Abstract
A new seeded velocity measurement technique, N2O molecular tagging velocimetry (MTV), is developed to measure velocity in wind tunnels by photochemically creating an NO tag line. Nitrous oxide “laughing gas” is seeded into the air flow. A 193 nm ArF excimer laser dissociates the N2O to O(1D) that subsequently reacts with N2O to form NO. O2 fluorescence induced by the ArF laser “writes” the original position of the NO line. After a time delay, the shifted NO line is “read” by a 226-nm laser sheet and the velocity is determined by time-of-flight. At standard atmospheric conditions with 4% N2O in air, ∼1000 ppm of NO is photochemically created in an air jet based on experiment and simulation. Chemical kinetic simulations predict 800–1200 ppm of NO for 190–750 K at 1 atm and 850–1000 ppm of NO for 0.25–1 atm at 190 K. Decreasing the gas pressure (or increasing the temperature) increases the NO ppm level. The presence of humid air has no significant effect on NO formation. The very short NO formation time (<10 ns) makes the N2O MTV method amenable to low- and high-speed air flow measurements. The N2O MTV technique is demonstrated in air jet to measure its velocity profile. The N2O MTV method should work in other gas flows as well (e.g., helium) since the NO tag line is created by chemical reaction of N2O with O(1D) from N2O photodissociation and thus does not depend on the bulk gas composition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Accurate and reliable velocimetry measurements are essential in our understanding of the fluid-mechanical and thermochemical properties of experimental gas flow fields. Nonintrusive velocimetry methods have been developed that do not perturb the interrogated flow field and provide a high degree of accuracy. Most quantitative laser methods for gas flow velocity measurements are based on scattering of light from particles seeded in the gas flow such as particle imaging velocimetry [1]. With the large light-scattering cross section of particles, particle-based methods can provide very accurate velocity measurements. However, in high-speed flows, the particle seed does not always track the gas velocity leading to errors [2].
Laser-based nonintrusive velocity measurements have been developed without the addition of particles. These molecular velocity methods sometimes measure the Doppler shift of the molecularly scattered light [3]. These are particularly useful in high-speed compressible flows where the Doppler shift is large. In the resonantly excited Doppler shift methods, the fluorescent emission is measured with respect to the laser excitation wavelength to monitor the Doppler frequency shift. For example, Doppler shifts of sodium [4], iodine [5], copper [6], nitric oxide [7] and hydroxyl [8, 9] have been used to measure the gas flow velocity. The Doppler shift of Rayleigh-scattered laser light has been measured with Fabry–Pérot interferometers and molecular filters to give the velocity field [10, 11]. Doppler shift methods are often used in high-speed flows but are inaccurate at low velocities because the Doppler shift is extremely small.
Molecular tagging velocimetry methods are able to track both low and high velocities; they measure velocity by time-of-flight by writing a tag line into the flow and recording its displaced position after a fixed time delay. Molecular tagging methods have been developed based on oxygen in air. In the RELIEF method, O2 is vibrationally excited by stimulated Raman to the v=1 excited state that is a long-lived molecular tag in air [12]. Because of the non-linear writing process, very small grids can be written to resolve small turbulence scales [12], but large grids are problematic. In ozone tagging velocimetry (OTV), O2 is photodissociated to O atoms that then react to O3 in about 20 μs under atmospheric standard conditions [13]. After a time delay, a second laser reveals the displaced O3 tag line position by causing photodissociation of O3 and subsequent fluorescence of the vibrationally excited O2 photoproduct. Use of the OTV technique in low-temperature air flows, writing as many as six intersecting lines of 20–40 mm length, has been described and demonstrated [13–15]. The OTV method is well suited for low-temperature flows; however, at high temperatures, both the tag line lifetime and the peak concentration of O3 are reduced [14, 15]. Also, the formation time of ∼20 μs is generally too long for integration of high speed flows.
MTV methods have been developed based on photodissociation of water in gas flows to produce an OH tag. Boedecker photodissociated H2O with 2-photons of 248 nm light to produce an OH tag line [16]. Single-photon photodissociation of H2O with a 193 nm ArF laser was used to measure the line-of-sight averaged velocity in a shock tube by recording the displaced line position with UV absorption [17]. Hydroxyl tagging velocimetry (HTV) has been developed where a 193-nm ArF laser photodissociates H2O in a single-photon process to produce a large grid (up to 11×11 lines) of OH that is imaged by a second laser to determine the velocity in low- and high-temperature reacting flows in a two-dimensional plane [15, 18–22]. The HTV method has been used to measure supersonic flows in wind tunnels [20, 21] and rocket exhausts [22]. Although the OH tag can be long-lived at high temperature [15, 18], at room temperature the OH lifetime is about 20 μs [19], limiting low-temperature application to moderate- and high-speed flows. Below freezing, the water vapor concentration is insufficient to implement the HTV method.
Nitric oxide is a very stable MTV tag that has been used in both low- and high-temperature gas flows [23–31]. In low-pressure high-speed flows, NO can be naturally present (e.g., arc-heated tunnel) or directly added to the gas. When the NO is electronically excited along a line in this low quenching rate environment, the NO fluorescence lifetime is sufficient for the laser line displacement to be tracked in time [23–25]. In air flow without NO addition, a highly focused ArF laser beam can create photochemically produced NO along line [26]. Other MTV methods seed the flow with molecules that are photodissociated to form the NO tag. NO2 has been added and photodissociated to form either ground-state NO [25, 27–30] or vibrationally excited NO that can be imaged in a background of ground-state NO [25, 29]. Finally, tert butyl nitrate has been photodissociated to form an NO grid in a gas flow to measure velocity in 3D [31]. All of these NO tag MTV methods generally require the addition of a toxic gas [e.g., NO, NO2, (CH3)3CONO] to the flow, limiting their application due to safety concerns.
In this study, a new N2O MTV is developed based on photodissociation of N2O to chemically produce a stable NO tag. Nitrous oxide “laughing gas” is a non-toxic gas that is commonly inhaled as a mild anesthetic in dentistry and surgery or used as an aerosol agent in foods. N2O has a low boiling temperature (−88.5∘C @ 101.3 kPa) and can be added to low-temperature, high-speed flows without condensation. Using a 193-nm ArF excimer laser, NO is photochemically created from N2O; the initial and displaced NO lines are imaged with laser-induced fluorescence. The NO formation time is measured in the laboratory and compared to chemical kinetic simulations. Also, the effect of the initial temperature and pressure on the NO formation time and concentration is investigated. Finally, N2O MTV is applied to an air jet to measure its velocity profile.
2 Experimental system
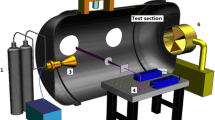
Experiments were conducted in a jet flow of air seeded with 4% N2O gas issuing from 12.5 mm diameter inner tube of a ∼25 mm circular Hencken burner. The central 12.5 mm inside diameter tube of the Hencken burner contains a honeycomb matrix with a micro-tube inserted in each honeycomb channel. When it is used as burner, oxidizer is fed into the honeycomb channels, the fuel is fed into the micro-tubes and they mix at the burner exit. In this work, the air and N2O gases were mixed well upstream of the burner and the mixture was fed into the oxidizer input. The burner also has a 4-mm annulus for a shield gas which was not used. The air and N2O gas flow rates were monitored with Teledyne-Hastings HFM-201 flow meters (accurate to 1% full scale) to produce the jet flow of a mixture of air and N2O gases at the surface of the burner at atmospheric pressure (101 kPa).
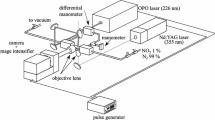
A schematic of the experimental system is shown in Fig. 1. The N2O molecular tagging velocimetry optical system consists of two pulsed laser light sources. The “write” laser is a 193-nm, ArF broadband excimer laser (Lambda Physik COMPex-Pro 50F) with unstable resonator optics (0.4×0.4 mrad far field divergence), 0.5 nm bandwidth, 20 ns duration and 30 mJ/pulse. The ArF laser is focused 20 mm above the surface of a Hencken burner with 0.5 m focal length spherical lens to write tag line of 0.47 mm diameter across the flow field. The 193-nm light travels about 2.5 m in air from the laser to the jet flow and O2 absorption reduces the ArF laser energy to 5 mJ in the measurement volume.
The “read” laser system consists of a frequency-doubled Continuum Powerlite Nd:YAG laser (420 mJ @ 532 nm) pumping a Continuum ND6000 dye (Rhodamine 590) laser whose output (35 mJ @ 574 nm) is frequency doubled to 287 nm. The 287-nm light is then frequency mixed with residual 1064 nm IR from the Nd:YAG laser to produce 0.8 mJ of tunable light at ∼226 nm. The 226-nm laser light is focused by a 0.3 m f.l. cylindrical lens to a sheet of 12 mm by 0.5 mm cross section.
NO fluorescence images are collected with a multi-element UV lens (Nikon UV-Nikkor, f/4.5, 105 mm) coupled to a Princeton Instruments PI-MAX ICCD camera (1024×1024 pixels) typically gated for 50 ns. Because of the broad range of NO fluorescence (230–300 nm), a Schott UG-5 filter (3 mm thick) was fitted to the camera to create a band pass around 260 nm, the region of NO fluorescence [32, 33]. Timing for all systems was controlled by 4-channel Stanford Research DG535 pulse generators and monitored with a Tektronix 4-channel 200 MHz oscilloscope.
3 N2O MTV photochemistry
A conceptual drawing of N2O MTV technique is shown in Fig. 2. The air is seeded by 4% N2O gas by volume. A pulse of 193-nm light from an ArF excimer laser writes a line of NO in the air–N2O mixture flow where the NO line is written by two sequential reactions [34]:


In reaction (R1), a single photon of 193-nm light photodissociates N2O into N2 and O(1D) with a cross section of σ 1=8.95×10−20 cm2/molecule at 302 K [35]. The NO formation rate is governed by the reaction rate of reaction (R2), where k 2=7.2×10−11 cm3/molecule-s [34]. The photochemical tagged line is convected downstream and is read by 226-nm light sheet at a prescribed time-of-flight delay.
4 NO tag spectral identification, formation, and lifetime
Figure 3 shows the simulated and measured fluorescence excitation spectra for NO flow tag measured in air jet seeded with 4% N2O gas (297 K) for over a ∼0.5-nm range of tunable dye laser. The dye laser does have a wider tuning range; however, to keep the dye laser output relatively strong (and close to its peak value), it was tuned over a ∼0.5-nm range, between ∼226.23 nm (44202 cm−1) and ∼226.69 nm (44113 cm−1). The spectral signature of NO-LIF is clearly identified in this excitation and transitions are assigned by comparing the experimental results (Fig. 3b) to a simulated spectrum (Fig. 3a) for the same initial conditions using the LIFBASE software (version 2.0.63, 2011). As seen from the measured and simulated spectra, the Q1(1) (44197.86 cm−1 or 226.255 nm) is the strongest line to pump at 297 K.
The dissociated N2O fraction can be calculated by [15]:
in the limit of weak absorption (σ i n i L<1) where in the ith vibrational level, n i is the number density of N2O, n di is the number density of dissociated N2O, σ i is the absorption cross section, E is the laser pulse energy, h is Planck’s constant, υ is the frequency of light, L is the length of the N2O region through which the laser beam passes, and A is the area of the laser beam. At room temperature (300 K), ∼85% of the N2O molecules are in the ground vibrational state with three fundamental frequencies (υ 1,υ 2,υ 3) [35]. For 193-nm, the total absorption cross section is σ i =8.95×10−20 cm2 at 302 K [35], and leads to about 25% dissociation of N2O gas in 4% N2O–air mixture for typical experimental conditions (5 mJ/pulse, 0.470 mm dia. laser beam).
A series of single-shot images of the experimental flow field (4% N2O in air jet) were taken to determine the signal-to-noise ratio (SNR) of the tag images and the expected accuracy of the velocity measurement. Example single-shot undelayed and delayed images recorded with the 226-nm LIF are shown Fig. 4. Since there was no vibrations induced by the experimental apparatus, these images were taken at different times and they are not a linked pair. The ICCD camera images a 36 mm × 22.7 mm region (746×474 pixels) of the air jet flow; thus each camera pixel corresponds to a 48 μm × 48 μm sample of the flow field. The average maximum signal above the background (S p ) and average background noise level (N p ) were determined from a 4.7-mm (98 pixel) length of the tag line for 100 single-shot images taken at each condition. The statistics for average signal and noise are given in Table 1.
N2O MTV single-shot images in a 4% N2O–air jet flow at room temperature taken ∼2 diameters downstream of the jet exit. Flow is moving vertically with inclined “write” laser line and “read” laser sheet. Write laser: ArF excimer at 193 nm. Read laser: mixing after doubling dye laser at 226 nm. Delays: a 0 μs; b 80 μs. Average signal, noise, and signal-to-noise statistics for these conditions are given in Table 1
In the undelayed image (0 μs), the maximum average signal (S p =3486 counts) is very strong and dominated by ArF laser-induced O2 fluorescence [36] that is transmitted through the Schott UG-5 filter onto the camera detector. As will be seen below, NO is formed in <10 ns and ArF laser pulse is 20 ns. Thus some of the fluorescence seen in Fig. 4a could be coming from 193-nm excitation of the D←X(0,1) band of NO [37]. The 226-nm read sheet was not needed to locate the initial line position. In the delayed image (80 μs), the maximum signal is much lower (S p =79 counts) and is due to the weaker NO fluorescence induced by the 226-nm laser sheet. The signal-to-noise ratio (SNR) is defined as S p /N p where N p is peak-to-peak background noise estimated by 4σ (σ is standard deviation of the background noise). The SNRs for undelayed and delayed images are SNR=7.5 and SNR=3.5, respectively. For a delayed image with SNR=3.5, we expect to be able to determine the displacement of a NO tag grid intersection to 0.1–0.2 pixel accuracy using either template matching or cross-correlation methods [38]. In the gas flow of ∼15 m/s with a time delay of 80 μs, the tag line displaces about 1 mm (21 pixel) and one would expect a velocity accuracy of <1%.
By double-pulsing the intensifier, both of the images shown in Fig. 4 could be imaged together on the ICCD camera; the initial line located by 193-nm induced O2 fluorescence and the delayed line located by 226-nm induced NO fluorescence. Only two lasers are needed. For large wind-tunnel experiments where there is significant vibration, this method could be employed to reduce the deleterious effects of vibration.
To determine the experimental NO PPM level in the tagging experiments, we calibrated the NO LIF system using a calibration bottle of 1000 PPM of NO in nitrogen and the results are shown in Fig. 5. The air jet is seeded with various levels of NO and the NO LIF signal is measured. For the tagging experiments, these are the maximum NO LIF signals obtainable from the NO tag line when the write laser line and read sheet are perfectly overlapped.
Next we determined the NO formation time and the effect of delay time on the NO tag signal strength. The strength of the NO tag “read” signal depends on the initial mole fraction of N2O present in the air, the percentage of N2O dissociated by the write beam, the chemical reaction rates, and the delay time between the write and read laser pulses. The signals of a typical laser line segments (4.7 mm, 98 pixels) in the single-shot NO tag images were integrated and averaged at various times to determine the time of formation and the lifetime of the integrated signal. A value for background (fairly constant from shot-to-shot) was subtracted from a corresponding total signal to yield experimental points on the curve shown in Fig. 6.
Normalized N2O MTV signal intensity (points) for 100 image average, and NO concentration (lines) are plotted versus time delay (a, μs) and (b and c, ns) between the write and read lasers for room air temperature (297 K, 38% RH, 1 atm) with 4% N2O seeded in air. The CHEMKIN predictions are normalized by 1000 PPM and calculated for 25% dissociation of N2O including humid air with and without H2O dissociation. Experimental measurements are averaged and normalized by the NO LIF signal intensity for 750 PPM of NO (see Fig. 5)
To avoid the strong O2 LIF signal from the ArF write laser, the first NO tag signal strength measurement was taken 50 ns after the ArF laser pulse (the shortest gate time width of the ICCD camera is 50 ns). As seen in Fig. 6, the predicted NO formation time is less than 50 ns. The formation time and lifetime of NO tag were simulated at constant pressure and enthalpy using CHEMKIN thermodynamic database (version 10101) and chemical kinetics solver. The air-chemistry reaction mechanism of reference [19] was modified by adding the following reactions with reaction rates given in [34, 39–43] using units as in reference [19].Footnote 1
A constant pressure adiabatic reactor system was simulated that initially contains an equilibrium humid air mixture and N2O to which is added a certain amount of O(1D). As calculated above, 25% of the 4% of N2O gas is photodissociated into O(1D) and N2 according to the reaction (R1) by the ArF write laser.
The effect of H2O dissociation by the ArF excimer laser in the N2O–air mixture was included by calculating the dissociated H2O fraction in the limit of weak absorption using (1). At room temperature (300 K), all the H2O molecules are in the ground vibrational state with three fundamental frequencies (ν 1,ν 2,ν 3) for its three modes: symmetric stretch (ν 1=3651.7 cm−1), symmetric bend (ν 2=1595.0 cm−1), and asymmetric stretch (ν 3=3755.8 cm−1). For 193-nm, the ground state (0, 0, 0) absorption cross section is small, 8×10−22 cm2 [44]. With room air conditions (298 K and 38% RH), 0.22% of the H2O vapor will be dissociated leading to a OH number density of ∼6.1×1014 molecules/cm3 (25 ppm).
Figure 6 shows a comparison between the CHEMKIN predictions and measurements for NO concentration (with either 0 or 0.22% dissociation of the H2O content) with initially 25% of N2O being dissociated into O(1D) and N2. Both are normalized to one at zero time and plotted as a function of delay time between the write and read lasers. The results show a good agreement between the normalized experimental results and the predictions up to 2 μs delay time. As indicated in the calibration curve shown in Fig. 5, the experimentally determined NO tag signal intensity at zero time delay corresponds to 750 PPM which compares to the simulation prediction of 1000 PPM as seen in Fig. 6. The lesser amount of NO measured in the experiment is attributed to the imperfect overlap of the tag line and the read sheet in the experiments. The dissociation of H2O in the humid air has no significant effect on the NO concentration simulation. As can be seen from Figs. 6b–c, in all cases (with and without H2O dissociation) the NO is rapidly formed in about 10 ns.Footnote 2 The NO tag is very stable and lasts for a long time. The NO concentration after 100 ms delay is 520 PPM (not shown in Fig. 6).
The 193 nm laser absorption will heat the gas and the N2O MTV method is not completely nonintrusive. In the CHEMKIN predictions, the gas temperature increased 30–40 K due to recombination after N2O and H2O dissociation by the ArF laser. The dissociation of O2 by the broadband ArF excimer laser will also heat the gas about 30 K based on previous work [13]. Thus the total heating effect due to 193-nm laser absorption is estimated to as much as 50–60 K.
Since NO is formed from the reaction of O(1D) with N2O (R2), the NO concentration will depend the percentage of the N2O dissociated; both 0 and 100% N2O dissociation will give zero NO. Figure 7 illustrates the prediction of the NO concentration as a function of the percent of N2O dissociated. The results indicate that the NO concentration (and hence, the NO signal) is maximum at 50% N2O dissociation. In the current experiments, we estimated that 25% of the N2O was dissociated. Based on Fig. 7, the NO levels and corresponding signal levels in the tag line could be substantially increased by dissociating 50% of the N2O.
To determine the effect of temperature on the NO tag time of formation and the tag lifetime, the NO time histories were simulated a dry N2O-air mixture at several different initial temperatures ranging from 190 to 750 K at one atmosphere pressure (see Fig. 8). For the initial 4% N2O in the dry air mixture, 25% of the N2O is assumed to be dissociated into O(1D)+N2. Increasing the initial mixture temperature from 190 to 750 K, the initial NO concentration is increased by 33.3% from 800 to 1200 PPM. At low initial mixture temperature, there is a ∼15% surge in NO concentration at short times (<1 μs) that then relaxes to a nearly constant value.
Figure 9 shows the effect of gas pressure on the NO tag-time relation, where the NO time relation was simulated at different gas pressures (0.25 to 1 atm) for the two gas temperatures of 190 and 298 K, under the same 25% photodissociation of N2O in the dry air mixture. The results show that at the lower gas temperature of 190 K, decreasing the gas pressure increases the initial NO concentration and gives a more stable NO lifetime (Fig. 9a). At higher pressure (0.75–1.0 atm), the NO concentrations show a slight decline at a time delays greater than 30 μs. At a higher gas temperature of 298 K (Fig. 9b), the results are qualitatively the same but decreasing the pressure leads to a lower enhancement of the NO concentration. These chemical kinetic simulations indicate that the N2O MTV method can be used to measure velocity in low-pressure (0.25 atm) and low-temperature (190 K) high speed wind tunnels.
5 N2O MTV imaging and velocity measurement in an air jet
Tagging measurements were made in the 4% N2O in air jet created by the inner tube (12.5 mm diameter) of a small Hencken burner nozzle (∼25 mm overall diameter including a 4-mm co-flow annulus with zero gas velocity) emanating into a quiescent surrounding environment at 297 K and 1 atm. The measured flow rates were 96 slpm of air and 3.8 slpm of N2O where the mass flow meters record standard liters per minute at 0∘C and 1 atm. This yields an average jet exit velocity of U ave=15±0.5 m/s based on the uncertainty in the mass flow meter readings and the actual jet exit inner diameter. Variable time delays between the write and read lasers were used to study the NO tag’s lifetime. A single tag line was created by the 193-nm write laser beam focused by 500 mm f.l. lens. This line is imaged after a time delay by the 226 nm read laser sheet to determine displacement. Figure 10 shows typical average images of 100 single-shot images of the undisplaced (0 μs) and displaced (80 μs) N2O MTV tag lines. This is for a location immediately downstream from the nozzle’s exit (∼2 diameters) near the air jet’s centerline and the flow is vertically upward. Running left to right in Fig. 1, the 193-nm “write” laser line is slightly inclined downwards and the 226-nm laser “read” sheet is inclined upwards. These inclinations do not affect the ability to measure the vertical displacement of the line.
N2O MTV averaged images in 4% N2O–air jet flow at room temperature taken ∼2 diameters downstream of the jet exit. 100 single-shot images are averaged. Flow is moving vertically with inclined “write” laser line and “read” laser sheet. Write laser: ArF excimer at 193 nm. Read laser: mixing after doubling dye laser at 226 nm. Delays: Delays: a 0 μs; b 80 μs. Average signal, noise, and signal-to-noise statistics for these conditions are given in Table 1
Based on the initial and final locations of the tag lines and knowing the time delays, one can establish velocity profiles assuming the gas flow is vertical. Figure 11 shows an example of the velocity profile at ∼2 diameters downstream of the jet exit for flow rates of 96 slpm of air and ∼3.8 slpm of N2O using a time delay of 60 μs. The multi-element Hencken burner produces a slightly asymmetric profile. The velocity average over the potential core of the jet (±3 mm) is 15.4 m/s which is in agreement with the expected average jet exit velocity of 15±0.5 m/s based on the mass flow meter readings. For future work, we would write grids into the gas flow which would allow us to measure the velocity in two dimensions and one component of vorticity [38].
Radial profile of axial velocity of jet flow issuing from inner nozzle (12.5 mm diameter) of Hencken burner, downstream from the nozzle’s exit (∼2 diameters) using a 60 μs time delay. The average bulk velocity is 15±0.5 m/s based on the mass flow meter measurement (96 slpm of air and 3.8 slpm of N2O)
6 Summary and conclusions
The N2O MTV method is a new nonintrusive instantaneous molecular diagnostic for measurement of velocity in either low- or high-temperature flow fields in humid or dry air with a NO tag photochemically produced from the non-toxic N2O seed. A 193-nm ArF excimer laser photodissociates a fraction of the N2O seed into O(1D) that subsequently reacts with N2O to form a tag line of NO molecules. O2 fluorescence induced by the 193-nm laser writes the original position of the NO line. A sheet of light from a 226-nm tunable dye laser reads the final position of the tagged line after a time of delay. Recording the fluorescence from the initial and final lines on an ICCD camera gives the velocity. Based on a detailed chemical kinetic simulation, the NO rapidly forms (∼10 ns) to chemically stable levels in dry and humid air. Dissociation of the H2O in humid air into OH+H due to the 193-nm laser has no significant effect on the NO concentration level. The NO tag mole fraction (PPM) level is nearly constant over the pressure range of 0.25–1.0 atm at 300 K and temperature range of 190–750 K at 1 atm. The NO mole fraction has a moderate increase with increasing initial temperature (190–750 K) or with deceasing gas pressure (1.0–0.25 atm). The time-of-flight for the N2O MTV method can be very short or long (∼100 ns≤Δt≤∼100 ms), making accurate measurement of low- and very high-velocity flows possible. N2O MTV is demonstrated in an air jet to measure its velocity profile. The single-shot images of the undelayed and delayed tag lines have signal-to-noise ratios of 7.5 and 3.5, respectively. This should lead to a ∼1% velocity measurement using template matching data analysis. The signal-to-noise of the delayed tag line can be improved by increasing N2O dissociation from 25 to 50% which is the optimal value based on the chemical kinetic simulations. Although the technique is demonstrated in air, N2O MTV can be applied to tunnels using other gases such as high-speed helium tunnels. The NO tag line is formed by chemical reaction of N2O with O(1D) created by N2O photodissociation and thus does not depend directly on the bulk gas chemical composition.
Notes
k=AT nexp(−E/RT), reaction rates for A are in [cm3-mol-sec-K] units, T [K], E [cal/mol].
NO formation time shown in Fig. 6c is consistent with an estimate based on the (R2) rate constant of k 2=7.2×10−11 cm3/molecules [34] under standard conditions. With this rate constant, the time of formation of 63% of the steady-state NO concentration (at STP) is \(1/(2k_{2}n_{\mathrm{N}_{2}\mathrm{O}}) \approx 10\ \mbox{ns}\) where \(n_{\mathrm{N}_{2}\mathrm{O}}\) is the N2O number density after dissociation.
References
R.J. Adrian, J. Westerweel, Particle Image Velocimetry (Cambridge University Press, New York, 2010)
S. Koike, H. Takahashi, K. Tanaka, M. Hirota, K. Takita, G. Masuya, AIAA J. 45, 2770 (2007)
R.B. Miles, W.R. Lempert, J.N. Forkey, Meas. Sci. Technol. 12, R33 (2001)
M. Zimmermann, R.B. Miles, Appl. Phys. Lett. 37, 885 (1980)
J.C. McDaniel, B. Hiller, R.K. Hanson, Opt. Lett. 8, 51 (1983)
W.J. Marinelli, W.J. Kessler, M.G. Allen, S.J. Davis, S. Arepalli, C.D. Scott, in 29th AIAA Aerospace Sciences Meeting (1991), paper 91-0358
P.H. Paul, M.P. Lee, R.K. Hanson, Opt. Lett. 14, 417 (1989)
M. Allen, S. Davis, W. Kessler, H. Legner, K. McManus, P. Mulhall, T. Parker, D. Sonnenfroh, AIAA J. 32, 1676 (1994)
K.G. Klavuhn, G. Gauba, J.C. McDaniel, J. Propuls. Power 10, 787 (1994)
R.G. Seasholtz, F.J. Zupanc, S.J. Schneider, J. Propuls. Power 8, 935 (1992)
J.N. Forkey, N.D. Finkelstein, W.R. Lempert, R.B. Miles, AIAA J. 34, 442 (1996)
R.B. Miles, W. Lempert, B. Zhang, Fluid Dyn. Res. 8, 9 (1991)
R.W. Pitz, T.M. Brown, S.P. Nandula, P.A. Skaggs, P.A. DeBarber, M.S. Brown, J. Segall, Opt. Lett. 21, 755 (1996)
L.A. Ribarov, J.A. Wehrmeyer, F. Batliwala, R.W. Pitz, P.A. DeBarber, AIAA J. 37, 708 (1999)
R.W. Pitz, J.A. Wehrmeyer, L.A. Ribarov, D.A. Oguss, F. Batliwala, P.A. DeBarber, S. Deusch, P.E. Dimotakis, Meas. Sci. Technol. 11, 1259 (2000)
L.R. Boedecker, Opt. Lett. 14, 473 (1989)
D.F. Davidson, A.Y. Chang, M.D. DiRosa, R.K. Hanson, Appl. Opt. 30, 2598 (1991)
J.A. Wehrmeyer, L.A. Ribarov, D.A. Oguss, R.W. Pitz, Appl. Opt. 38, 6912 (1999)
L.A. Ribarov, J.A. Wehrmeyer, R.W. Pitz, R.A. Yetter, Appl. Phys. B 74, 175 (2002)
R.W. Pitz, M.D. Lahr, Z.W. Douglas, J.A. Wehrmeyer, S. Hu, C.D. Carter, K.Y. Hsu, C. Lum, M.M. Koochesfahani, Appl. Opt. 44, 6692 (2005)
M.D. Lahr, R.W. Pitz, Z.W. Douglas, C.D. Carter, J. Propuls. Power 26, 790 (2010)
M.C. Ramsey, R.W. Pitz, T.P. Jenkins, Y. Matsutomi, C. Yoon, W.E. Anderson, Shock Waves 22, 39 (2011)
A.F.P. Houwing, D.R. Smith, J.S. Fox, P.M. Danehy, N.R. Mudford, Shock Waves 11, 31 (2001)
P.M. Danehy, S. O’Byrne, A.F.P. Houwing, J.S. Fox, D.R. Smith, AIAA J. 41, 263 (2003)
A.G. Hsu, R. Srinivasan, R.D.W. Bowersox, S.W. North, Appl. Opt. 48, 4414 (2009)
N. Dam, R.J.H. Klein-Douwel, N.M. Sijtsema, J.J. ter Meulen, Opt. Lett. 26, 36 (2001)
C. Orlemann, C. Schulz, J. Wolfrum, Chem. Phys. Lett. 307, 15 (1999)
S. Nakaya, M. Kasahara, M. Tsue, M. Kono, Heat Transf. Asian Res. 34, 40 (2005)
A.G. Hsu, R. Srinivasan, R.D.W. Bowersox, S.W. North, AIAA J. 47, 2597 (2009)
N. Jiang, M. Nishihara, W.R. Lempert, Appl. Phys. Lett. 97, 221103 (2010)
S. Krüger, G. Grünefeld, Appl. Phys. B 69, 509 (1999)
U. Westblom, M. Aldén, Appl. Opt. 29, 4844 (1990)
C.D. Carter, R.S. Barlow, Opt. Lett. 19, 299 (1994)
M. Tsuji, J. Kumagae, T. Tsuji, T. Hamagami, J. Hazard. Mater. B 108, 189 (2004)
G. Selwyn, J. Podolske, H.S. Johnston, Geophys. Res. Lett. 4, 427 (1977)
G. Laufer, R.L. McKenzie, W.M. Huo, Opt. Lett. 13, 99 (1988)
A.M. Wodtke, L. Huwel, H. Schluter, G. Meijer, P. Andresen, H. Voges, Opt. Lett. 13, 910 (1988)
M.C. Ramsey, R.W. Pitz, Exp. Fluids 51, 811 (2011)
R. Atkinson, D.L. Baulch, R.A. Cox, R.F. Hampson Jr., J.A. Kerr, M.J. Rossi, J. Troe, J. Phys. Chem. Ref. Data 26, 1329 (1997)
R.F. Heidner III, D. Husain, Int. J. Chem. Kinet. 5, 819 (1973)
V.M. Doroshenko, N.N. Kudryavtsev, V.V. Smetanin, High Energy Chem. 26, 227 (1992)
A.A. Borisov, V.M. Zamanskii, G.I. Skachkov, Kinet. Catal. 19, 26 (1978)
W. Tsang, J.T. Herron, J. Phys. Chem. Ref. Data 20, 609 (1991)
M.S. van Hemert, R. van Harrevelt, private communication, Leiden Institute of Chemistry, Gorlaeus Laboratories, Leiden, The Netherlands (1999)
Acknowledgements
Dr. Ayman M. ElBaz is grateful to the Fulbright Egyptian Scholar Program for supporting his research experience at Vanderbilt University. RWP acknowledges support by the Air Force Office of Scientific Research, Combustion and Diagnostics Program. The authors thank Dr. Campbell Carter at the Air Force Research Laboratories for the suggesting photodissociation of N2O as a possible MTV method. We also thank Mr. Marc Ramsey at Vanderbilt University for technical advice and help in this effort.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
ElBaz, A.M., Pitz, R.W. N2O molecular tagging velocimetry. Appl. Phys. B 106, 961–969 (2012). https://doi.org/10.1007/s00340-012-4872-5
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-012-4872-5