structure transitioned to a monoclinic phase as the Ba content was increased beyond 1.8, the excitation spectrum of the monoclinic phase could be explained in terms of the covalency, site symmetry, and crystal field strength. However, the emission spectrum, which displayed a large red-shift, could not be explained by these factors, and differences in the Stokes shifts of the tetragonal and monoclinic structures were invoked. Including consideration of the effect of Stokes shift, the characteristics of the emission spectrum from the monoclinic phase also could be well explained.
References
E. Fred Schubert, Light-Emitting Diodes (Cambridge University Press, Cambridge, 2003)
S. Shionoya, W. Yen, Phosphor Handbook (CRC Press, Boston, 2000)
B. Blasse, B. Grabmaier, Luminescent Materials (Springer, Berlin, 1994)
P. Atkins, T. Overton, J. Rourke, M. Weller, F. Armstrong, Inorganic Chemistry (Oxford University Press, New York, 2006)
D. He, Y. Shi, D. Zhou, T. Hou, J. Lumin. 122–123, 158 (2007)
M. Zhang, J. Wang, W. Ding, Q. Zhang, Q. Su, Opt. Mater. 30, 571 (2007)
T. Aitasalo, J. Hölsä, T. Laamanen, M. Lastusaari, L. Lehto, J. Niittykoski, F. Pellé, Suppl. Issues Z. Kristallogr. 23, 481 (2006)
P. Dorenbos, Phys. Rev. B 65, 235110 (2002)
S. Poorr, G. Biassr, J. Lumin. 72–74, 247 (1997)
G. Blasse, Photochem. Photobiol. 52, 417 (1990)
G. Dieke, H. Crosswhite, H. Crosswhite, Spectra and Energy Levels of Rare Earth Ions in Crystals (Wiley, New York, 1968)
Y. Liu, C. Shi, Mater. Res. Bull. 35, 689 (2000)
C. Wickleder, J. Alloys Compd. 374, 10 (2004)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, T., Kim, Y. & Kang, S. Luminescence properties of Eu2+ in M2MgSi2O7 (M=Ca, Sr, and Ba) phosphors. Appl. Phys. B 106, 1009–1013 (2012). https://doi.org/10.1007/s00340-012-4914-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-012-4914-z