Abstract
In this manuscript, we demonstrate high-speed (10-kHz-acquisition rate) planar laser-induced fluorescence (PLIF) imaging of formaldehyde (CH2O) in turbulent non-premixed flames. Using the unique pulse-burst laser system (PBLS) at Ohio State University, high-energy laser pulses (∼100 mJ/pulse) at 355 nm with 100 μs pulse separation are generated and used to measure the time-varying CH2O distributions in attached and lifted methane-based turbulent flames. By taking advantage of the tunable, narrow spectral linewidth of the PBLS at 355 nm, the laser output can be frequency-tuned and adjusted to overlap with absorption “peaks” within the tail of the A–X transition of CH2O near 355 nm, thus increasing the acquired signal by as much as a factor of three. The reported signal-to-noise ratio (SNR) exceeds 55, which represents one of the highest SNR reported to date for kilohertz-rate imaging of scalars for comparable spatial resolution. Potential applications and pairings with other diagnostic approaches for high-speed reaction rate and multi-scalar imaging also are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Formaldehyde (CH2O) is an important combustion intermediate occurring within the “cool flame” region prior to the primary reaction zone within hydrocarbon-fueled systems. CH2O plays a critical role in several lower-temperature combustion processes including fuel oxidation (decomposition), auto-ignition, lifted flame stabilization, and engine knock. In addition, CH2O is conducive to laser-based measurements including laser-induced fluorescence, cavity ringdown absorption spectroscopy, and tunable diode laser absorption spectroscopy, as well as other methods as discussed in Refs. [1] and [2]. For turbulent flames, planar measurements are highly desired to visualize the spatially and temporally complex structure of the scalar field and thus, planar laser-induced fluorescence (PLIF) is the primary method used for CH2O measurements in turbulent environments. An additional utility of CH2O PLIF measurements is the potential for local heat release and reaction rate measurements in turbulent flames by taking the product of simultaneous CH2O and OH PLIF signals as shown previously by Paul and Najm [3]. The use of CH2O/OH imaging for examining instantaneous heat release and reaction rates in turbulent flames has been demonstrated successfully under premixed, partially premixed, and non-premixed flame conditions e.g. [4–10]. Because turbulent combustion processes are highly transient, species concentration and reaction rate measurements that are resolved in both space and time are highly desired. In addition, many of the processes in which CH2O plays an important role are not statistically stationary and thus should be characterized with a series of temporally correlated (not statistically independent) measurements. In this manner, data acquisition of scalar fields, CH2O in this case, should be at a much higher sampling rate than typical turbulent time scales (≫1 kHz).
Recently, developments in Q-switched, diode-pumped, solid-state (DPSS) laser and high-speed CMOS camera sources have permitted the extension of a subset of planar laser-based imaging methods such as OH PLIF and particle imaging velocimetry (PIV) to kilohertz repetition rates. Numerous examples and applications using these technologies have been reported in the recent review article by Bohm et al. [11]. However, it is noted that a major limitation of the commercially available DPSS lasers is that the available pulse energies at kilohertz repetition rates are limited to a few milliJoules. Thus many techniques that require higher pulse energies (and are common at low repetition rates) are not possible at high repetition rates using DPSS lasers.
To the authors’ knowledge, only two previous examples of high-speed planar formaldehyde imaging have been presented. A first demonstration of high-speed CH2O PLIF measurements was performed using the frequency-tripled output of a DPSS Yb:YAG disk laser operating at 1 kHz. Results from a series of conference proceeding papers have been presented in laminar-like “cool” flames, technical combustors, droplet combustion environments, and microgravity conditions using the 343 nm output to excite the \(\tilde{A}^{1}A_{2}\)–\(\tilde{X}^{1}A_{1},4_{0}^{3}\) transition with approximately 1–2 mJ/pulse e.g., [12, 13]. However, it is difficult to ascertain the utility of these measurements as the only results appearing in an archival journal consist of a single six-frame, 1-kHz image sequence in a laminar-like dimethyl ether flame [14] with little information concerning the signal-to-noise ratio, spatial resolution, or applicability at higher repetition rates. A more notable demonstration of high-speed CH2O imaging was performed previously at Lund University using their well-known multi-YAG laser cluster with output at 355 nm [15]. Using four double-pulsed, Q-switched Nd:YAG lasers, eight sequential laser pulses were generated with pulse energies of 25 mJ and temporal separations of 80 μs (12.5 kHz). This output was used to excite the tail of the \(\tilde{A}^{1}A_{2}\)–\(\tilde{X}^{1}A_{1},4_{0}^{1}\) transition of formaldehyde and image the CH2O distribution as a function of crank angle in a single-cylinder spark ignition engine operating under ethanol-fueled, combusting conditions. The results were quite favorable with a reported signal-to-noise ratio (SNR) of 10.
In this paper, we present recent results from our laboratory using the OSU pulse-burst laser system e.g., [16–24] to generate a series of high-energy laser pulses at 355 nm (∼100 mJ/pulse) with 100 μs separation (10 kHz). These pulse trains are subsequently used for high-speed CH2O PLIF imaging in attached and lifted turbulent methane non-premixed flames.
2 Experimental details
In this paper, we consider high-speed (kHz-acquisition rate) imaging of formaldehyde in turbulent non-premixed methane flames issuing from a circular 8-mm-diameter tube into a low-speed (0.3 m/s) co-flowing stream of air. Two different Reynolds number cases corresponding to Re=6000 and 13,500, respectively are considered. The first case represents an attached turbulent flame and the second flame condition corresponds to a stable, lifted flame. For both cases, the images are centered on the jet centerline and at x/D=8, where x is the axial position and D is the tube diameter. The Reynolds number of the second flame was determined by systematically increasing the flow rate of the methane fuel until the average observed liftoff position intersected with the laser sheet used for the high-speed CH2O imaging.
2.1 Pulse-burst laser system
The pulse-burst laser system (PBLS) at Ohio State, shown schematically in Fig. 1a, has been described in detail previously in Ref. [16] and thus will only be described briefly here. The laser system is a master oscillator, power amplifier (MOPA) design, which consists of a single-frequency (<10−3 cm−1) cw diode-pumped ring laser operating at 1064 nm serving as the primary oscillator, an electro-optic dual pockels cell pulse slicer, and a series of flashlamp-pumped Nd:YAG amplifiers. The cw laser is initially pre-amplified in a double-pass flashlamp-pumped amplifier and then formed into a “burst” of laser pulses by rapidly rotating the polarization of the pre-amplified pulse by one of two pockels cells as described by Wu et al. [25]. In the present experiment, the slicing process creates a train of 10-ns wide pulses, which are separated by 100 μs, corresponding to a repetition rate of 10 kHz. The pulse train is then further amplified by a series of four additional flashlamp-pumped Nd:YAG amplifiers, resulting in a system gain of >108. The pulse trains are limited in the present study to 1 ms, which corresponds to ten temporally sequential laser pulses and images. However, a new system is under development, which will produce a 20+ ms pulse train, corresponding to >200 temporally sequential laser pulses/images at a 10-kHz-acquisition rate.
To reduce amplified spontaneous emission (ASE) buildup in the system (which ultimately limits the total system gain), a phase conjugate mirror (PCM) is placed between amplification stages 3 and 4 [16]. The PCM is an optical cell filled with a high index-of refraction liquid (FC-75) that uses the principle of stimulated Brillouin scattering (SBS) to act as an intensity filter and break the unwanted ASE growth. When the pump beam intensity is above a minimum threshold, a coherent beam is backscattered at 180∘ and the desired high-intensity laser burst is backscattered toward the final amplifier stages, while the sources of low-intensity background (e.g., ASE) do not exceed minimum threshold and pass through the PCM cell to a beam dump. Finally, the series of 1064-nm laser pulses are frequency-tripled to 355 nm with a pair KD*P crystals, which are enclosed within a temperature-stabilized housing. In the current work, the individual energies of the 355-nm pulses are approximately 100 mJ. An example series of 355-nm pulses are shown in Fig. 1(b), where the intensity difference between the pulses is less than 10%. Previously, this system has been used to perform high-repetition-rate (10 to 50 kHz) OH and CH PLIF imaging in turbulent flames e.g., [17, 20], NO PLIF imaging in hypersonic flows [18, 22], Raman scattering measurements in isothermal turbulent jets [19], mixture fraction imaging in turbulent non-reacting flows using planar Rayleigh scattering [21], velocity measurements in high-speed flows using planar Doppler velocimetry [23], and molecular tagging velocimetry (MTV) of NO2 [24].
2.2 CH2O excitation
There are numerous excitation strategies for CH2O PLIF measurements (see Ref. [2] for a detailed listing) including \(4_{0}^{1}\) transition centered near 353 nm e.g., [4, 5, 26–28], the \(4_{0}^{3}\) transition near 339 nm e.g., [3, 29, 30], and the \(4_{1}^{0}\) transition of the so-call “hot bands” near 370 nm e.g., [31–33]. While each of these excitation strategies has their advantages and disadvantages, one of the more prevalent strategies is excitation via the \(4_{0}^{1}\) transition, and in particular the use of the output from a frequency-tripled Nd:YAG laser near 355 nm to excite the weak sidebands of this transition as reviewed by Harrington and Smyth [26] and Brackmann et al. [34]. In principle, it is possible to generate a burst of kHz-rate pulses near 339, 353, or 370 nm using an optical parametric oscillator similar to that described in Refs. [20] and [35]; however, the potential benefits of stronger absorption cross sections and lower C2 or Raman interference levels associated with these alternative excitation strategies are negated by the poorer beam quality, higher pulse-to-pulse energy fluctuations, and two orders of magnitude lower levels of pulse energy as compared to using the third harmonic output of the PBLS near 355 nm directly.
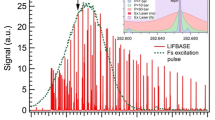
One important facet of the PBLS is that the output is spectrally narrow and tunable over a small (∼1 cm−1) frequency range. The linewidth of the second harmonic output (Δν 532) output at 532 nm of the PBLS was measured previously using a 2 GHz free spectral range confocal spectrum analyzer [35] and found to be approximately 320 MHz. The linewidth of the third harmonic output (Δν 355) is then estimated as the RMS of the linewidths of the fundamental and second harmonic beams, which is equivalent to Δν 355=1.22Δν 532 or ∼400 MHz. While not immediately obvious, this potentially is important for excitation of the sidebands of the \(4_{0}^{1}\) transition as there is a somewhat resolvable excitation spectrum for CH2O laser-induced fluorescence near the 355-nm output of the frequency-tripled Nd:YAG as shown by Brackmann et al. [36] and within our own research group when using narrow frequency laser sources. For example, Fig. 2 shows an excitation spectrum (dashed black line) of CH2O centered at 28183 cm−1 taken in our own laboratory from a ϕ=1.3 premixed dimethyl ether flame using a commercially available, tunable, injection-seeded Nd:YAG operating at 10 Hz with a spectral linewidth of ∼150 MHz. Overlaid on Fig. 2 is the estimation of the spectral distribution of the PBLS assuming a Voigt profile with a full width at half maximum (linewidth) of 400 MHz. Although the excitation spectrum of Fig. 2 shows that there is not a true “off resonance” due to congested spectral features of the CH2O absorption lines, the narrowband output of the PBLS could potentially fall between two absorption “peaks” and limit the emitted (and collected) PLIF signal. However, the PBLS can be frequency-tuned near the CH2O peaks to maximize the emitted PLIF signal. Due to the finite tuning range of the cw laser that serves as the primary oscillator, the output from the PBLS is tuned to 28182.6 cm−1 as shown in Fig. 2, which is quite near one of the two aforementioned peaks at 28182.5 cm−1.
2.3 Optical arrangement, detection, and image processing
A schematic of the optical arrangement used for these experiments is given in Fig. 3. The 355-nm output of the pulse-burst laser system (PBLS) is vertically polarized and formed into a 25×0.3 mm2 laser sheet via combination of plano-concave cylindrical and spherical lenses. The emitted fluorescence from the \(\tilde{A}^{1}A_{2}\)–\(\tilde{X}^{1}A_{1}\) electronic transition of CH2O occurs between 350 and 550 nm and is collected with a high-speed CMOS camera (Vision Research, Phantom v710) coupled to a high-speed image intensifier (LaVision Inc., HS-IRO) operating at 10,000 frames per second. The camera system is outfitted with an 85 mm f/1.4 Nikon camera lens and Schott GG385 longpass filter. The magnification is approximately 1:2.8 yielding an imaged field-of-view of approximately 57×37 mm2 (before cropping) with a camera pixel resolution of 56 μm (before binning). No attempt was made to quantify the in-plane spatial resolution of the intensified camera system, which is expected to be greater than the CMOS camera pixel resolution due to the use of an image intensifier. However, it is expected that the out-of-plane spatial resolution, as determined by the laser sheet thickness (300 μm), determines the limiting spatial resolution of the measurements. The synchronization of the camera acquisition to the PBLS output is controlled using a commercially available high-speed camera controller (HSC, LaVision, Inc.).
Although no attempt is made in this paper to place the CH2O PLIF signals on an absolute scale, it is important that the recorded signal is due to CH2O alone in order to assess the measurement quality, and in the future, use the high-speed imaging to gain insight into turbulent flame dynamics and transient processes in which CH2O plays an important role. The camera lens used for this experiment has a visible anti-reflection coating that absorbs ultra-violet light at wavelengths below 400 nm. In this manner, the camera lens itself acts as a filter for Rayleigh scattering interference at 355 nm with transmission of <10%. This, combined with the ∼104 rejection of 355-nm light when using the longpass filter renders interference from Rayleigh scattering negligible.
Other potential sources of interference arise from spontaneous Raman scattering and LIF interferences from C2 or PAH e.g., [2, 26, 34]. While the pure rotational Raman scattering is effectively rejected with the longpass filter, interferences due to vibrational Raman scattering of N2 (2330 cm−1), O2 (1556 cm−1), CO2 (1388, 1285 cm−1), CO (2145 cm−1), H2O (3657 cm−1), H2 (4160 cm−1), and CH4 (2915, 3017 cm−1) are possible in the non-premixed flames considered within this study with the current filtering scheme. However, an estimate of the degree of interference can be made by considering the product of the filter and camera lens transmission at the various vibrational frequencies, the ratio of the vibrational Raman cross sections to the Rayleigh scattering cross section of N2, the expected peak mole fractions in the measurement volume, and a calibration measurement of the Rayleigh scattering from the co-flowing air stream. Using this methodology in relation to the measured peak signal (presumably from the CH2O PLIF), it is expected that the contribution of the O2, N2, CO2, CO, H2O, H2, and CH4 Raman signals are <7% collectively, with the largest potential interference arising from CH4 (∼4%) and H2O (∼2%). This is verified using the sample results (Fig. 4) discussed in Sect. 3 by considering the collected signal in regions with no known CH2O concentrations. First, in the co-flowing region, contributions corresponding to the Rayleigh and vibrational Raman scattering of N2+O2 are measured to be <3% of the peak signal assigned to CH2O (PLIF). It is noted that the number density of N2+O2 will significantly decrease in the higher-temperature regions, thus any contribution from N2+O2 will be negligible where CH2O exists. Second, we consider the acquired signal on centerline (again where no CH2O is observed). In this region, we would expect that the major contribution to the signal would arise from CH4 Rayleigh/Raman scattering. As expected, a small signal (<5% of the peak signal) is measured on centerline. However, we note again that if this signal arises from CH4, it will decrease significantly in regions where CH2O exists due to the chemical conversion of the fuel to intermediates and products.
Other LIF interferences such as that from PAH and C2 are negligible due to (1) the fact that the measurements were taken in the non-sooting or “blue” portion of the flames, (2) the PAH LIF signal quickly saturates at very low energies (tenths of a milliJoule) relative to CH2O PLIF [26, 34]; thus for the 355-nm pulse energies used in this experiment, the resulting PAH signal should be minimal, (3) the absorption cross sections for PAH and C2 at 355 nm are relatively small in comparison to other excitations strategies, and (4) the fact that the PBLS is narrowband and “tuned” to a peak within the CH2O spectrum (see Fig. 2) near 355 nm, thus maximizing the CH2O contribution of the collected signal. With all of these factors in mind, we can conclude that the majority (>95%) of the collected signal is due to the CH2O PLIF signal alone. Finally, it is noted that by gating the image intensifier for 100 ns, flame luminosity is effectively rejected.
Following the acquisition of the PLIF signal, subtraction of the background signal (darkfield image) is performed followed by 5×5 pixel binning to increase the signal-to-noise ratio. The 5×5 binning results in an in-plane spatial resolution (<280 μm) which is nearly matched to the out-of-plane spatial resolution defined by the laser sheet thickness. Again, in this manuscript, there is no attempt to place the CH2O PLIF signals on an absolute scale, so corrections for sensor non-uniformity (i.e., “flatfield” corrections), non-uniformities in the laser sheet intensity distribution, and image intensifier charge depletion [37] are not made.
3 Sample results and discussion
Figure 4 shows an example of a full ten-frame temporal sequence of 2D CH2O PLIF images from the Re=6000 attached methane flame, with inter-pulse spacing of 100 μs and average pulse energy of ∼100 mJ/pulse. Each image is taken on centerline and represents a 20×38 mm field-of-view. As expected, the CH2O distribution is located within the preheat zone (between fuel core and high-temperature reaction zone) and although not completely distributed in space, it is quite broader than distributions of radicals such as CH which occur within the highest temperature regions. The signal-to-noise ratio (SNR), defined as the peak CH2O PLIF signal, divided by the standard deviation of the back ground signal (measured in the “fuel core” of the flame) is 55, which not only represents the highest attained for high-speed CH2O, but for kilohertz-rate PLIF imaging in general. The high SNR is most likely due to the combination of ultra-high spectral irradiance of the PBLS output (∼2×109 W/cm2⋅cm−1) and the high saturation limit of CH2O (measured as >1011 W/cm2⋅cm−1 in our laboratory).
Figure 5 shows another ten-frame image sequence of CH2O PLIF images, this time in a lifted CH4 jet flame operating at a Reynolds number of 13,500. This image sequence highlights the potential utility of high-speed CH2O PLIF imaging for studying lifted flame stabilization and/or auto-ignition (i.e., the process before the lifted flame is established and stabilized). As shown in the image sequence, the CH2O not only represents low-temperature chemistry on the fuel-side of the high-temperature reaction zone (near the jet centerline), but represents the lean reaction zones at the outer edge (air side) of the stabilized flame. Previously, at low repetition rates, Santoro et al. [38] used CH2O as a marker for an edge flame and more recently Joedicke et al. [10] used CH2O PLIF as a marker for the lean reaction zone and the low-temperature chemistry in lifted flames to help to identify the so-called triple flame structure and the corresponding “triple point”, where flame stabilization occurs. By imaging CH2O in “real time” the dynamics of flame stabilization can be examined by monitoring the time-varying nature of the intersection between the low-temperature chemistry and the lean reaction zone, identified as the most upstream portion of the CH2O distribution [10].
Potential improvements in the high-speed CH2O imaging will come from utilizing a new longpass filter with a cut-on of greater than 400 nm and horizontal polarization of the 355-nm laser pulses. In this manner, all potential Raman/Rayleigh interference would be negligible. In addition, higher pulse energies (>300 mJ) at 355 nm are expected from a next-generation pulse-burst laser system which currently is being constructed at OSU. The new PBLS is not only designed for higher pulse energies, but increased pulse-burst durations (>200 pulses/burst) as well. Finally, it is noted that the high-speed CH2O can be combined with high-speed OH PLIF (as demonstrated previously in Ref. [17]) measurements using the PBLS to measure temporally correlated image sequences of the reaction rate and heat release in turbulent non-premixed flames.
References
A.C. Eckbreth, Laser Diagnostics for Combustion Temperature and Species, 2nd edn. (Gordon and Breach, New York, 1996)
K. Kohse-Hoinghaus, J.B. Jeffries (eds.), Applied Combustion Diagnostics (Taylor and Francis, London, 2002)
P.H. Paul, H.N. Najm, Proc. Combust. Inst. 27, 43 (1998)
S. Böckle, J. Kazenwadel, T. Kunzelmann, D.-I. Shin, C. Schulz, J. Wolfrum, Proc. Combust. Inst. 28, 279 (2000)
B.O. Ayoola, R. Balachandran, J.H. Frank, E. Mastorakos, C.F. Kaminski, Combust. Flame 144, 1 (2006)
A. Fayoux, K. Zähringer, O. Gicquel, J.C. Rolon, Proc. Combust. Inst. 30, 251 (2005)
M.J. Dunn, A.R. Masri, R.W. Bilger, R.S. Barlow, Flow Turbul. Combust. 85, 621 (2010)
R.L. Gordon, A.R. Masri, E. Mastorakos, Combust. Flame 155, 181 (2008)
R.L. Gordon, A.R. Masri, E. Mastorakos, Combust. Theory Model. 13, 645 (2009)
A. Joedicke, N. Peters, M. Mansour, Proc. Combust. Inst. 30, 901 (2005)
B. Bohm, C. Heeger, R.L. Gordon, A. Dreizler, Flow Turbul. Combust. 86, 313 (2011)
W. Paa, W. Triebel, in SPIE Proceedings, Solid State Lasers and Amplifiers, ed. by A. Sennaroglu, J.G. Fujimoto, C.R. Pollack, September 2004
W. Paa, D. Mueller, A. Gawlik, W. Triebel, in SPIE Proceedings, Optical Diagnostics, ed. by L.M. Hanssen, P.V. Farrell, August 2005
A. Burkert, W. Paa, G. Schmidl, W. Triebel, Ch. Eigenbrod, Acta Astronaut. 55, 199 (2004)
J. Olofsson, M. Richter, M. Alden, M. Auge, Rev. Sci. Instrum. 77, 013104 (2006)
B.S. Thurow, N. Jiang, M. Samimy, W.R. Lempert, Appl. Opt. 43, 5064 (2005)
J.D. Miller, M. Slipchenko, T.R. Meyer, N. Jiang, W.R. Lempert, J.R. Gord, Opt. Lett. 34, 1309 (2009)
N. Jiang, M. Webster, W.R. Lempert, Appl. Opt. 48, B23 (2009)
K.N. Gabet, N. Jiang, W.R. Lempert, J.A. Sutton, Appl. Phys. B 101, 1 (2010)
N. Jiang, R.A. Patton, W.R. Lempert, J.A. Sutton, Proc. Combust. Inst. 33, 767 (2011)
R.A. Patton, K.N. Gabet, N. Jiang, W.R. Lempert, J.A. Sutton, Appl. Phys. B (2011). doi:10.1007/s00340-011-4658-1
N. Jiang, M. Webster, W.R. Lempert, J.D. Miller, T.R. Meyer, C.B. Ivey, P.M. Danehy, Appl. Opt. 50, A20 (2011)
B. Thurow, N. Jiang, W. Lempert, M. Samimy, AIAA J. 43, 500 (2005)
N. Jiang, M. Nishihara, W.R. Lempert, Appl. Phys. Lett. 97, 221103 (2010)
P. Wu, W.R. Lempert, R.B. Miles, AIAA J. 38, 672 (2000)
J.E. Harrington, K.C. Smyth, Chem. Phys. Lett. 202, 196 (1993)
S. Böckle, J. Kazenwadel, T. Kunzelmann, D.-I. Shin, C. Schulz, Appl. Phys. B 70, 733 (2000)
A. Burkert, D. Grebner, D. Müller, W. Triebel, J. König, Proc. Combust. Inst. 28, 1655 (2000)
R. Schießl, P. Pixner, A. Dreizler, U. Mass, Combust. Sci. Technol. 149, 339 (1999)
D.I. Shin, T. Dreier, J. Wolfrum, Appl. Phys. B 72, 257 (2001)
R. Bombach, B. Käppelli, Appl. Phys. B 68, 251 (1999)
R.J.H. Klein-Douwel, J. Luque, J.B. Jeffries, G.P. Smith, D.R. Crosley, Appl. Opt. 39, 3712 (2000)
J. Luque, J.B. Jeffries, G.P. Smith, D.R. Crosley, Appl. Phys. B 73, 731 (2001)
C. Brackmann, J. Nygren, X. Bai, Z. Li, H. Bladh, B. Axelsson, I. Denbratt, L. Koopmans, P.-E. Bengtsson, M. Alden, Spectrochim. Acta Part A 59, 3347 (2003)
N. Jiang, W.R. Lempert, G.L. Switzer, T.R. Meyer, J.R. Gord, Appl. Opt. 47, 64 (2008)
C. Brackmann, Z. Li, M. Rupinski, N. Docquier, G. Pengloan, M. Alden, Appl. Spectrosc. 59, 763 (2005)
V. Weber, J. Brubach, R.L. Gordon, A. Dreizler, Appl. Phys. B 103, 421 (2011)
V. Santoro, A. Linan, A. Gomez, Proc. Combust. Inst. 28, 2039 (2000)
Acknowledgements
Acknowledgment is made to the Air Force Office of Scientific Research (Julian Tishkoff/Chiping Li—Technical Monitors) and to the Donors of the American Chemical Society Petroleum Research Fund for partial support of this research. The authors acknowledge previous financial support from NASA (Paul Danehy—Technical Monitor), the U.S. Air Force Research Laboratory—Propulsion Directorate (James Gord—Technical Monitor), the Air Force Office of Scientific Research (J. Schmisseur—Technical Monitor), and the National Science Foundation Major Research Instrumentation program for the development of the pulse-burst laser system. KNG acknowledges support from the Department of Energy (DOE) Office of Science Graduate Fellowship Program administered by the Oak Ridge Institute for Science and Education for the DOE.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gabet, K.N., Patton, R.A., Jiang, N. et al. High-speed CH2O PLIF imaging in turbulent flames using a pulse-burst laser system. Appl. Phys. B 106, 569–575 (2012). https://doi.org/10.1007/s00340-012-4881-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-012-4881-4