Abstract
With the continuous utilization of green energy resource, energy storage devices are becoming one of the main issues in the field of energy. Supercapacitors have achieved great popularity owing to their several advantages in energy storage. For determining the efficiency of the supercapacitor, the electrode material is considered as the core element. Herein, mesoporous nanostructure of nickel ferrite (NiFe2O4) immobilized on boron, nitrogen and fluorine tri-doped CeO2 (NFTDNC) was prepared and scrutinized as supercapacitor material. The structural and compositional study of the synthesized material was done using characterizations, such as SEM, XRD, TEM, XPS and FTIR. The synthesized NFTDNC electrode demonstrates good electrochemical performance and shows 81.81% capacitance retention after 3000 GCD cycles. The achieved results verify the capability of the prepared NFTDNC electrode to be efficiently employed as cathode in the fabrication of high-performance supercapacitors. Moreover, the proposed material offers new opportunities in the domain of energy storage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the present era, the concept of sustainable development intrigued scientists the most. They are continuously striving towards it by refining the existing technologies and materials for the same [1]. The continuously growing population needs task-specific materials for realizing their needs. Traditional routes for the generation of energy lead to problems such as fossil fuel depletion along with affecting climatic and environmental conditions. Therefore, continuous efforts are made to find an alternate route for sustainable and clean energy production [2]. Researchers worldwide are trying to make advanced energy devices with improved capacity to address the expanding energy concerns of the world. For many years, batteries and capacitors-based technological devices served as essential components for electrical energy storage. However, drawbacks such as inadequate energy and power density make them inferior to renewable energy systems [3]. Supercapacitor, one of the highly promising devices for storing renewable energy offers benefits including high power density, a quick charging, a high rate capacity, as well as good cyclic performance [4]. Numerous efforts are made to find suitable active material for increasing the specific power and capacitance of the device. Materials such as RuO2, MnO2, NiCo2O4, NiO, and Co3O4 with high power as well as high specific capacitance, are favoured for the fabrication of supercapacitors [5]. CeO2, with significant chemical stability, outstanding redox properties and minimal toxicity acted as electrode in a variety of acidic and alkaline electrolytes [6]. Nanomaterials due to their distinctive physical and chemical characteristics, are potential applicants for assembling electronics supercapacitors. When supercapacitor is engineered at nano-level, its nanoscale dimensions influence displayed an increase in activity owing to high volume to surface ratio as well as quantum confinement phenomena [7]. CeO2 was modified with dopants for generation of new active sites in addition to the primary sites, introducing the crystal strain along with lowering of activation energy [8]. The effect of co-doping in the crystal lattice of CeO2 influencing its specific capacitance is under investigation. Ionic liquids (ILs), also known as “neoteric solvents” and “liquid salts,” are polar organic salts with unique properties; serve the dual purpose as template as well as doping agents resulting in the formation of a desired nanomaterial. They act as a versatile doping agent by offering dopants such as B, N, F, P and S simultaneously [9]. Nickel ferrite, a soft magnetic material having unique properties such as high specific capacitance, low operating potential, economical as well as non-toxicity is frequently utilized as rectangular hysteresis material, microwave absorbing material, catalyst as well as electrodes in supercapacitors [10].
In this study, we report the synthesis of mesoporous nanostructure of NiFe2O4 immobilized on B, N and F tri-doped CeO2. The synthesized material was pasted on flexible conductive fabric and scrutinized for its structural, compositional and electrochemical properties as supercapacitor electrode material. The electrochemical performance was investigated in 6 M KOH electrolytic solution employing three-electrode configuration.
2 Experimental
2.1 Materials
Butyl-4-methylpyridinium tetrafloroborate (≥ 97) was procured from Sigma-Aldrich. Anhydrous (FeCl3, 98%) and nickel chloride hexahydrate (NiCl2·6H2O, 97%) and hydrazine hydrate were purchased from HiMedia. Other chemicals used in the preparation of pyrazolopyranopyrimidine and 1H-pyrazolo[1,2-b]phthalazine-5,10-dione derivatives were procured from Alfa Aesar, Avra Synthesis and LobaChemie chemical company, respectively. Chemicals were used without processing.All the experimental procedure was carried out in the triply distilled water.
2.2 Characterization
Shimadzu spectrometer was used for the recording of the Fourier transform infrared (FTIR) spectra. The material was prepared via mixing sample (5 mg) with KBr (200 mg) to form pellets for measurement. The BET specific surface area was analyzed using nitrogen adsorption–desorption isotherm at 77 K by employing Belsorb Mini-X instrument. The sample was pretreated in a vacuum for 4 hat 200 °C to dehydrate the catalysts before N2adsorption. The Thermofisher Scientific Nexsa baseX-ray photoelectron spectroscope was used to analyze the X-ray photoelectron spectrum of the nanostructure. Transmission electron micrographs (TEM) were obtained on FEI, Tecnai G2, F30 transmission electron microscope having field emission gun working at 300 kV. It was also utilized to perform the EDX mapping and line scan of the sample. SEM results were obtained on an FEG-SEM-JSM-7600F. The powder X-ray diffraction (PXRD) spectroscopy was carried out on Smart lab, 9 kW rotating anode, at scanning rate of 2° min−1 from 20 to 80 with Cu Kα radiations (λ = 1.5148 Å).
2.3 Catalyst synthesis
2.3.1 Synthesis of nanoceria (NC)
In the synthesis of nanoceria [1], the solution of cerium (IV) sulfate (5.2 g) in distilled water (50 mL) was formed with constant stirring for an hour. After this, pH 10 was maintained by dropwise addition of NH3 solution followed by constant stirring for 4 h at 70 °C. The mixture was filtered under reduced pressure for obtaining a solid residue followed by heating at 600 °C for 2 h under an air atmosphere. The obtained material was grounded and repeatedly washed with 1 M HCl solution followed by distilled water. Then, it was dried for 12 h at 50 °C using an oven.
2.3.2 Synthesis of tridoped nanoceria (TDNC)
For tri-doping of ceria, ionic liquid 1-butyl-4-methylpyridinium tetrafloroborate (1 g) was added to the already prepared nanoceria (5 g) in a silica crucible. This mixture was thoroughly mixed with grinding accompanied by calcination at 600 °C for 3 h. It was washed with water and dried using an oven at 80 °C for 24 h.
2.3.3 Synthesis of NiFe2O4/B, N and F-tridoped nanoceria (NFTDNC)
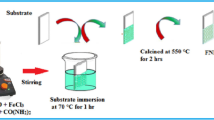
First of all, NiFe2O4 NPs were synthesized according to an earlier reported method. A solution of 0.04 mol FeCl3 (6.488 g) in 25 ml distilled water was made under continuous stirring. Similarly, 0.02 mol NiCl2·6H2O (4.754 g) was added in 25 ml distilled water and stirred to form its solution. Then, these solutions were mixed and stirr at 60 °C for 40 min. until a homogenous yellow-colored stock solution was formed. Its pH was maintained at 11 by the dropwise addition of NH3 solution. It leads to the formation of reddish-brown color precipitates in the reaction mixture. After complete precipitation, these precipitates were filtered under reduced pressure and thoroughly washed with DI water as well as ethanol. The obtained sample was centrifuged for 10 min at 1000 rpm along with drying at 100 °C in an oven for 10 h. It was then ground in an agate mortar for obtaining fine powder.
NiFe2O4 NPs (1 g) and B, N and F tridoped nanoceria (5 g) were mixed in a silica crucible and heated at 650 °C for 3 h using muffle furnace. The contrived nanomaterial, i.e., NiFe2O4/B, N,F-tridoped nanoceria, was repeatedly washed with deionized water along with ethyl acetate and ethanol followed by drying under vacuum at normal conditions.
2.4 Fabrication of electrode
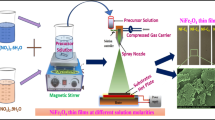
The synthesized nanoparticles were ground into a fine powder before being mixed with graphite and polyvinyl alcohol (PVA) in N-Methyl-2-Pyrrolidone (NMP). PVA is used as a binder instead of other binders, such as poly(vinylidene fluoride) (PVDF), since it is less costly. NMP is a polar solvent with distinct characteristics. It has a wide range of applications owing to its exceptionally high solvency, low freezing point, ease of handling, and high boiling point. It is miscible with water as well as the majority of popular organic solvents. It also belongs to the dipolar aprotic solvent class, which includes dimethylformamide and dimethyl sulfoxide. It is utilized as a solvent in the petrochemical, polymer, and battery areas owing to its non-volatility as well as ability to dissolve various compounds. To manufacture a suitable electrode, 0.025 g of graphite powder, 0.2 g of synthesized nanoparticles, and 0.025 g of PVA powder were mixed in NMP and heated to form a paste. Finally, coat the conductive fabric using this paste. A suitable electrode for further electrochemical analysis is produced by placing the coated conductive fabric at 40° C, for 1 h.
3 Results and discussion
3.1 PXRD and FTIR characterizations
The phase purity and the effect of dopants on the crystal structure of synthesized nanomaterial (NFTDNC) were examined using the PXRD characterization. The diffractogram, Fig. 1a, of NFTDNC displayed peaks at 2 θ = 28.5, 33.1, 47.49, 56.36, 59.1, 69.5, 76.7, 79.06, 88.4 and 95.4o. They are in coordination with the planes (111), (200), (220), (311), (222), (400), (331), (420), (422) and (521) of crystalline FCC CeO2 (JCPDS no. 34–0394) [11]. There were no signs of any phase adulterations. It showed that the doping elements and the spinel structure of NiFe2O4 were integrated into the host CeO2. In the crystal lattice of NFTDNC, microstrain, particle size, and intrinsic strain all contributed to the XRD peak broadening. It was estimated using Williamson–Hall (W–H) method, according to the following equation:
Here, k represents Scherrer constant (0.89), λ (nm) is wavelength of the Cu Kα radiations (0.15405 nm), βst corresponded to total broadening, because if crystallite size, Dav represents crystallite size, whereas 2θ signifies the diffraction angles. The W–H curve displayed in Fig. 1b was linearly fitted using Eq. 1 to determine the crystallite size and the intercept, which were both found to be 42.72 nm and 0.00339. Moreover, a negative slope of 0.00096 is also noted. It reveals the occurrence of micro flaws in NFTDNC, such as the narrowing of the lattice spacing of CeO2 host nanostructures. The doping of the CeO2 host nanostructure further lead to the narrower bandgap in NFTDNC. [12]
Figure 1c exhibits the Fourier-transform infrared spectroscopy (FTIR) spectrum of nanomaterial NFTDNC. It displayed strong absorption bands at 570, 3400, 1633, 1515, 1265, 1039, 939 and 1157 cm−1. The peak at 3400 cm−1 was attributed to stretching vibration of water and O–H functional groups. The corresponding in-plane and out-of-plane bending bands correlated with O–H functional group were found at 1630, 1408, and 1039 cm−1, correspondingly. After doping, a peak attributed to O–Ce–O bonds was observed at 1152 cm−1 [13]. CeO2 nanoparticles were responsible for peaks at 1515, 1265, 1130, 1064, 952 and 669 cm−1. The peaks at 669 and 570 cm−1 were attributed to the NiFe2O4 cation–oxygen bond's stretching vibration.
EDS characterization of the synthesized NFTDNC electrode was also done and the obtained results are displayed in Fig. 1d.
3.2 SEM and TEM characterizations
SEM characterization was used to investigate the shape and surface morphology of synthesized nanomaterial (NCTDNF), Fig. 2a, b. They revealed a spherical morphology with a porous and composite character. Furthermore, it was noticed that the spinel nanostructures of NiFe2O4 were consistently dispersed over tri-doped ceria, modifying the intrinsic characteristics of CeO2 host nano-framework including porosity, excellent dispersion, and robustness. The synthesis of 3-D porous of nickel ferrite consolidated into the framework of tri-doped nanoceria was facilitated by interactions among the support and metal oxide nanoparticles. HR-TEM micrographs were examined for an insight into the structure, particle size, and distribution of NiFe2O4 over tri-doped ceria. The immobilization of NiFe2O4 nanoparticles on tri-doped ceria in the synthesized material was confirmed in Fig. 2c, d. The selected area electron diffraction (SAED) pattern as shown in Fig. 2e confirmed the polycrystallinity of the material. The diffraction rings of lattice planes viz. (111), (220) and (331) are observed in Fig. 2e. These correspond to the cubic structure of CeO2 in the synthesized material. In addition, TEM micrographs were analyzed for the determination of d-spacing value in NFTDNC and the values come out to be 0.17 nm and 0.21 nm which are related to (422) and (400) planes of the NiFe2O4 in NFTDNC confirming the immobilization of NiFe2O4 over tri-doped nanoceria [14, 15].
3.3 XPS characterization
XPS analysis was utilized to determine the configuration and chemical states of surface elements present in the assembled nanostructure. The XPS results of the prepared material as demonstrated in Fig. 3a–g exhibits spectra correlated with the Ce 3d, Ni 2p, F 1s, N 1s, B 1s, and Fe 2p. Figure 3a reveals that Ce4+ chemical state was present in the Ce 3d. Furthermore, it displayed six peaks attributed to the Ce 3d3/2 and Ce 3d5/2 core levels. It showed two conventional photo-electron peaks (889.48 and 907.58 eV), along with shakeup and shakedown peaks attributed to the spin–orbit constituent of Ce4+ in various electron configuration states (898.58 eV, 916.98 eV, and 882.58 eV, 901.08 eV, respectively) [16, 17]. The peak at 916.98 eV further indicates the presence of only Ce4+ in synthesized NFTDNC material [18]. The XPS spectra of Ni 2p is shown in Fig. 3b. It demonstrates a peak at 856.02 eV along with satellite peak at 861.86 eV with respect to Ni 2p3/2. In addition, it also exhibited a peak at 873.68 eV along with satellite peak at 879.18 eV related to Ni 2p1/2. Such peaks confirmed that Ni was present in NFTDNC in Ni2+ as well as Ni3+ oxidation state [19]. Figure 3c illustrates the XPS spectrum of Fe 2p. It displayed the peak correlated with Fe 2p3/2 and Fe 2p1/2 spin–orbit peaks around 711.1 and 724.2 eV, correspondingly signifying the presence of Fe (III) oxidation states in the material [19]. Besides, the presence of a satellite peak at 718.6 eV confirmed the presence of Fe in Fe2+ as well as in Fe3+ state [20]. Figure 3d shows the XPS spectrum of N 1s. It revealed a peak at 397.1 eV ascribed to Ce–N bonds. The peak observed at 400.1 eV was attributed to chemisorbed N2 in the synthesized material [21]. The XPS spectrum of O 1s (Fig. 3e), displayed two peaks at binding energies 529.99 and 531.7 and 532.7 eV, respectively. The peak at 529.99 eV was attributed to the oxygen of crystal lattice and the peak at 531.7 eV was due to the hydroxyls-like defect sites. Four peaks were obtained in XPS result of B 1s, Fig. 3f. It revealed peak associated with the B–Ce bond at 190.64 eV and peaks associated with the B–N, B–O and B–F bond are at 190.8, 192.08, and 193.6 eV, respectively [22, 23]. XPS spectra F 1s, Fig. 3g, demonstrate a peak at 689.7 eV.
4 Electrochemical characterizations
The electrochemical properties of the prepared NFTDNC electrode was scrutinized via an electrochemical instrument (CHI 660E) in a three-electrode configuration in 6 M potassium hydroxide electrolytic solution. The Pt wire, synthesized electrode, and saturated calomel electrode (SCE) were employed as counter, working, and reference electrodes, respectively. Cyclic voltammetry (CV) was done at different scanning rates (10, 20, 30, 40, 50, 60, 80 and 100 mV/s). The galvanostatic charge–discharge (GCD) results for the NFTDNC material were investigated as the function of current density (A/g). In addition, electrochemical impedance spectroscopy (EIS) was done within the frequency range of 100 kHz to 0.1 Hz with AC voltage at 5 mV. The active mass of the synthesized NFTDNC pasted on the conductive substrate was ≈ 0.5 mg.
As a function of current density, the values of specific capacitance (C) of the NFTDNC electrode of mass m (g) were calculated from GCD results using the equation written below [24]:
Here C (F/g) stands for specific capacitance, I stand for the discharging current, Δt is the discharging time, whereas ΔV is the potential window. Furthermore, the coulombic efficiency (η, %) is estimated using the following equation:
Here, td represents discharge and tc stands for charge time.
4.1 Cyclic voltammetry
CV is a powerful as well as popular electrochemical technique usually employed to study the reduction and oxidation processes and the storage mechanism in the synthesized material. The electrochemical performance of the NFTDNC electrode was investigated using 6 M KOH electrolytic solution. The CV results obtained within the potential window of 0 to 0.5 V are illustrated in Fig. 4a as a function of change in scan rate from 10 to 100 mV/s. The existence of two major redox peaks in each CV result reveals the pseudocapacitive behavior of synthesized material because of the Faradaic reduction–oxidation reactions irrespective of the rectangular shape for EDLC behaviour.
In addition, with an increase in scan rates, the CV results demonstrates broader separation among the oxidation and reduction peaks, signifying high power character along with high electrochemical reversibility of the material attributed to the kinetics of interfacial Faradaic redox and fast rates of electronic and ionic transportation. Moreover, on increasing the scan rates, the anodic and cathodic peaks exhibit symmetric characteristics revealing good electrochemical properties. Furthermore, to comprehend the mechanism of charge-storage in the synthesized NFTDNC electrode, Dunn’s method is employed to know either the overall capacity is because of diffusion-controlled process (b = 0.5) or surface capacitive behavior (b = 1) or due to both [25, 26]. In general, the capacitive effects can be scrutinized with the help of CV results according to the power law given below [27]:
here ip represents peak current (A), v represents scan rates (V/s), whereas a and b are adjustable quantities. The b value is determined using the slope of log ip vs log v graph. Figure 4b, c illustrates the log ip vs log v results for cathodic and anodic peaks and it was observed b = 0.69 for anodic peak, whereas b = 0.61 for cathodic peak, indicating diffusion-controlled phenomenon [28] and battery-type characteristics [29].
4.2 Electrochemical impedance spectroscopy
It is a highly sensitive technique utilized to make the electric response of chemical systems in a non-destructive way. The high capacitance of the synthesized material is attributed to the fast and more efficient ionic diffusion as well as low resistance which results in high conductivity. The EIS measurements are conducted within the frequency window of 100 kHz to 0.1 Hz. The obtained Nyquist plot for the NFTDNC electrode is illustrated in Fig. 4d that reveals a quasi-semicircle at higher frequency, whereas a straight line in low frequency region whose slope is attributed to Warburg resistance (Zw) demonstrating the diffusion of electrolytic ions in the material. In the region of high frequency, the intercept of Z′ (X-axis) reveals bulk resistance (Rb) which is the combined result of resistance of active electrode, electrolytic solution and interface of the active material–current collector interaction. The attained Rb is 1.09 Ω signifying low resistance and hence high conductivity of synthesized NFTDNC material.
4.3 Galvanostatic charge–discharge
This characterization was performed to scrutinize the electrochemical properties of the synthesized sample in detail. Figure 5a, b demonstrates the GCD profiles of the NFTDNC electrode at varied current densities (A/g) ranging from 4 to 100 A/g within the potential window of 0–0.4 V. The non-linear GCD profiles also reveal that slow-kinetic process takes place in active electrode. The specific capacitances of the NFTDNC electrode were evaluated with the help of Eq. 3 and it comes out to be 631.8, 642, 652, 665.25, 663, 658, 656, 648, 645, 622.5, 600, 575, 555, 542.5, 520 and 500 F/g at 4, 6, 8, 10, 12, 14, 16, 18, 20, 30, 40, 50, 60, 70, 80 and 100 A/g, respectively. Values of specific capacitance as a function of current densities are also plotted, as demonstrated in Fig. 5c. At 100 A/g, the synthesized positive electrode reveals high rate capability with 79.13% retentively in the capacitance obtained at 4 A/g. In addition, cyclic performance is also a crucial parameter for determining the efficiency of the electrode material. The cycling performance was determined via repeated charging–discharging of the electrode at 0.065 A for 3000 cycles. As demonstrated in Fig. 5d, 81.81% capacitance retention was attained. The reduction in specific capacitance is attributed to some minor changes in active electrode due to frequent phase variation at the time of reduction–oxidation process. In addition, the electrolytic solution of KOH retained its limpidity after 3000 GCD cycles, signifying no dissolution of active material into the electrolytic solution, confirming high cyclic performance of the synthesized material [30]. From the abovementioned results, it was found that the proposed NFTDNC electrode demonstrates high specific capacitance along with good cycling properties, and exhibits the potential to be employed as cathode with good electrochemical performance in supercapacitors.
5 Conclusion
Mesoporous nanostructure of nickel ferrite immobilized on, N and F tri-doped CeO2 was successfully synthesized using simple and economical approach and scrutinized electrochemically to investigate its performance as supercapacitor electrode material. It was observed ftrom the CV results that the synthesized material exhibits the potential to effectively operate in positive potential range with specific capacitance of 665.25 F/g at 10 A/g as well as shows 81.81% retentivity after 3000 GCD cycles in three-electrode configuration. Taking benifits of positive operating potential, it was supposed that the prepared NFTDNC electrode exhibits great potential to be employed as supercapacitor cathode. Such a facile and economical approach that also results in good electrochemical performance opens various opportunities for next-generation high-performance supercapacitors.
Data availability statement
The raw/processed data required to reproduce these findings will be available on the reasonable request.
References
F. Hermundsdottir, A. Aspelund, Sustainability innovations and firm competitiveness: a review. J. Clean. Prod. 280, 124715 (2021)
G. Velvizhi, C. Goswami, N.P. Shetti, E. Ahmad, K.K. Pant, T.M. Aminabhavi, Valorisation of lignocellulosic biomass to value-added products: paving the pathway towards low-carbon footprint. Fuel 313, 122678 (2022)
H.D. Yoo, E. Markevich, G. Salitra, D. Sharon, D. Aurbach, On the challenge of developing advanced technologies for electrochemical energy storage and conversion. Mater. Today 17(3), 110–121 (2014)
M.A. Azam, N.S.N. Ramli, N.A.N.M. Nor, T.I.T. Nawi, Recent advances in biomass-derived carbon, mesoporous materials, and transition metal nitrides as new electrode materials for supercapacitor: a short review. Int. J. Energy Res. 45(6), 8335–8346 (2021)
R. Liang, Y. Du, P. Xiao, J. Cheng, S. Yuan, Y. Chen, J. Chen, Transition metal oxide electrode materials for supercapacitors: a review of recent developments. Nanomaterials 11(5), 1248 (2021)
A.K. Das, U.N. Pan, V. Sharma, N.H. Kim, J.H. Lee, Nanostructured CeO2/NiV–LDH composite for energy storage in asymmetric supercapacitor and as methanol oxidation electrocatalyst. Chem. Eng. J. 417, 128019 (2021)
K. Khan, A.K. Tareen, M. Aslam, A. Mahmood, Y. Zhang, Z. Ouyang, H. Zhang, Going green with batteries and supercapacitor: two dimensional materials and their nanocomposites based energy storage applications. Progr. Solid State Chem. 58, 100254 (2020)
M. Sun, Z. Li, H. Li, Z. Wu, W. Shen, Y.Q. Fu, Mesoporous Zr-doped CeO2 nanostructures as superior supercapacitor electrode with significantly enhanced specific capacity and excellent cycling stability. Electrochim. Acta 331, 135366 (2020)
K. Yavir, Ł Marcinkowski, R. Marcinkowska, J. Namieśnik, A. Kloskowski, Analytical applications and physicochemical properties of ionic liquid-based hybrid materials: a review. Anal. Chim. Acta 1054, 1–16 (2019)
M. Fu, W. Chen, X. Zhu, Q. Liu, One-step preparation of one dimensional nickel ferrites/graphene composites for supercapacitor electrode with excellent cycling stability. J. Power Sources 396, 41–48 (2018)
K. Deori, D. Gupta, B. Saha, S.K. Awasthi, S. Deka, Introducing nanocrystalline CeO2 as heterogeneous environmental friendly catalyst for the aerobic oxidation of para-xylene to terephthalic acid in water. J. Mater. Chem. A 1(24), 7091–7099 (2013)
C. Feng, J. Duan, G. Liu, Crystal lattice spacing shrinkage and band-gap narrowing phenomena in In-doped SnO2 nanoparticles. Mater. Res. Express 2(4), 045008 (2015)
J. Iqbal, N.S. Shah, M. Sayed, J.A. Khan, N. Muhammad, Z.U.H. Khan, K. Polychronopoulou, Synthesis of nitrogen-doped Ceria nanoparticles in deep eutectic solvent for the degradation of sulfamethaxazole under solar irradiation and additional antibacterial activities. Chem. Eng. J. 394, 124869 (2020)
M.S. Matseke, H. Luo, L. Wen, H. Zheng, The upgraded performance of the NiFe2O4/C electrocatalyst using Co substitution for the oxygen reduction reaction in an alkaline solution. J. Phys. Chem. Solids 165, 110644 (2022)
L. Li, S. Lu, Y. Dai, H. Li, X. Wang, Y. Zhang, Controlled synthesis of hierarchical nanostructured metal ferrite microspheres for enhanced electrocatalytic oxygen evolution reaction. ACS Appl. Nano Mater. 6, 2184–2192 (2023)
J.J. Li, E.Q. Yu, S.C. Cai, X. Chen, J. Chen, H.P. Jia, Y.J. Xu, Noble metal free, CeO2/LaMnO3 hybrid achieving efficient photo-thermal catalytic decomposition of volatile organic compounds under IR light. Appl. Catal. B 240, 141–152 (2019)
C.M. Sims, R.A. Maier, A.C. Johnston-Peck, J.M. Gorham, V.A. Hackley, B.C. Nelson, Approaches for the quantitative analysis of oxidation state in cerium oxide nanomaterials. Nanotechnology 30(8), 085703 (2018)
V. Stetsovych, F. Pagliuca, F. Dvořák, T. Duchoň, M. Vorokhta, M. Aulická, V. Matolín, Epitaxial cubic Ce2O3 films via Ce–CeO2 interfacial reaction. J. Phys. Chem. Lett. 4(6), 866–871 (2013)
M. Hua, L. Xu, F. Cui, J. Lian, Y. Huang, J. Bao, H. Li, Hexamethylenetetramine-assisted hydrothermal synthesis of octahedral nickel ferrite oxide nanocrystallines with excellent supercapacitive performance. J. Mater. Sci. 53, 7621–7636 (2018)
N. Sharma, M. Gupta, B. Chowhan, A. Frontera, Magnetically separable nanocatalyst (IL@ CuFe2O4-L-Tyr-TiO2/TiTCIL): preparation, characterization and its applications in 1, 2, 3-triazole synthesis and in photodegradation of MB. J. Mol. Struct. 1224, 129029 (2021)
A.B. Jorge, J. Fraxedas, A. Cantarero, A.J. Williams, J. Rodgers, J.P. Attfield, A. Fuertes, Nitrogen doping of ceria. Chem. Mater. 20(5), 1682–1684 (2008)
C.H. Wei, X.H. Tang, J.R. Liang, S.Y. Tan, Preparation, characterization and photocatalytic activities of boron-and cerium-codoped TiO2. J. Environ. Sci. 19(1), 90–96 (2007)
J. Qu, Q. Li, C. Luo, J. Cheng, X. Hou, Characterization of flake boron nitride prepared from the low temperature combustion synthesized precursor and its application for dye adsorption. Coatings 8(6), 214 (2018)
S. Verma, V. Gupta, A. Khosla, S. Kumar, S. Arya, High performance asymmetric supercapacitor based on vertical nanowire arrays of a novel Ni@Co–Fe LDH core@ shell as negative and Ni(OH)2 as positive electrode. Nanotechnology 31(24), 245401 (2020)
V.K. Mariappan, K. Krishnamoorthy, P. Pazhamalai, S. Sahoo, S.J. Kim, Electrodeposited molybdenum selenide sheets on nickel foam as a binder-free electrode for supercapacitor application. Electrochim. Acta 265, 514–522 (2018)
T. Brezesinski, J. Wang, J. Polleux, B. Dunn, S.H. Tolbert, Templated nanocrystal-based porous TiO2 films for next-generation electrochemical capacitors. J. Am. Chem. Soc. 131(5), 1802–1809 (2009)
J. Xie, P. Yang, Y. Wang, T. Qi, Y. Lei, C.M. Li, Puzzles and confusions in supercapacitor and battery: theory and solutions. J. Power Sources 401, 213–223 (2018)
Z. Chang, T. Li, G. Li, K. Wang, One-pot in-situ synthesis of Ni(OH)2–NiFe2O4 nanosheet arrays on nickel foam as binder-free electrodes for supercapacitors. J. Mater. Sci. Mater. Electron. 30(1), 600–608 (2019)
Y. Wang, Y. Song, Y. Xia, Electrochemical capacitors: mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 45(21), 5925–5950 (2016)
S. Verma, A. Khosla, S. Arya, Performance of electrochemically synthesized nickel–zinc and nickel–iron (Ni–Zn//Ni–Fe) nanowires as battery type supercapacitor. J. Electrochem. Soc. 167(12), 120527 (2020)
Acknowledgements
We are grateful to acknowledge AMRC, IIT Mandi for P-XRD and XPS analysis. We acknowledge DST and SAIF, IIT Bombay for HR-TEM, FEG–SEM, elemental mapping and STEM and Head, IIC, IIT Roorkee P-XRD studies. Neha Sharma (SRF Fellowship, Reference no.: 19/06/2016(i) Eu-V), are grateful to UGC New Delhi, India, respectively, for financial support. The corresponding author acknowledges the Science and Engineering Research Board (SERB), India for the support (File no. EEQ/2021/000172). This work was also supported by JK Science Technology and Innovation Council, Department of Science and Technology, JKUT.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, N., Verma, S., Singh, A. et al. High-performance wearable supercapacitor electrodes developed from nickel ferrite immobilized on boron, nitrogen and fluorine tridoped nanoceria (NFTDNC). Appl. Phys. A 129, 457 (2023). https://doi.org/10.1007/s00339-023-06733-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-06733-8