Abstract
The effect of the dielectric and chemical properties of the surrounding medium on the extinction spectra of silver island films is determined. The shift of the plasmon resonance maximum towards low energies with an increase in the permittivity of the medium (for SiO2 and Al2O3) corresponds to the calculated value, essentially depending on the shape of nanoparticles. Simultaneously with the shift, an increase in the optical density at the plasmon resonance frequency is observed. For nanoparticles immersed in an anisotropic medium, liquid crystal in particular, the shift of the plasmon resonance maximum depends on the local permittivity in the immediate vicinity of nanoparticles, i.e., on how exactly the surrounding molecule interacts with the substrate and the nanoparticle. In this case, the displacement can either be completely absent or be significantly greater than the displacement in isotropic media, depending on the shape of nanoparticles. The increase in optical density also depends on the local permittivity. The partial oxidation of nanoparticles leads to an insignificant but noticeable shift towards higher energies. In this case, the plasmon lifetime in the nanoparticle increases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In most applications of metal nanoparticles, they are located in various media, the permittivity of which differs from that for vacuum. This applies biology and medicine [1,2,3,4], catalysis [5, 6], to sensors for chemical analysis [7,8,9], etc. This environment can consist of both simple and very complex molecules.
As a rule, due to the presence of localized plasmon resonance (LPR), metal nanoparticles are used to amplify certain signals. The position of the LPR in the extinction spectra, its width and amplitude depend both on the permittivity of the medium and on the nature of its molecules interaction with the nanoparticle. This problem has been the subject of numerous publications over the past decades. For example, review [10] describes the possibilities of controlling an LPR, in particular, by immersion in a dielectric medium with variable parameters. In [11], a comparison is made for the plasmon resonance width of silver clusters with a diameter of about 2 nm in different states (in the free state, on a substrate, and as embedded in fused quartz). In [12], the influence of adsorbed gasses on the position of plasmon resonances of metal nanoparticles is studied. In [13], the plasmon resonance wavelength of the spherical nanoparticles immersed in a medium with a refractive index n as function of n is described by the linear dependence based on the Drude model. The results of calculations demonstrate on the influence of a substrate with a known permittivity on the plasmon resonance frequency. It follows from the formula [13] that the dependence of this wavelength on the refractive index should be linear. It is argued that a similar dependence is also preserved for nonspherical nanoparticles. For particles of more complex shape, the behavior of plasmon resonances is described by more complex calculations in [13, 14].
Of particular interest are the properties of metal nanoparticles in a shell of other substances, such as a semiconductor or dielectric. In [15], the properties of metal nanoparticles in a fullerene matrix were studied at different metal-to-fullerene ratios. In [12], the influence of the oxide of the same metal on the LPR of sodium nanoparticles was described, demonstrating low energy shift of the resonance wavelength, which should occur with an increase in the permittivity of the medium surrounding the particle. A theoretical substantiation of this phenomenon is given.
Although the influence of the environment on the LPR of nanoparticles has been studied in detail, both theoretically and experimentally, the discrepancy between the results of calculations and an experiment is sometimes quite large. The discrepancy in the experimental results of different authors is also great. Mostly, only the main regularities coincide—such as, the linearity of the LPR peak position dependence on the refractive index. Apparently, such parameters as the position and width of the LPR, depend to a large extent on the experimental conditions.
The purpose of this work is to experimentally determine how the plasmon resonance of silver nanoparticles formed on various dielectric substrates is changed after immersion in various media, to determine the factors (except for the dielectric permittivity) that affect the LPR parameters, and to explain these changes within the framework of the model ideas about the influence of the dielectric environment on the spectral position and intensity of the localized plasmon resonance.
2 Experimental details
Silver island films were obtained by thermal evaporation in a Kurt Lesker vacuum setup. The films were deposited on polished substrates (sapphire or fused quartz, refractive index 1.772 and 1.458, respectively), and on about 100-nm thick films of ITO (indium tin oxide) and on ITO films coated with a thin film of carbon. ITO contained 90 wt% In2O3 and 10 wt% Sn2O and was transparent in the visible region. ITO films were formed by magnetron sputtering.
Before sputtering, the substrates were cleaned in an ultrasonic bath in ethanol and distilled water. The deposition rate of silver films was varied from 0.1 to 0.4 Å/s and controlled by a quartz microbalance. The same microbalance was used to measure the equivalent thickness of the deposited film. The thickness of silver films varied from 2 to 6 nm. Deposited films could be heated (annealed) in a vacuum chamber at a temperature 200–250 °C. Refractory dielectrics (quartz and sapphire) were deposited by the electron beam evaporation in the same chamber.
In addition to quartz and sapphire, a nematic liquid crystal ZhK-1282 (NIOPIK, Moscow) and a thin polymethyl methacrylate (PMMA) film formed by applying a solution in its own monomer, were used as immersion media in contact with the surface of silver granular films.
These substances do not absorb light in the visible region. ZhK-1282 is cyanobiphenyl-based electropositive crystal (ordinary and extraordinary refractive indices 1.50 and 1.67, respectively, dielectric anisotropy Δε = 9.9 (ε ||= 15.5 and ε ┴ = 5.6) [16]. The polarizability of LC molecules with positive dielectric anisotropy (Δε > 0) along the director direction is larger than in the perpendicular direction [17]. The ZhK-1282 consists of alkoxycyanobiphenyls (80%), Demus ether (16%), and Gray ether (4%). Its nematic phase exists in the temperature range from 253.1 to 335.1 K. The mixture is characterized by the presence of both strongly and weakly polar components [18].
The dye 3,3'-diethyl-thiadicarbocyanine iodide (dicarbocyanine) was also deposited on silver films according to the procedure described in [19].
Nanoparticles were oxidized in a vacuum chamber of laboratory design [20]. For oxidation, two procedures were applied: a film placed in an oxygen atmosphere was either illuminated in the wavelength range of 300–350 nm with an UV light of a lamp [20], or it was slightly heated in the oven inside the chamber. Ultrapure oxygen was run from a cylinder into the vacuum chamber to a pressure of several units to several hundred Torr.
The extinction spectra were obtained on an SF-56 spectrophotometer of OKB Spektr-LOMO. SEM images of nanoparticles on a sapphire substrate and ITO were obtained using a Merlin scanning electron microscope (Carl Zeiss).
3 Major results
To understand the shape and size of nanoparticles obtained during deposition and annealing, SEM images of silver island films were taken (Fig. 1).
It can be seen that the unannealed film (Fig. 1a) is a set of shapeless nanoparticles. At the same time, the surface area occupied by the islands is larger than for the annealed film (Fig. 1b), that is, these islands are flatter than the islands of the annealed film. Most islands of the annealed film are round and 20–50 nm in diameter.
In our experiments, the influence of the dielectric environment on the position and shape of the silver nanoparticle localized plasmon resonance was determined primarily for isotropic media (quartz and sapphire). For depositing a silver film with an effective thickness of 5 nm, we used four substrates for each medium (fused quartz and sapphire). Two films on each substrate were annealed at a temperature of 200 °C. Thick (more than 10 nm) films of the corresponding substrate (quartz or sapphire) were deposited on half of each of the four silver films so that silver nanoparticles could be considered completely immersed in a particular medium. The other half of the substrate was masked with a foil during sputtering.
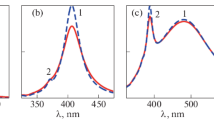
The results of the experiment are shown in Fig. 2.
Full immersion in a dielectric medium leads to a shift in the plasmon resonance peak, in accordance with the theory [13]. Table 1 lists the position of the LPR peaks for films deposited on quartz and sapphire, both unannealed and annealed, and the shift of these peaks owing to the burying into quartz or sapphire, respectively.
First of all, we note that the positions of the LPR peaks of both annealed and unannealed films on quartz and sapphire substrates differ slightly, only in hundredths of eV. Consequently, the dielectric properties of the substrate exert slight effect on the LPR position. The same can be said about the resonance half-width. At the same time, for nanoparticles fully buried into the corresponding medium, the peak is already shifted significantly and the half-width is increased. The shift for a nanoparticle completely “buried” into quartz is less than for a nanoparticle “buried” into sapphire, which may be expected due to their refractive indices (1.772 for sapphire and 1.458 for quartz).
At the same time, for annealed nanoparticles the LPR shift is almost twice as large compared with unannealed particles for both materials. Therefore, the dielectric medium acts on a spherical particle more strongly than on a flat particle. In addition, the absorption of silver nanoparticles noticeably increases after complete immersion, and this increase is also greater for annealed films.
In the case of immersion of nanoparticles in an anisotropic medium, the results were somewhat different. Nematic liquid crystals (LCs) are viscous liquids with dielectric properties, which are characterized by a long-range orientational order and complete freedom of movement of gravity centers of individual molecules in space. The predominant direction of LC molecules orientation can be obtained as a result of their interfacial interaction with a solid anisotropic surface, which orients the molecules near the surface along a certain direction. The forces of intermolecular interaction orient the molecules in the volume in the same direction [16, 21, 22]. The result of the experiments with a film deposited on a sapphire substrate and immersed in a liquid crystal are shown in Fig. 3.
Like immersion in isotropic medium, the immersion in anisotropic medium causes nanoparticle plasmon resonance to shift, with some differences observed. For initial particles, the resonance shift before annealing is either very small or absent, whereas that for the annealed particles, is larger than for immersion in isotropic medium. (For the island film in Fig. 3, the shift for initial and annealed particles is 0 and 0.30 eV, respectively). Consequently, for immersion in anisotropic medium the dependence of LPR shift on the shape of nanoparticles is more pronounced compared to immersion in isotropic medium.
The shape of nanoparticles, is more important than how they are obtained. In our case, it does not matter whether the annealing process took place or the spherical islands were formed by different processes. On some surfaces with weak adhesion, carbon films in particular, the islands acquire a close to spherical shape without annealing, immediately after deposition (Fig. 4).
On immersion in LC, the extinction spectrum on such a substrate changes in the same way as the spectrum of an annealed film. After sputtering, LC was poured onto the surface of the substrates (ITO and ITO + C). The film deposited on carbon immediately changed color. For the film deposited on ITO, no visible changes were observed. The extinction spectra of both films are plotted in Fig. 5. As seen, the spectrum of the film on carbon has changed significantly: the resonance is shifted to the red side and the extinction is increased. The spectrum of the film on ITO almost did not change.
In addition to LC, the influence of another substance with a complex structure, polymethyl methacrylate, on the position of the LPR was studied. It is not clear how the polymer chain affects the permittivity in the vicinity of a nanoparticle. It is possible that during the coating of the island film, when PMMA is dissolved, the chain breaks up and methyl methacrylate monomers are formed. As for the LPR, PMMA produces an effect largely similar to that produced by a liquid crystal. The peak shift is 0.07 eV due to pouring PMMA onto unannealed film deposited on quartz, whereas it is 0.37 eV due to pouring onto the annealed film. For the annealed film the absorption is increased nearly two-fold, whereas for the unannealed film, it is increased insignificantly.
Of particular interest is how dyes, another type of organic molecules with intense absorption and luminescence bands in the visible spectral region, affect the absorption of nanoparticles. Numerous studies have been carried out with a significant overlap of the absorption band of dyes and plasmon resonance of nanoparticles to obtain strong coupling [23,24,25] and laser generation [26,27,28]. In our experiment, the absorption spectra overlapped, however, to small extent. We used a silver island film annealed at temperatures below 200 °C, i.e., with nanoparticles far from spherical in shape and isolated from each other. The dicarbocyanine dye was selected, because its absorption band slightly overlaps the LPR peak of the film, which allows determination of the dye effect on nanoparticles (Fig. 6). The effect of photoluminescence on plasmon resonance is absent, because photoluminescence is located spectrally in the longer wavelength region.
Figure 6 shows the extinction spectra of a silver island film before and after applying the dye layer. The LPR peak is shifted by 0.135 eV. For nanoparticles the whose shape is far from spherical, this is a rather large shift. The absorption in this spectral region is also increased (Note, the absorption in the dye band is increased tremendously). That is, the effect of the dye on the absorbance of the island film is similar to that of other organic molecules such as LC and PMMA.
Partial oxidation of the films gives somewhat unexpected results: the oxidation leads to the appearance of a relatively thin coating around the metal nanoparticle (Fig. 7).
This “shirt” has permittivity, greater than that of the nanoparticle. It should lead to a long-wave shift of the plasmon resonance. However, the effect is opposite (Fig. 8).
As seen, the plasmon resonance half-width is slightly decreased, and the maximum is shifted towards short wavelengths. Obviously, there is a parameter whose change is opposite to the effect of the dielectric environment on the plasmon resonance position and shape. The oxidation of films on substrates made of another material (quartz) and UV oxidation in an oxygen atmosphere lead to the same results.
There is another phenomenon, the explanation of which is not obvious. It is known [30] that the lifetime of a plasmon in a nanoparticle is determined, among other properties, by the state of the nanoparticle surface, since electrons are scattered on the surface. Therefore, it is natural to assume that the plasmon lifetime in a nanoparticle with a thin oxide layer on the surface is shorter than in an non-oxidized nanoparticle. The plasmon lifetime was determined for the experiments conducted by the method of spectral hole burning described in [30, 31]. Figure 8b shows the difference spectra obtained by single-pulse irradiation before and after the oxidation. It turned out that the width of the peak in the difference spectrum for the oxidized film is smaller, although not much, than for the non-oxidized one. Therefore, the plasmon lifetime in the oxidized film is somewhat longer.
4 Discussion
We determined that the immersion of a metal nanoparticle in a medium with a permittivity different from that for vacuum leads to a shift in the LPR maximum, an increase in its half-width, and an increase in absorption. It is interesting to compare the shift of the LPR maximum in our case with the calculated and experimental data available.
Our calculations by the Mie theory [32] coincide with the experiment in order of magnitude. At the same time, the calculated peak positions of resonances for spherical nanoparticles immersed in quartz and sapphire have an energy of 2.85 and 2.62 eV, respectively, which exceeds the experimental values for the annealed particles: 2.63 and 2.56 (Table 1). (These results are for the annealed particles, with close to spherical shape). The diameter of the particles substituted in the formula was 50 nm. Most of the nanoparticles have a smaller diameter (see Fig. 1). At the same time, the contribution of large nanoparticles to the optical density largely exceeds the contribution of smaller ones, since the extinction cross section is proportional to volume. Therefore, this approximation is justified. The discrepancy can be attributed to the deviation of nanoparticles from the spherical shape. Apparently, these particles are slightly flattened, which is not visible in the SEM image.
In [14], DDA calculations was made for a silver nanoparticle in the form of a truncated tetrahedron on substrates with different refractive indices. The graph shows both theoretical and experimental values of the LPR maximum position depending on the refractive index [14]. In the same work, the calculated and experimental results are presented for the complete immersion of a nanoparticle in media with different refractive indices. The theoretical values of the position of the LPR maximum differ noticeably from the experimental ones. If the difference in the positions of the LPR maxima on different substrates of experimental values [14] is compared with the results, presented in this study, then in [14] this difference is three times larger (0.06 and 0.02 eV, respectively). The results for complete immersion of the nanoparticle in the medium also differ by approximately a factor of three. The discrepancy can be explained by the fact that when quartz or sapphire is deposited on an island film, a porous structure is formed with a refractive index lower than that of solid materials [33]. However, the difference is visible only in the second decimal place. The refractive index of quartz and sapphire films was estimated in the experiments conducted from the interference oscillations of the optical density of these films deposited on the corresponding substrates and the Sellmeier equation. Therefore, for massive fused quartz, the refractive index is 1.458, while for a porous film it is near 1.440. The difference in the shift of the LPR maximum in this case should be 3 nm (calculated by the Mie formula [32]). The smallest LPR shift (Fig. 2a) observed for an unannealed silver film on a fused quartz substrate and the same film immersed in quartz—was 26 nm, which is much larger than 3 nm. In the case of annealed film or film on sapphire, the shift was even greater.
The discrepancy can also be explained by the difference in the shapes of the nanoparticles. In our case, the particles, apparently, have a shape close to spheroid. In this case, the area of contact with the substrate is small and, thus, the effect of the substrate on the position of the LPR maximum is small. A possible reason may also be the deviation from linearity in the dependence of the LPR wavelength on the refractive index. In our case, the LPR maximum is at shorter wavelengths than in [14].
At the same time, we see that changes in the LPR spectrum depend not only on the dielectric permittivity, but also on the degree of anisotropy of the immersion medium. For different anisotropic media, these changes can be significantly different. In addition, we see that changes in the LPR spectrum depend on the nanoparticle shape.
The causes of changes in the extinction spectra of nanoparticles by the example of immersion in a liquid crystal should be revealed. The experiments performed show that these changes can be either very significant, or absent. This depends on the orientation of LC molecules near the nanoparticle [34]. In the LC we used, the molecules have a permanent dipole moment parallel to the axis. In the case of unannealed films, particles of small height and large area, the orientation of the dipoles perpendicular to the substrate predominates. In this case, the polarizability of LC molecules in the direction of polarization of a normally incident electromagnetic wave is minimal. This leads to minimal changes in the extinction spectra or the complete absence of such changes. Annealed films are composed of particles with a shape close to spherical. In this case, the fraction of molecules oriented parallel to the electric field strength is large. This leads to an increase in the permittivity in the vicinity of the nanoparticle, which, in turn, shifts the plasmon resonance in the extinction spectrum and increases the optical density at the frequency of this resonance.
In addition, it should be considered that the orientation of LC molecules is affected not only by the shape of the island, but also by the degree of filling of the substrate with metal nanoparticles. During annealing, the degree of filling decreases significantly, and the area of the unfilled substrate surface increases (Fig. 1). Orientation of LC molecules on conducting and dielectric surfaces differs significantly. On smooth metal surfaces, homeotropic orientation with weak adhesion is common. On a smooth metal surface of spherical shape, the LC molecules are oriented along the normal to the tangent in the absence of other influences.
Cyanobiphenyl liquid crystals, to which ZhK-1282 belongs, have high adhesion forces to the surface of such dielectrics as quartz, which ensures a planar orientation with strong coupling on a pure quartz substrate [35, 36]. Thus, the ordering of LC molecules is affected not only by the shape of the island, but also by the filling factor, i.e., area of the “clean” surface of the substrate, which increases significantly upon annealing. This leads to the maximum interaction with the light wave and, consequently, to the maximum changes in the extinction spectra (Fig. 9).
The extinction spectra of the nanoparticles formed during deposition depend on their shape, since the plasmon resonance energy depends on the shape of the island [14, 37, 38]. In turn, the structure of the films and the shape of the islands obtained by sputtering are largely determined by the surface of the substrate. Thus, the spectra of films deposited on ITO differ significantly from the spectra of films deposited on a carbon substrate. The plasmon resonance for unannealed films deposited on carbon is much narrower, which indicates a greater homogeneity of island shapes than for films deposited on ITO. SEMs show that the islands on carbon are isolated to larger extent (Fig. 4). Many of them have a rounded shape, that is, they look like islands of an annealed film. Hence, there is a completely different nature of the LC action on films (Fig. 5).
Immersion of nanoparticles in media with an increased refractive index always leads to an increase in the optical density in the absorption bands associated with the LPR excitation. However, the degree of this increase, as well as the shift of the LPR maximum, depends on the shape of the nanoparticles. This is well illustrated by Figs. 2, 3 and 5. That is, the conditions for increasing the optical density are the same as the conditions for shifting the LPR maximum. The nature of this increase can be explained as follows: the molecules of the environment are polarized under the influence of light. In the vicinity of the nanoparticle at the plasmon resonance frequency, this polarization is greater due to the increase in the field near the nanoparticle. The greater polarization, the greater the polarizability of the molecules surrounding the nanoparticle.
The effect on the extinction spectra of a liquid crystal is an extreme case. Many other complex molecules have less dipole moment and less polarizability than liquid crystal molecules. However, the nature of their interaction with nanoparticles does not fundamentally change. An example are dye molecules, which interaction with nanoparticles also leads to a shift in the LPR maximum and an increase in absorption. It should be noted that the orientation of the studied cyanine dye and its aggregates is the same both on conductive and dielectric substrates—the dipole moments of molecules have significant deviations relative to the normal to the surface (of the order of 50–80°), depending on their structure [29].
For a metal nanoparticle surrounded by a shell of its own metal oxide, the situation is quite different. The LPR shift occurs in the direction opposite to that predicted by the theory. One of the possible explanations for this behavior can be simply a decrease in the particle size, which leads to a decrease in the width of the resonance and its shift towards short wavelengths [7, 39]. However, an insignificant depth of oxidation (of the order of 1 nm, i.e., 1–3% of the particle diameter) is unlikely to lead to a noticeable decrease in size. In addition, the maximum optical density of the oxidized nanoparticle is no less than that of the non-oxidized one (Fig. 8a). Probably, the oxidation occurs unevenly in thickness. Non-smooth, protruding areas of the nanoparticle surface are oxidized larger. In this case, metal fractions of nanoparticles become more uniform in shape, which should lead to a decrease in the LPR width. This process is opposite to the shift of the LPR towards low energies due to the immersion of the particle in a medium with a permittivity larger than that of the air.
5 Conclusion
The dependence of the spectral position of the plasmonic absorption band on the dielectric environment and the shape of the particles constituting the island film is experimentally demonstrated by the example of the most commonly used sapphire and quartz substrates.
For nanoparticles immersed in a medium of elongated, polarized and, at the same time, mobile molecules, changes in the extinction spectra, i.e., a shift in the plasmon resonance and an increase in extinction at the resonance frequency, can differ significantly from calculations made only considering the permittivity.
The reason for the differences is the local change in the refractive index of the medium in close proximity to the metal particles, The change is determined both by the shape of the particles and by the filling factor of the substrate and its material. If the resulting distribution of medium molecules with anisotropic polarizability near the metal film is dominated by an orientation parallel to the electric vector of the incident electromagnetic wave, then the spectral changes are maximum. This orientation is facilitated by the spherical shape of the metal islands and the low filling factor of the substrate, which occurs in the case of annealed films. For unannealed films, on the contrary, molecules are oriented in such a way that their electronic polarizability in the direction of the electric wave vector is minimal predominate. The wave does not interact with them and there is no weakening.
Partial oxidation of nanoparticles (formation of an oxide shell) leads to results opposite to those predicted by the naive theory, considers only the change in the dielectric environment of particles of an unchanged shape. The experiment showed that a change in the shape and dimensions of the metal core also plays a significant role. The LPR shifts towards higher energies, and its half-width decreases. The lifetime of a plasmon in a nanoparticle increases.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
J. Wolfram, M. Zhu, Y. Yang, J. Shen, E. Gentile, D. Paolino, Y. Zhao, Curr. Drug Targets (2015). https://doi.org/10.2174/1389450115666140804124808
L. Zhang, F.X. Gu, J.M. Chan, A.Z. Wang, R.S. Langer, O.C. Farokhzad, Clin. Pharmacol. Ther. (2008). https://doi.org/10.1038/sj.clpt.6100400
M. Kim, J.H. Lee, J.M. Nam, Adv. Sci. (2019). https://doi.org/10.1002/advs.201900471
M. De, P.S. Ghosh, V.M. Rotello, Adv. Mater. (2008). https://doi.org/10.1002/adma.200703183
F. Tao, Metal Nanoparticles for Catalysis: Advances and Applications (The Royal Sosiety of Chemistry, Cambridge, 2014), p.269
L.K. Khorashad, L.V. Besteiro, M.A. Correa-Duarte, S. Burger, Z.M. Wang, A.O. Govorov, J. Am. Chem. Soc. (2020). https://doi.org/10.1021/jacs.9b11124
R. Gordon, D. Sinton, K.L. Kavanagh, A.G. Brolo, Acc. Chem. Res. (2008). https://doi.org/10.1021/ar800074d
M. Matuschek, D.P. Singh, H.H. Jeong, M. Nesterov, T. Weiss, P. Fischer, F. Neubrech, N. Liu, Small (2018). https://doi.org/10.1002/smll.201702990
Y. Guan, Z. Wang, B. Ai, C. Chen, W. Zhang, Y. Wang, G. Zhang, A.C.S. Appl, Mater. Interfaces (2020). https://doi.org/10.1021/acsami.0c15955
N. Jiang, X. Zhuo, J. Wang, Chem. Rev. (2018). https://doi.org/10.1021/acs.chemrev.7b00252
H. Hovel, S. Fritz, A. Hilger, V. Kreibig, M. Vollmer, Phys. Rev. B (1993). https://doi.org/10.1103/PhysRevB.48.18178
T. Brandt, W. Hoheisel, A. Iline, F. Stietz, F. Trager, Appl. Phys. B (1997). https://doi.org/10.1007/s003400050349
K.L. Kelly, E. Coronado, L.L. Zhao, G.C. Schatz, J. Phys. Chem. B (2003). https://doi.org/10.1021/jp026731y
M.D. Malinsky, K.L. Kelly, G.C. Schatz, R.P. van Duyne, J. Phys. Chem. B (2001). https://doi.org/10.1021/jp002906x
R.A. Dynich, A.D. Zamkovets, A.N. Ponyavina, E.M. Shpilevsky, Int. J. Nanosci. (2019). https://doi.org/10.1142/S0219581X19400295
L.P. Amosova, P.S. Parfenov, M.V. Isaev, J. Opt. Technol. (2014). https://doi.org/10.1364/JOT.81.000686
V.P. Shibaev, Soros Educ. J. 11, (1996)
V.V. Surnychev, D.L. Bogdanov, V.V. Belyaev, Tech. Phys. Lett. (2005). https://doi.org/10.1134/1.1931789
A.A. Starovoytov, R.D. Nabiullina, I.A. Gladskih, V.A. Polischuk, P.S. Parfenov, A.N. Kamalieva, Proc. SPIE (2018). https://doi.org/10.1117/12.2307246
N.B. Leonov, Opt. Spectrosc. (2019). https://doi.org/10.1134/S0030400X19100321
I. Zgura, T. Beica, S. Frunza, L. Frunza, P. Ganea, F. Ungureanu, C. Negrila, A. Nuta, A.-A. Sorescu, I. Bunea, C.N. Zaharia, Optoelectron. Adv. Mater. Rapid Commun. 5, 318 (2011)
I. Karnei, E.A. Melnikova, O.G. Romanov, I.V. Stashkevitch, Proc. SPIE (2000). https://doi.org/10.1117/12.385698
Q. Zhao, W.J. Zhou, Y.H. Deng, Y.Q. Zheng, Z.H. Shi, L.K. Ang, L. Wu, J. Phys. D Appl. Phys. (2022). https://doi.org/10.1088/1361-6463/ac3fdf
R.D. Nabiullina, A.A. Starovoytov, I.A. Gladskikh, Opt. Quantum Electron. (2020). https://doi.org/10.1007/s11082-019-2161-9
M. Kumar, J. Dey, M.S. Verma, M. Chandra, Nanoscale (2020). https://doi.org/10.1039/D0NR01303J
S.I. Azzam, A.V. Kildishev, R. Ma, C. Ning, R. Oulton, V.M. Shalaev, M.I. Stockman, J. Xu, X. Zhang, Light Sci. Appl. (2020). https://doi.org/10.1038/s41377-020-0319-7
N.A. Toropov, A.N. Kamalieva, A.A. Starovoitov, S. Zaki, T.A. Vartanyan, Adv. Photonics Res. (2021). https://doi.org/10.1002/adpr.202000083
Z. Wang, X. Meng, A.V. Kildishev, A. Boltasseva, V.M. Shalaev, Laser Photonics Rev. (2017). https://doi.org/10.1002/lpor.201700212
E.N. Kaliteevskaya, V.P. Krutyakova, T.K. Razumova, A.A. Starovoytov, Opt. Spectrosc. (2018). https://doi.org/10.1134/S0030400X18090138
J. Bosbach, C. Hendrich, F. Stietz, T. Vartanyan, F. Trager, Phys. Rev. Lett. (2002). https://doi.org/10.1103/PhysRevLett.89.257404
T.A. Vartanyan, N.B. Leonov, Opt. Spectrosc. (2016). https://doi.org/10.1134/S0030400X1604024X
K. Ladutenko, Mie calculator. The Department of Physics and Engineering, ITMO University. https://physics.itmo.ru/ru/mie#/spectrum
I.A. Gladskikh, T.A. Vartanyan, Opt. Spectrosc. (2016). https://doi.org/10.1134/S0030400X16120109
L.P. Amosova, N.B. Leonov, N.A. Toropov, Opt. Spectrosc. (2016). https://doi.org/10.1134/S0030400X16120031
W.R. Liou, C.-Y. Chen, J.-J. Ho, C.-K. Hsu, C.-C. Chang, R.Y. Hsiao, S.-H. Chang, Displays (2006). https://doi.org/10.1016/j.displa.2005.11.001
K.C. Kim, H.J. Ahn, J.B. Kim, B.H. Hwang, H.K. Baik, Langmuir (2005). https://doi.org/10.1021/la050839y
E. Ringe, J.M. McMahon, K. Sohn, C. Cobley, Y. Xia, J. Huang, G.C. Schatz, L.D. Marks, R.P. Van Duyne, J. Phys. Chem. C (2010). https://doi.org/10.1021/jp104366r
D.E. Mustafa, T. Yang, Z. Xuan, S. Chen, H. Tu, A. Zhang, Plasmonics (2010). https://doi.org/10.1007/s11468-010-9141-z
N.B. Leonov, I.A. Gladskikh, V.A. Polishchuk, T.A. Vartanyan, Opt. Spectrosc. (2015). https://doi.org/10.1134/S0030400X15090179
Acknowledgements
The study was supported by the Russian Science Foundation grant No. 21-72-10098, https://rscf.ru/project/21-72-10098/. The authors are grateful to V. Polishchuk for SEM—images of films, T. Vartanyan and V. Krutiakova for careful reading of the paper and discussion of the results.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Leonov, N.B., Gladskikh, I.A. & Starovoytov, A.A. Effect of media on plasmon resonance of silver nanoparticles. Appl. Phys. A 129, 430 (2023). https://doi.org/10.1007/s00339-023-06715-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-06715-w