Abstract
The structural and electrical characteristics of the glassy system with the chemical formula (18.75Li2O–18.75Na2O–25V2S5–37.5P2S5 mol%) and its nano-crystallized glass–ceramics have been investigated. The glass sample was prepared via the conventional melt-quenching technique. The corresponding nano-crystallized glass–ceramics were prepared via a heat treatment (HT) procedure at 405 °C for diverse times in the air. The effect of HT time on nanostructure and the dc conductivity for the nano-crystallized glass–ceramics were investigated. X-ray diffraction (XRD) spectra and field emission scanning electron microscopy (FESEM) images confirmed the amorphous nature of the as-synthesized glass and the growth of nano-crystals after heat treatment within the glassy matrix. The nano-crystallized glass–ceramics exhibited considerably higher conductivity and lower activation energy than the initial glass. Furthermore, the dc conductivity was improved by nearly 57 times in the nano-crystallized glass–ceramic samples as compared with the initial glass. The activation energy was improved by utilizing the HT process and was found to be 0.535 eV for the as-prepared glass and between 0.272 and 0.462 eV for the corresponding nano-crystallized glass–ceramics. The dc conductivity increased with decreasing crystalline size in the case of nano-crystallized glass–ceramics, which may be accredited to the reduction in grain boundary scattering brought on by the smaller particle size. The conduction mechanism was emphasized to follow the non-adiabatic small polaron hopping (SPH).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Glasses and nano-crystallized glass–ceramics containing transition metal ions (TMIs), like Fe, V, Co, and W, are of tremendous interest owing to their possible applications [1,2,3,4,5,6]. They display semiconducting properties because of the existence of two diverse valance states of the TMIs, for instance, V4+ and V5+ in the case of vanadium or Fe2+ and Fe3+ in the case of iron. This charge transmission is commonly named SPH, in which the small polaron jumps from the low to the high valence state, and occurs electron conduction in the glasses [3,4,5,6]. Alkali phosphate glass, doped with TMI (such as V), is of considerable interest owing to its attractive optical, semiconductor, electrical, and thermal characteristics and their particular applications. Recently, this category of materials has made interesting candidates in several fields of application such as mechanical and thermal sensors, reflecting windows, optical filters, ray absorbers, non-linear optical and electronic devices, switching, memory elements, and batteries materials [7,8,9,10,11,12]. In the latest decades, glass–ceramics relying on alkali phosphate has been effectively optimized by a crystallization handling of the initial glass, and the conductivity of alkali ions has been investigated [7, 10,11,12]. The crystallization of glass is a technique to manufacture nano-materials and it is remarkable to optimize novel conductive materials with these nano-structures [7, 10,11,12]. In the glass–ceramic technique, it is conceivable to prepare the desired crystalline phase from the homogeneous glassy stage and to produce the size and form of the crystalline stage by controlled crystal nucleation and growth. Alkali ions modifiers are expected to inhabit non-bridging oxygen (NBO) sites in the glass phase [9,10,11]. This means the addition of alkali oxides such as Li2O and Na2O to the glassy matrix results in structural changes by the opening up of the glass network as NBO increases, thereby causing higher ionic conductivity and hence increasing electrical conductivity [13]. The applications of these glasses and corresponding nano-crystallized glass ceramics have also obtained increased attention owing to their possible use as electrodes in solid-state devices [13]. Heat treatment of the glassy stage nearby their crystallization temperature for diverse times fabricates nano-crystals entrenched in the specimens. The nano-crystalline phase is very important to enhance numerous properties, such as the electrical conductivity of the glass [10,11,12, 14,15,16,17,18,19]. Moreover, the alkali phosphate glass containing vanadium yields mixed ionic and electronic conductivity happening through the transport of electron hopping and alkali ions between vanadium ions centers [6, 11, 20]. However, the existence of sulfur in the glassy matrix leads to improve electrical conductivity. The attendance of S ions can enlarge the size of alkali ion transport passageways due to the larger diameters of S ions than O ions. In addition to that, the S ions have a high polarization aptitude than O ions, which leads to a weakening of the bond between alkali ions (Li+ and Na+) and the skeleton [21]. Moreover, the S atoms work as a reduction agent that reasons decreasing V ions from the higher to the lower valence states [6, 10]. This decrease is important to get compounds containing V3+ and V4+, consequently can boost electrical characteristics via easiness of the electrons and ions transmission [6, 11, 22]. Moreover, the electrochemical performance of energy storage devices (batteries or supercapacitors) depends greatly on the electrical conductivity of the electrode materials [23, 24], which makes the study of this conductivity very important.
In this study, the Li2O–Na2O–V2S5–P2S5 glass and its nano-crystallized glass–ceramics are synthesized. The improvement of the electrical conductivity due to the existence of the nano-crystalline phases inside the glassy matrix is investigated as a function of crystalline size. In addition, the mechanism of electrical conductivity of the as-synthesized glass and nano-crystallized glass–ceramics is clarified.
2 Experimental work
The glassy system with the chemical formula (18.75Li2O–18.75Na2O–25V2S5–37.5P2S5 mol%) was fabricated through the conventional melt-quenching technique. The corresponding nano-crystallized glass–ceramics were manufactured through the HT process of the glassy sample via annealing in the midair at 405 °C for 2, 5, 10, and 20 h. Further details concerning the glass and corresponding nano-crystallized glass–ceramics synthesis were given in our previous paper Ref. [11]. The density values of the glassy specimen and corresponding nano-crystallized glass–ceramics were scaled using the Archimedes technique in Toluene as a dipping liquid (d(Toluene) = 0.8669 g/cm3). XRD 2θ scans were carried out to examine the glassy/nano-crystalline phases in the prepared samples by utilizing a Panalytical X’pert Pro diffractometer. FESEM; JSM-7600F-JEOL) was utilized to study the surface morphology of samples. For dc conductivity measurements, bulk samples were highly polished and then coated with silver paste on both surfaces of the pellet. The dc conductivity values were estimated by both current and voltage values by applying a constant dc voltage of 2 V to the pellets at room temperature (RT).
3 Results and discussion
3.1 Characterization of glass and nano-crystallized glass–ceramics
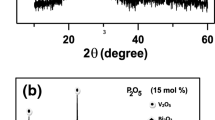
Figure 1 displays the XRD spectra of the as-synthesized glass sample and nano-crystallized glass–ceramics annealing at 450 °C, after the crystallization temperature (Tc) that was obtained from differential scanning calorimetry (DSC) as reported in our preceding work [25], for varied times (2, 5, 10, and 20 h). This figure exhibits the XRD of amorphous, Fig. 1a, and partially crystalline samples, Fig. 1b. It can be noticed that the glassy sample exhibited an amorphous halo exemplar for glass. The corresponding nano-crystallized glass–ceramics exhibited sharp reflections of diverse crystalline phases. The intensity and width of the reflections changed with diverse heat-treating times from 2 to 20 h. These crystalline phases indicated the structures of P4S5 (JCPDS#00-010-0329), Li4P2S6 (JCPDS#01-076-0992), Na2O (JCPDS#00-002-1288) and Li3VO4 (JCPDS#00-013-0249) [11, 25]. These nano-crystalline phases were different compared to the phases that were obtained in the previous papers [6, 26]. This may be due to the presence of mixed alkali oxides (Li2O and Na2O) and using P2S5 and V2S5 instead of P2O5 and V2O5. The crystalline size (D) of the nano-crystallized glass–ceramics was calculated by the full width at half maximum (FWHM) of the most intense peaks based on the average value of the several crystalline phases using the following equation [26]:
where c ≈ 0.9, λ = 0.15406 nm is the wavelength of X-ray used, β is the FWHM in radians, ϵ is the lattice strain, and θ is the Bragg angle. The ϵ in powders because of crystal distortion and crystal defect was estimated by utilizing the formula [26, 27]:
The D values for nano-crystallized glass–ceramics were obtained to be between 37 and 50 nm, as recorded in Table 1. These outcomes agree with the outcomes achieved by transmission electron microscopy (TEM) images reported in our previous work [11]. It is found that when the annealing time raised from 2 to 5 h, the D raised from 47 to 50 nm, respectively. After that, when the annealing time was raised to 10 h and 20 h, the D reduced to 42 nm and 37 nm, respectively. This result could be explained as follows: first, the heat-treating of the glass sample led to an increase in the atomic movement and boosted the capability of atoms to get the actively preferred sites and therefore the crystalline size increased with increasing HT time. Then, the increase in heat treatment time introduced many defects in the crystalline phase which destroyed the long-range clustering, causing the reduction of the crystal size [28, 29]. The variation of ϵ values for the as-synthesized nano-crystallized glass–ceramics with the difference in annealing time was displayed in Fig. 1c. The lattice strain of these nano-crystallized glass–ceramics was nearly similar (≈ 0.003), proposing that the lattice of the crystallite phase was not deformed with differences of D in the obtained crystalline phases (i.e., the as-synthesized nano-crystallized glass–ceramics had similar lattice constructions by variation of D in the obtained crystalline phases) [26, 30].
Figure 2 shows the FESEM images of the glass sample and nano-crystallized glass–ceramic annealing for 10 h. For the glassy sample, Fig. 2a, there are no discernible crystals, demonstrating an amorphous phase feature. The nano-crystallized glass–ceramic sample, Fig. 2b, on the other hand, had big secondary particles resulting from an aggregation of small elementary grains, and they did not have a regular form. The transport properties of nano-crystallized glass–ceramics are influenced by these grains. It is possible that the intrinsic features, such as conductivity, are responsible for the enhanced dynamics of alkali (such as Na and Li) ions, electrical properties, and electrochemical properties of these samples. Alkali ions do not disperse over a longer distance between the surface and the core during alkali transportation in grains with a relatively high surface area [11, 26]. This outcome showed that annealing may produce a comparatively large surface area, leading to elevated electronic and ionic conductivity, bigger capacity, and the highest rate ability [11, 16, 26]. As a result, these composites may be appropriate materials for use as electrodes in energy storage devices that need strong electronic and ionic conductivity (such as batteries or supercapacitors).
3.2 Density and molar volume
Utilizing toluene as a dipping liquid, Archimedes’ principle was used to compute the density (d) of the current glass and nano-crystallized glass–ceramics. The d values are scheduled in Table 1. The d of the glass sample was 2.58 g/cm3. However, the d values of nano-crystallized glass–ceramics were between 2.62 and 3.23 g/cm3. It was found that the densities of nano-crystallized glass–ceramics were greater than that of glass. The rise in density of nano-crystallized glass–ceramics might be owing to molecular rearrangement, which caused structural alterations. That is, the structure in nano-crystallized glass–ceramics was more tightly packed than in the comparable glass phase [31, 32]. As demonstrated in Table 1, for the nano-crystallized glass–ceramics, the d values followed the same crystalline size behavior with HT time, i.e., the variation in d values was owing to changes in the crystalline size caused by variations in the volume of these crystallite phases [6]. Table 1 shows that the magnitude of density was highest in nano-crystallized glass–ceramics with the biggest crystalline size, which is consistent with prior research [6, 16, 33]. For all samples, the molar volume (Vm) was computed using the formula [34]:
where M is molecular weight and d is density, respectively. The Vm of glass and corresponding nano-crystallized glass–ceramics are scheduled in Table 1. The relation between d and Vm with the change of HT time is displayed in Fig. 3. Since d is related to the mean molecular weight that is oppositely proportional to Vm, it is usually anticipated that d and Vm must display an inverse proportion [32].
3.3 DC conductivity
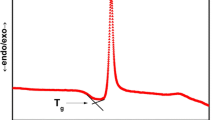
The reciprocal temperature reliance of the dc conductivity (σdc) for the as-prepared glass and corresponding nano-crystallized glass–ceramics that measured between 313 and 473 K was illustrated in Fig. 4. The figure displays that the chart became a straight line above a specified temperature, θD/2, where θD is the Debye temperature (listed in Table 1), suggesting that the σdc follows the Arrhenius relation [34]:
where k is the Boltzmann constant, W is the activation energy for conduction, and σ0 is the preexponential factor matching electrical conductivity at high temperatures. W is necessary to overawe the energy barriers and electrostatic force throughout the leap. Thus, as could be shown in Fig. 4, the σdc rose with temperature, which is a common feature of semiconducting glasses. Furthermore, the transportation is ascribed to electronic at low-temperature (i.e., T < θD/2); however, Li and Na ions might be moveable and take part in the conduction process at the high-temperature area (i.e., T > θD/2). Several phosphate glasses exhibiting TMI are comparable to this [35,36,37]. The slope of the plots (Fig. 4) was used to compute the W, but the intercept of the straight-line fits of the data was used to get the σ0. W values in the high-temperature areas are scheduled in Table 1. The σdc values of nano-crystallized glass–ceramics were greater than initial glass (Fig. 4). The existence of nano-crystals with average crystalline size in the range of 37–50 nm as observed in XRD was considered to be responsible for the improved conductivity. Other studies have reported remarkable increases in conductivity with the existence of nanostructure [16, 32, 35, 36]. To explain the electrical conductivity enhancement during nano-crystallization and decrease during colossal crystallization it is useful to refer to the well-established core–shell model. Pietrzaka et al. [37] have discussed this model. For this model a grain consists of an inner completely crystalline “core” and an outer highly disordered, defective, and non-stoichiometric “shell” [37]. The same “core–shell” model can clarify the conductivity improvement produced by nano-crystallization. The overlapping and intersecting defective shells around crystalline cores can form a complicated system of paths for facilitated electron transport. The mechanism of electronic transport in vanadium oxides consists of SPH between aliovalent V4+ and V5+ sites. In the regions where the local concentration of the V4+–V5+ pairs is high, the conductivity is also high. Such a circumstance takes place inside and around the defective grain-shell areas [37, 38]. In the case of colossal crystallization, the grains become well-developed crystallites with sharp edges. A negative result of this colossal crystallization is the decay of the “easy conduction paths” of the interfacial regions [37, 38]. However, the disappearance of the disordered shells due to colossal crystallization causes a considerable conductivity drop. On the other hand, heat-treated at 405 °C resulted in nanoparticles created in the glassy phase, which improved dc conductivity by forming a dense network of electrical conductivity routes between V2O5 nanoparticles [39]. Similar findings have recently been published in nano-crystallized glass–ceramics [16, 32]. Figure 5 depicts how changes in σdc and crystalline size with the change in HT time. The σdc and crystalline size clearly behaved in opposing manners. This suggests that grain boundary scattering had an influence on electrical conductivity. A potential barrier was created by the grain boundaries acting as electron traps. Conduction electrons scatter along grain boundaries as a result of the development of a potential barrier, reducing conductivity. As a result, the reduction in the scattering of grain boundary owing to decreasing crystalline size may be linked to the increase in conductivity [16, 32]. This result was in agreement with the results reported in previous works [6, 16, 28, 32, 35, 36, 38, 40]. With different HT times, the change in σdc of the present glass and its nano-crystallized glass–ceramics at a specific temperature (T = 409 K°) and W is shown in Fig. 6. According to Fig. 6, which conforms to the SPH conduction process in TMIs, the conductivity was lowest when the activation energy was higher. A conductivity of this nature results from the jumping of electrons between diverse valence states [34,35,36,37]. Consequently, the conductivity values at T over θD/2 were examined using the SPH model provided by Mott [41].
For the non-adiabatic system in the Mott model, the σdc for jumping to the closest neighbor at T > θD/2 was computed utilizing Eq. (4). The σ0 in Eq. (4) was calculated by this formula [32]:
where C is the fraction of TMI \((C = V^{ + } /\sum V )\), N is the TMIs density, R is the mean distance between TMIs, α is the tunneling factor, e is the charge of the electron and the optical phonon frequency, denoted by the symbol ν0, is calculated using the formula (kθD = hν0, where h is Plank's constant). The values of ν0 were found to be between 1.362 and 1.504 × 1013 Hz and are listed in Table 1.
The W could be given as [32]
where Wp is the binding energy, WH is the polaron hopping energy \(\left({W}_{\mathrm{H}}={W}_{\mathrm{P}}/2\right)\) and WD is the disorder energy that is defined as the variance in electronic energy between the two hopping locations. The values of WD are summarized in Table 2.
The αR in Eq. (5) is insignificant in the adiabatic hopping system [32, 42], and the σ0 is then described by the following formula [32, 34]:
The nature of the polaron hopping process might be ascertained from the graph of lnσdc versus W at a constant experimental T using the equation lnσdc = lnσ0 – W/2.303kTe (non-adiabatic or adiabatic) [6, 16, 42]. If the temperature (Te) that is determined by the plot is close to T then the hopping should take place in an adiabatic system. Otherwise, hopping will take place in a non-adiabatic system. Figure 7 illustrates the correlation between lnσdc and W for nano-crystallized glass–ceramics at a constant temperature (409 K). The results were linear over the whole range of W values, which was important to note. The Te was 323 K, which differed significantly from the specified temperature (409 K). Figure 8 displays the influence of HT time on σ0. This figure indicates that the σo changed with HT times. Based on both of these findings, we infer that non-adiabatic SPH was responsible for the conduction mechanism in the current samples. [5].
The following formula was used to calculate the number of ions per unit volume (N) [43, 44]:
where NA is the Avogadro number, P is the weight fraction of V2O5, M is its molecular weight, and d is the density. The average distance R between TMIs was determined through [43, 44]:
The R and N values for the current glass and nano-crystallized glass–ceramics are listed in Table 1.
For nano-crystallized glass–ceramics, Fig. 9 illustrates the correlation between R and W. W was affected by the site-to-site distance R in the measurement range. This result demonstrated that the relationship between W and R was positive. This result was consistent with previous reports [16, 32]. The nano-crystallized glass–ceramic annealing for 20 h had the lowest W (as presented in Fig. 9). This low W could be explained by a decrease in grain boundary scattering [16].
The polaron radius, rp, was computed using distance R from the formula [5, 6]:
The rp values are included in Table 2. It has been observed that for the present glass and its nano-crystallized glass–ceramics, rp < R. This indicates strong localization of the carriers in this system. The following relation was used to compute the polaron hopping energy (WH) [5, 6]:
where εP is the effective dielectric constant.
where εs and ε∞ are the static and high-frequency dielectric constants, respectively. εs was recorded at 120 Hz, whereas ε∞ was recorded at 100,000 Hz [32]. The computed WH and εp values are recorded in Table 2. To determine the density of states at the Fermi level, N(EF), the following formula could be used [6, 16, 32]:
The N(EF) values for the current glass and nano-crystallized glass–ceramics are scheduled in Table 2.
The small polaron coupling constant (γp), a measuring electron–phonon interaction, was obtained by utilizing the relationship [4]
The calculated values of γp for the current glass and its nano-crystallized glass–ceramics are summarized in Table 2. Generally, the γp > 4 referred to the strong electron–phonon interaction [5].
The polaron hopping model was studied by Emin and Holstein [45]. For the case of non-adiabatic hopping, they derived an equation for the σdc:
But for the adiabatic hopping:
where J denotes the polaron bandwidth associated with the overlap of the electron wave functions on the nearby sites. The nature of SPH conduction could also be examined using the polaron bandwidth, J, or the electron overlap integral, regardless of whether the system is adiabatic or non-adiabatic, as follows [45]:
For adiabatic
And for non-adiabatic
The values of J, which are estimated from the right-hand side of Eqs. (17a) or (17b), at 409 K for the current glass and nano-crystallized glass–ceramics, were found to be in the range of 0.0132–0.0165 eV. J must be calculated to clearly adopt whether the polaron is in an adiabatic or non-adiabatic system, and it could be determined via [34]:
The J values could be computed from Eq. (18) by utilizing the N(EF) and εp values for the current glass and nano-crystallized glass–ceramics. The J values obtained using Eq. (18) were approximately J < 0.001 eV. It can be noted that the J values for the current glass and nano-crystallized glass–ceramics (calculated by Eq. (18)) were clearly less than that of the right-hand side of Eqs. (17a) or (17b). So, for the current glass and corresponding nano-crystallized glass–ceramics, the theory of non-adiabatic hopping was the best suited to explain the phenomenon of polaron transport at great temperatures. Both of the above outcomes indicate that the current system conduction was owing to the non-adiabatic SPH of electrons.
The hopping carrier mobility, μ, in the non-adiabatic hopping system was obtained by the formula [5]:
In addition, the carrier density Nc was computed by the relationship [6]:
The μ and Nc values for the current glass and nano-crystallized glass–ceramics were listed in Table 3 and Fig. 10. These values were in agreement with the values reported in the previous papers [6, 16, 28, 40]. The electrons are strongly localized at the V-ion sites due to the strong electron–phonon interaction with large γp [16, 32], as evidenced by the very small of μ for the current system at 409 K. Owing to the localized condition for the conducting electrons being usual for μ < 0.01 cm2V−1 s−1, the creation of SP in the current glass and nano-crystallized glass–ceramics was reconfirmed [5]. Furthermore, since there was no significant change in the Nc values, the conductivity was principally decided by the hopping mobility [16].
The approach described by Greaves [46] as an adjustment to Molt's variable range hopping (VRH) model [47] may be used at intermediate temperature, and it gave the following formula for the σdc:
where A and B are constants. B is estimated by:
Figure 11 illustrates ln(σdc T1/2) as a function of T−1/4. Expression (21) provided an excellent match to the experimental data in the intermediate temperature region, indicating that Greaves’ VRH might be applicable to the current glass and its nano-crystallized glass–ceramic. Table 4 contains the parameters A and B derived from these curves. Expression (22) was utilized to determine the factor α. It is found that the α and N(EF) values were appropriate for the localized states [5, 16, 40].
4 Conclusions
18.75Li2O–18.75Na2O–25V2S5–37.5P2S5 glass and corresponding nano-crystallized glass–ceramics were successfully synthesized by utilizing melt-quenching and HT techniques at 405 °C for 2, 5, 10 and 20 h. The effects of the HT time on the structural and electrical features of the nano-crystallized glass–ceramics were investigated. XRD spectrum of the as-prepared glass promoted the amorphous nature. XRD spectra of the nano-crystallized glass–ceramics revealed nanocrystallites incorporated in the glassy phase with nano-crystalline size between 37 and 50 nm. The amorphous and crystalline natures of the as-synthesized glass and nano-crystallized glass–ceramics were also verified by FESEM images. The nano-crystallized glass–ceramics demonstrated higher dc conductivity and lower activation energy for conduction than the as-prepared glass because between V2O5 nanoparticles there had formed a vast and dense network of electronic conductivity tracks. The conductivity and crystalline size exhibited opposite behaviors to each other (i.e., the grain boundary scattering affected electrical conductivity). The nano-crystallized glass–ceramic annealing for 20 h which had the lowest particle size, demonstrated the highest dc conductivity and the lowest activation energy. This outcome could be ascribed to the reduction in the scattering of grain boundary owing to the decrease in particle size. The conductivity was principally determined by hopping carrier mobility, and it was verified that the conduction obeyed non-adiabatic SPH. The Greaves VRH model was found to be suitable at intermediate temperatures.
Data availability
Data will be made available from the corresponding author upon reasonable request.
References
M.M. El-Desoky, Small polaron transport in V2O5–NiO–TeO2 glasses. J. Mater. Sci. 14, 215–221 (2003). https://doi.org/10.1023/A:1022981929472
M.S. Al-Assiri, M.M. El-Desoky, Grain-size effects on the structural, electrical properties and ferroelectric behaviour of barium titanate-based glass–ceramic nano-composite. J. Mater. Sci. 24, 784–792 (2013). https://doi.org/10.1007/s10854-012-0810-x
K. Fukuda, A. Ikeda, T. Nishida, A significant improvement of the electrical conductivity of semiconducting vanadate glasses caused by heat treatment. Solid State Phenom. 90–91, 215–220 (2003). https://doi.org/10.4028/www.scientific.net/SSP.90-91.215
N.F. Mott, Electrons in disordered structures. Adv. Phys. 16, 49–144 (1967). https://doi.org/10.1080/00018736700101265
I.G. Austin, N.F. Mott, Polarons in crystalline and non-crystalline materials. Adv. Phys. 18, 41–102 (1969). https://doi.org/10.1080/00018736900101267
M.M. El-Desoky, A.M. Al-Syadi, M.S. Al-Assiri, H.M. Hassan, Effect of sulfur addition and nanocrystallization on the transport properties of lithium–vanadium–phosphate glasses. J. Mater. Sci.: Mater. Electron. 29, 968–977 (2018). https://doi.org/10.1007/s10854-017-7994-z
L. Jlassi, H. Elhouichet, A comparative investigation of optical and electrical properties of vanadate zinc lithium phosphate glass and nanostructured glass–ceramics. Braz. J. Phys. 52, 1–13 (2022). https://doi.org/10.1007/s13538-021-01005-3
S. Mandaland, S. Hazra, Structural and physical properties of Fe2O3-doped lead vanadate glass. J. Mater. Res. 15, 218–221 (2000). https://doi.org/10.1557/JMR.2000.0035
S. Yusub, P.S. Rao, D.K. Rao, Ionic conductivity, dielectric and optical properties of lithium lead borophosphate glasses combined with manganese ions. J. Alloys and Compounds 663, 708–717 (2016). https://doi.org/10.1016/j.jallcom.2015.12.147
A.M. Al-Syadi, M.S. Al-Assiri, H.M. Hassan, G. El Enany, M.M. El-Desoky, Effect of sulfur addition on the electrochemical performance of lithium-vanadium-phosphate glasses as electrodes for energy storage devices. J. Electroanal. Chem. 804, 36–41 (2017). https://doi.org/10.1016/j.jelechem.2017.09.041
A.M. Al-Syadi, Electrochemical performance of Na2O–Li2O–P2S5–V2S5 glass–ceramic nanocomposites as electrodes for supercapacitors. Appl. Phys. A 127, 755 (2021). https://doi.org/10.1007/s00339-021-04899-7
T. Honma, K. Nagamine, T. Komatsu, Fabrication of olivine-type LiMnxFe1−xPO4 crystals via the glass–ceramic route and their lithium ion battery performance. Ceram. Int. 36, 1137–1141 (2010). https://doi.org/10.1016/j.ceramint.2009.10.003
S.J. Japari, A.K. Yahya, R. Hisam, Effects of mixed-alkali oxides on AC conductivity and dielectric properties of xNa2O-(20–x)K2O–30V2O5–50TeO2 glasses. Results Phys. 16, 102905 (2020). https://doi.org/10.1016/j.rinp.2019.102905
J.E. Garbarczyk, P. Jozwiak, M. Wasiucionek, J.L. Nowinski, Enhancement of electrical conductivity in lithium vanadate glasses by nanocrystallization. Solid State Ionics 175, 691–694 (2004). https://doi.org/10.1016/j.ssi.2004.08.025
J.E. Garbarczyk, P. Jozwiak, M. Wasiucionek, J.L. Nowinski, Nanocrystallization as a method of improvement of electrical properties and thermal stability of V2O5-rich glasses. J. Power Sources 173, 743–747 (2007). https://doi.org/10.1016/j.jpowsour.2007.05.069
A.M. Al-Syadi, M.S. Al-Assiri, H.M. Hassan, M.M. El-Desoky, Grain size effects on the transport properties of Li3V2(PO4)3 glass–ceramic nanocomposites for lithium cathode batteries. J. Mater. Sci.: Mater. Electron. 27, 4074–4083 (2016). https://doi.org/10.1007/s10854-015-4266-7
M.Y. Murashkin, I. Sabirov, X. Sauvage, R.Z. Valiev, Nanostructured Al and Cu alloys with superior strength and electrical conductivity. J. Mater. Sci. 51, 33–49 (2016). https://doi.org/10.1007/s10853-015-9354-9
A. Gupta, V. Choudhary, Electrical conductivity and shielding effectiveness of poly(trimethylene terephthalate)/multiwalled carbon nanotube composites. J. Mater. Sci. 46, 6416–6423 (2011). https://doi.org/10.1007/s10853-011-5591-8
S.A. Abdelwahab, A. Ali, K. Abdelwahed, I. Ahmed, A.I. Ali, Influence of TiO2/GO weight ratio on the structure, mechanical, and electrical properties of SiO2–Al2O3 glass–ceramics. J. Mater. Sci.: Mater. Electron. 32, 11092–11106 (2021). https://doi.org/10.1007/s10854-021-05774-0
P. Jozwiak, J.E. Garbarczyk, Mixed electronic–ionic conductivity in the glasses of the Li2O–V2O5–P2O5 system. Solid State Ionics 176, 2163–2166 (2005). https://doi.org/10.1016/j.ssi.2004.06.028
C. Cao, Z.-B. Li, X.-L. Wang, X.-B. Zhao, W.-Q. Han, Recent advances in inorganic solid electrolytes for lithium batteries. Front. Energy Res. 2, 25 (2014). https://doi.org/10.3389/fenrg.2014.00025
R. Barik, P.P. Ingole, Challenges and prospects of metal sulfide materials for supercapacitors. Curr. Opin. Electrochem. 21, 327–334 (2020). https://doi.org/10.1016/j.coelec.2020.03.022
L.C.R. Schneider, C.L. Martin, Y. Bultel, D. Bouvard, E. Siebert, Discrete modelling of the electrochemical performance of SOFC electrodes. Electrochim. Acta 52, 314–324 (2006). https://doi.org/10.1016/j.electacta.2006.05.018
C.W. Kim, J.S. Park, K.S. Lee, Effect of Fe2P on the electron conductivity and electrochemical performance of LiFePO4 synthesized by mechanical alloying using Fe3+ raw material. J. Power Sources 163, 144–150 (2006). https://doi.org/10.1016/j.jpowsour.2006.02.071
S. Kumar, B. Kishore, N. Munichandraiah, Electrochemical studies of non-aqueous Na–O2 cells employing Ag-RGO as the bifunctional catalyst. RSC Adv. 6, 63477–63479 (2016). https://doi.org/10.1039/C6RA13596J
A.M. Al-Syadi, M.S. Al-Assiri, H.M. Hassan, M.M. El-Desoky, Grain size effects on dynamics of Li-ions in Li3V2(PO4)3 glass-ceramic nanocomposites. Ionics 22, 2281–2290 (2016). https://doi.org/10.1007/s11581-016-1772-4
V.D. Mote, Y. Purushotham, B.N. Dole, Williamson-Hall analysis in estimation of lattice strain in nanometer-sized ZnO particles. J. Theor. Appl. Phys. 6, 1–8 (2012). https://doi.org/10.1186/2251-7235-6-6
A.E. Harby, A.E. Hannora, M.S. Al-Assiri, M.M. El-Desoky, Correlation between grain size and transport properties of lead titanate based-glass–ceramic nano-composites. J. Mater. Sci.: Mater. Electron. 27, 8446–8454 (2016). https://doi.org/10.1007/s10854-016-4859-9
A.A. Bahgat, Sh. Heikal, I.A. Mahdy, A.S. Abd-Rabo, A.A. Ghany, Cyclic electrical conductivity in BaTiO3–PbTiO3–V2O5 glass-ceramic nanocomposite. Physica B 447, 23–29 (2014). https://doi.org/10.1016/j.physb.2014.04.048
E.N. Brown, P.J. Rae, D.M. Dattelbaum, B. Clausen, D.W. Brown, In-situ measurement of crystalline lattice strains in polytetrafluoroethylene. Exp. Mech. 48, 119–131 (2008). https://doi.org/10.1007/s11340-007-9075-3
T. Takada, S.F. Wang, S. Yoshikawa, S.J. Jang, R.E. Newnham, Effect of glass additions on BaO–TiO2–WO3 microwave ceramics. J. Am. Ceram. Soc. 77, 1909–1916 (1994). https://doi.org/10.1111/j.1151-2916.1994.tb07070.x
A.A. Bahgat, M.G. Moustafa, E.E. Shaisha, Enhancement of electric conductivity in transparent glass–ceramic nanocomposites of Bi2O3–BaTiO3 glasses. J. Mater. Sci. Technol. 29, 1166–1176 (2013). https://doi.org/10.1016/j.jmst.2013.08.005
Y.G. Wang, S.P. Lau, H.W. Lee, S.F. Yu, B.K. Tay, X.H. Zhang, H.H. Hng, Photoluminescence study of ZnO films prepared by thermal oxidation of Zn metallic films in air. J. Appl. Phys. 94, 354–358 (2003). https://doi.org/10.1063/1.1577819
N.F. Mott, Conduction in glasses containing transition metal ions. J. Non-Cryst. Solids 1, 1–17 (1968). https://doi.org/10.1016/0022-3093(68)90002-1
J.E. Garbarczyk, T.K. Pietrzak, M. Wasiucionek, A. Kaleta, A. Dorau, J.L. Nowiński, High electronic conductivity in nanostructured materials based on lithiumiron-vanadate-phosphate glasses. Solid State Ionics 272, 53–59 (2015). https://doi.org/10.1016/j.ssi.2014.12.019
T.K. Pietrzak, E. Jerzye, M. Wasiucionek, J.L. Nowiński, Nanocrystallisation in vanadate phosphate and lithium iron vanadate phosphate glasses. Phys. Chem. Glasses-Eur. J. Glass Sci. Technol. Part B 57, 113–124 (2016). https://doi.org/10.13036/17533562.57.3.038
T.K. Pietrzak, J.E. Garbarczyk, I. Gorzkowska, M. Wasiucionek, J.L. Nowinski, S. Gierlotka, P. Jozwiak, Correlation between electrical properties and microstructure of nanocrystallized V2O5–P2O5 glasses. J. Power Sources 194, 73–80 (2009). https://doi.org/10.1016/j.jpowsour.2009.02.031
M.S. Al-Assiri, M.M. El-Desoky, Correlation between nanostructural and electrical properties of barium titanate-based glass–ceramic nano-composites. J. Alloy. Compd. 509, 8937–8943 (2011). https://doi.org/10.1016/j.jallcom.2011.06.079
M.Y. Hassaan, F.M. Ebrahim, A.G. Mostafa, M.M. El-Desoky, Effect of sulfur addition and heat treatment on electrical conductivity of barium vanadate glasses containing iron. Mater. Chem. Phys. 129, 380–384 (2011). https://doi.org/10.1016/j.matchemphys.2011.04.029
M.S. Al-Assiri, S.A. Salem, M.M. El-Desoky, Effect of iron doping on the characterization and transport properties of calcium phosphate glassy semiconductors. J. Phys. Chem. Solids 67, 1873–1881 (2006). https://doi.org/10.1016/j.jpcs.2006.04.015
N.F. Mott, Conduction in non-crystalline materials: III. Localized states in a pseudogap and near extremities of conduction and valence bands. Philos. Mag. 19, 835–852 (1969). https://doi.org/10.1080/14786436908216338
H.H. Qiu, H. Sakata, T. Hirayama, Electrical conductivity of Fe2O3-PbO-Bi2O3 glasses. J. Ceram. Soc. Jpn. 104, 1004–1007 (1996). https://doi.org/10.2109/jcersj.104.1004
M. Pant, D.K. Kanchan, P. Sharma, M.S. Jayswal, Mixed conductivity studies in silver oxide based barium vanado–tellurite glasses. Mater. Sci. Eng., B 149, 18–25 (2008). https://doi.org/10.1016/j.mseb.2007.11.037
H. Mori, T. Kitami, H. Sakata, Electrical conductivity of V2O5- Sb2O3- TeO2 glasses. J. Non-Cryst. Solids 168, 157–166 (1994). https://doi.org/10.1016/0022-3093(94)90132-5
D. Emin, T. Holstein, Studies of small-polaron motion IV. Adiabatic theory of the Hall Effect. Ann. Phys. 53, 439–520 (1969). https://doi.org/10.1016/0003-4916(69)90034-7
G.N. Greaves, Small polaron conduction in V2O5 P2O5 glasses. J. Non-Cryst. Solids 11, 427–446 (1973). https://doi.org/10.1016/0022-3093(73)90089-6
N.F. Mott, E.A. Davis, Electronic Processes in Non-crys-talline Materials (Oxford University Press, Oxford, 2012)
Acknowledgements
The authors are thankful to the Deanship of Scientific Research at Najran University for funding this work, under the General Research Funding program grant code (NU/DRP/SERC/12/11).
Funding
The authors are thankful to the Deanship of Scientific Research at Najran University for funding this work, under the General Research Funding program grant code (NU/DRP/SERC/12/11).
Author information
Authors and Affiliations
Contributions
AMA contributed to the conception of formal analysis, methodology, investigation, project administration, conceptualization, validation, writing—original draft, writing—review, editing, and revision of the manuscript. MA contributed to the conception of validation, investigation, writing—review, and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Ethical approval
The work does not require ethical approval.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Al-Syadi, A.M., Abaker, M. Effect of heat treatment time on the transport properties of nano-crystallized Li2O–Na2O–V2S5–P2S5 glass–ceramics. Appl. Phys. A 129, 425 (2023). https://doi.org/10.1007/s00339-023-06702-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-06702-1