Abstract
Cancer-related deaths are rising every day due to the lack of efficient detection methods in the primary stages, especially before metastasis, which is the main reason for the uncontrollability of cancer. Thus, a novel method is urgently required to efficiently and accurately identify circulating cancer cells scattered in the blood of patients with the primary stages of cancer. Here, in this work, a new fluorescent biosensor based on amine-functionalized graphene quantum dot (af-GQD) was constructed through a hydrothermal method from graphene oxide. Afterward, af-GQD was grafted with HA (GQD-HA) for specific detection through a ligand–receptor concept in which HA recognizes CD44 receptors, which are overexpressed on certain cancer cells such as A549, MDA-MB-231, MCF-7, and HeLa. Then, the intensity of fluorescent signals detected from each cell line media was assessed and quantified. Besides, the designed nanocomposite can be used as a bioimaging agent due to its remarkable properties, such as high fluorescent emission, good compatibility, low cytotoxicity, high resistance to photobleaching, and chemical stability. The results indicated that the designed nanocomposite could act either as an efficient a bioimaging agent or as a biosensor with high specificity and sensitivity with a linear detection range from 500 to 5000 cells/ml for the detection of CTCs. The limit of detection for cell lines was 95, 98, 112, 123 cell/ml for MDA-MB-231, A549, MCF-7, and HeLa, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
GLOBOCAN 2020 has reported 19.2 million new cases and approximately 10 million deaths by cause of cancer in 2020 [1]. Cancer is fatal, mainly because of metastasis. In this process, cancer cells undergo EMT (epithelial–mesenchymal transition), become motile, and enter the blood or lymph stream, where they are called CTCs. CTC (circulating tumor cells) is a term referring to tumor cells that are shed from the primary tumor site, move through the blood and lymph stream, and may form secondary tumors in other organs [2, 3]. Studies have shown that, despite our previous thoughts that CTCs are only seen in later stages of cancer development, their presence can be detected in the early stages, even before the primary tumor [4]. Contrary to the millions of WBC and RBC found in 1 ml of blood, CTCs are rare (100–1000 CTCs per ml for an active tumor) [5]. Thus, designing a low-cost, non-invasive, rapid, and sensitive method for the detection of CTCs in the bloodstream is urgently needed for cancer diagnosis and prognosis.
CD44 (cluster of differentiation 44) is a multi-structural and multi-functional adhesion glycoprotein that passes through the membrane and acts as a receptor [6]. It is also seen in different human cells, including embryonic stem cells and other differentiated cells [7]. CD44 is overexpressed in cancer cells, especially CTCs [8, 9] and it is involved in tumor initiation and EMT regulation [7] and can be used as a tumor biomarker for cancer diagnosis, prognosis, and therapy [10, 11]. Hyaluronic acid macromolecules (HA), which act selectively for targeting cancer cells with increased expression of CD44 receptors, are found in the extracellular matrix of normal tissues, [12]. HA is biocompatible and does not arouse an immune response. As a result, it can be used as a functionalizing agent that can target specific cancer cells.
Nanomaterials have various applications[13,14,15], in particular, they have been considerably employed for biomedical applications such as constructing nanofibers [16,17,18,19], tissue engineering scaffolds [20, 21], cancer therapy [22], drug delivery [23], developing vaccines [24], and designing biosensors [25,26,27]. Lately, nanomaterials have found particular applications in construction of biosensors for detection of cancer [28]. GQD (graphene quantum dot)-based biosensors have shown great promise in monitoring and capturing of CTCs [28, 29] because of their exceptional characteristics [30, 31]. GQDs are nanometer-sized graphene sheets with many functional groups on their surface. They have a high surface-to-volume-area ratio and their size is less than 10 nm [32]. GQDs have attracted lots of attention because of their unique properties related to their structure, such as low toxicity, excellent biocompatibility, and resistance to photobleaching which makes them excellent candidates to be employed as a biosensor [30, 33].
Recent studies show the crucial role of GQD in the development of novel biosensors for circulating cancer cell detection. For example, in a study by Lyu et al. [34], PEGylated GQDs have been employed for EpCAM (epithelial cell adhesion molecule) detection, which is overexpressed in some breast cancer cells, such as MCF-7. In this study, PEG-GQD is furnished on the MoS2 sheet surface, and the GQD fluorescence signal is consequently quenched. When exposed to cancer cells with EPCAM proteins, the PEG-GQDs detach from the MoS2 surface because of their higher affinity for EPCAM, and therefore, the fluorescence intensity is restored. In another work, Ruiyi et al. [31] designed a core–shell nanostructure for CTC detection in blood. First, they grafted GQD with folic acid (FA) and Glutamic acid (Glu), then functionalized it with palladium (Pd) and gold (AU). The resulting nanocomplex, FA/Glu-GQD-pd@Au, was used as a potential biosensor for electrochemical detection of circulating HepG2 cancer cells in human blood with ultra-high sensitivity.
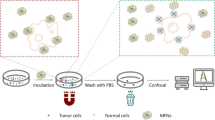
Now, in this study, we make a new nanocomposite that can find and image CTCs accurately. In our research, a GQD-based bio-safe biosensor with fluorescent properties was developed for CD44 high cancer cell detection. First, GQD was synthesized from GO using a hydrothermal method. Second, a hydrothermal strategy was employed for GQD amination and constructing af-GQD. Then, af-GQD was directly furnished with HA (GQD-HA). The functionalization process of af-GQD was not mediated by any molecule, thanks to the amide bonds provided by the amino groups on the surface of af-GQD. Our investigation determined that GQD-HA can recognize human cancer cells rich in CD44 receptors (MDA-MB-231 [11], A549 [35], MCF-7 [36], HeLa [37]) with high sensitivity and specificity. Furthermore, this nanocomposite acted as an efficient in vitro fluorescence bioimaging agent (Fig. 1).
2 Materials and methods
Graphite meshes (size < 200 mm), ammonia (25%), sulfuric acid (95–97%), NaNO3, H2O2 (30%), and HCl (97%) were purchased from Merck; HA powder (50 kD), glutaraldehyde (GA), dimethylformamide (DMF), dimethyl sulfoxide (DMSO), EDC, NHS, and MES buffer were provided by Sigma-Aldrich; 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) powder was purchased from Merck; Trypsin–EDTA solution, DMEM, FBS, and penicillin–streptomycin for cell culture were provided by Bio Idea Company.
2.1 GQD synthesis
Initially, GO was produced by oxidizing graphite through a modified Hummers method [38, 39] in a hydrothermal autoclave reactor (Zarir Pars Company). In summary, 1 g of graphite powder was added to a beaker containing 46 ml sulfuric acid and stirred for 15 min at 5 °C. Subsequently, 1 g of NaNO3 was added to the solution, while the stirring was maintained for 10 min. Then, 6 g of KMnO4 was gradually added to the mixture, and consequently, the temperature was elevated (20 °C). Meanwhile, the circumstances remained as they were; the stirring was carried out for another 20 min and, afterward, for 3 h at 35–40 °C. To reduce the concentration of the solution, DI water was added in two steps while stirring continued. In the first step, the beaker containing the solution was placed into an oil bath at 90 °C, and then 80 ml of DI was poured into the mixture. In the second step, 200 ml DI was added, and the beaker was placed on the stirrer at room temperature. After 30 min, 6 ml of H2O2 was added drop by drop to the mixture, resulting in a yellow–brown solution. After that, filter paper was used to separate the solid particles from the liquid. In the next step, HCL solution (10%) was used to wash the remaining precipitate on the filter paper. Thenceforth, washing with DI was done to increase the precipitate’s PH to 5–6. The washed precipitate found on the filter paper is graphite oxide that should be collected, sonicated, and dried (< 38 °C) to yield GO.
GQD was synthesized using a hydrothermal method. For this purpose, 270 mg of the GO powder was dissolved in 10 ml DMF, sonicated for 30 min, and then transferred to a PTFE reactor with a capacity of 30 ml. The reactor was heated to 200 °C for 5 h. Following this, the solution (of an orange color) was collected and passed through a 0.22 filter, and then freeze-dried for 24 h. The resultant sediment is GQD and kept in a refrigerator (4 °C) until use.
SEM and TEM (scanning and transmission electron microscopy) images of nanoparticles were obtained by electron microscope (Philips XL30, Netherlands, and Philips Electron Optics) to characterize the size and morphology of nanoparticles. Besides, the synthesized nanoparticles’ hydrodynamic diameter and zeta potential were estimated by the SZ-100z Dynamic Light Scattering and Zeta potential analyzer (Horiba Jobin Jyovin)). Moreover, EDX and FESEM MIRA3 were applied to study the single and conjugated nanoparticles.af-GQD synthesis
A hydrothermal method [40] was utilized for adding amine groups to GQD and producing af-GQDs. Briefly, 8 mg of GQD was dissolved in 3 ml DI, and then 3 ml ammonia was poured into the mixture. Afterward, the solution was stirred for 15 min and, next, sonicated for 30 min, and subsequently transferred to a 40 ml PTFE reactor to maintain the hydrothermal reaction at 180 °C for 10 h. Then, the reactor was stored at room temperature to cool. To eliminate any remaining ammonia, the solution was heated to 100 °C on a stirrer until the PH dropped to 7. The resulting solution, which contains af-GQD, was freeze-dried to be used in further experiments.
2.2 GQD-HA synthesis
To bind GQD to HA, first, the HA’s surface carboxyl groups were activated using the N-ethyl-N′-(3-(dimethylamino) propyl) carbodiimide/N-hydroxysuccinimide (EDC/NHS) coupling agent. Briefly, 10 mg HA was solubilized in 5 ml of MES buffer (0.1 M pH 5.5), and then EDC/NHS with the concentration of 0.8 and 1.2 mg/ml (this amount has been adjusted in our previous study[23]), respectively, were added into the HA solution, and stirred slowly for 1 h at room temperature. Alongside 20 mg af-GQD was dissolved in 5 ml MES buffer (0.1 M pH 8) and sonicated for 15 min. Then, af-GQD solution was added to the HA solution and reacted overnight on a slow stirrer in the dark. Finally, the solution was purified through a dialysis bag against PBS buffer (pH 7.4) for 24 h to remove free af-GQD. The purified solution was collected for the next experiment. Fourier Transform Infrared Spectroscopy (FTIR) spectra were measured by (Thermo Nicolet Nexus 670, USA) before and after adding HA. Moreover, the optical properties of GQD, af-GQD, and GQD-HA were studied by UV/Vis absorption (SPECORD 50 PLUS, Germany) and photoluminescence (PL) spectroscopy (Varian Cary Eclipse, USA) and Citation 3 Bio Tek.
2.3 Cell culture
MDA-MB-231 [11], A549 [35], MCF-7 [36] and HeLa [37] cancer cells as CD44+ samples, and L929 cells [41] as CD44− samples (control) were cultured in DMEM medium enriched with 10% FBS and 1% antibiotics and incubated at 37◦C in a balanced air incubator with 5% CO2. All cell images in this paper were obtained from a confocal laser scanning microscope, Leica TCS SPE (Germany).
2.4 Cytotoxicity test
The toxicity of the synthesized nanoparticles, GQD, af-GQD, and GQD-HA, was measured by MTT assay using L929 cells. First, the cells were cultured in 96-well plates with a concentration of 10,000 cells per well for 24 h. Second, the washing step was carried out by collecting each well upper medium and rinsing with PBS. Then, specific concentrations (0.25, 0.5, 1, and 2 mg/ml, respectively) from each of our nanoparticles were applied to the cells in each well. After 24 h of incubation in an incubator, the washing step was repeated. Afterward, 20 μl of MTT solution (5 mg/ml in PBS buffer pH 7.4) and 180 μl of DMEM were applied to each well on the plate and stored in an incubator for 4 h. Subsequently, the upper medium was removed. In the final step, to dissolve the formazan crystals formed in the living cells, equal volumes (200 μl) of DMSO were added to each well. A microplate reader was used to evaluate the rate of living cells by absorbance measurement at 570 nm. The toxicity results of the nanocomposite were measured by the microplate reader EPOCH2 (BioTek, American).
2.5 Fluorometry assay
MDA-MB-231, A549, MCF-7, HeLa, and L929 cell lines were cultured for 24 h, then specific numbers of 500, 1000, 3000, 5000, 10,000, 25,000, 50,000, and 100,000 cells were transferred to different microtubes and centrifuged 1500g for 5 min. Notably, six replicates were considered for each concentration. Then, all the cells were washed with PBS, equal volumes of GQD-HA solution (2 mg/ml) were added to each microtube, and then incubated for half an hour. Afterward, microtubes were centrifuged 1500 g for 5 min and the upper medium was collected and transferred to a 96-well black-colored quartz plate in order to measure the fluorescent emission spectrum using CYTATION 3 multiplate reader.
2.6 Confocal microscopy study
MDA-MB-231, A549, MCF-7, HeLa, and L929 cell lines were cultured in a 24-well plate on a coverslip with 100,000 cell density and incubated for 24 h in an incubator. Then, GQD-HA solution (2 mg/ml) was added to each well and incubated for half an hour. To remove the unattached GQD-HA, the upper medium was gathered cautiously, and the cells were washed twice with PBS. For cell fixation, the cells were then exposed to paraformaldehyde at 4% for 20 min. Finally, the cells were washed twice with PBS, and 200 µl PBS was added to each well before imaging by confocal laser scanning microscope.
In order to evaluate our GQD-HA cancer cells selectivity, 100 A549 and 100 MDA-MB-231 cancer cells were separately mixed with 200,000 L929 normal cells and cultured on a coverslip placed in a 24-well cell culture plate. Then, they were applied with a specific concentration of GQD-HA and monitored with laser confocal microscopy imaging.
3 Result and discussion
The SEM images of GO are shown in Fig. 2a. The GO morphology displays a thin, layered structure, endorsing that the graphite oxidation process has been completed. Figure 2b. display TEM image GQD. It is clear from the results that GQD nanoparticles possess a circular shape with a size of 3.9 ± 1.1 nm obtained using imageJ software.
The existence and percentage of each element in the analyzed nanocomposites were determined by employing EDX (Fig. 3). The number of oxygen groups in GQD samples was 43.12%, which confirms the presence of hydroxyl and carboxyl groups on the edges of GQD particles. GQD’s EDX chart shows a low level of nitrogen, which may be attributed to the free nitrogen being absorbed by the surface. The practical completion of the amination process is determined by an increase in the surface-nitrogen concentration of 7.43 percent in the af-GQD sample. The number of carbon molecules in GQD-HA samples has been shown to rise (66.81%), which may be related to the addition of carbon molecules conforming to HA in the samples. Furthermore, the decrease in nitrogen amount of GQD-HA may be attributable to their binding to the carboxylic groups in HA molecules.
Figure 4 exhibits the results of the zeta potential and DLS of nanostructures. GQD has a zeta potential of − 20 mv, which is because of the carboxyl and hydroxyl groups available on its surface. The aminated GQD displays a more positive zeta potential of − 1.1 mV because of the positive charge of amine groups. The addition of HA to af-GQD contributes to lower zeta potential due to negatively charged hydroxyl groups in the hyaluronic acid chemical structure. In each stage, the shift in GQDs’ charge indicates that the reactions were carried out successfully. The DLS results suggest that the GQD size was 5.3 nm and the af-GQD particles were sized at 2.2 nm. In fact, the decrease in the mean size of af-GQD compared to GQD stems from the fact that for the amination procedure, another hydrothermal process was carried out, which reduced the size as the same results were also reported in other studies [42]. On the other hand, the conjugation of HA to the af-GQD surface increases the nanocomposite diameter with a more even size distribution, declaring successful fabrication of the nanocomposite. The DLS results also confirmed the TEM result.
To analyze the surface functional groups of GQD, af-GQD, and GQD-HA, FTIR spectroscopy was performed (Fig. 5). Through exploring the HA curve [23, 43,44,45], a broad peak at 3569 cm−1 indicates the presence of hydroxyl groups (OH). The appearance of the peak at 1621 cm−1 are attributable to the companionship of carboxyl groups. In addition, obtaining four dominant peaks at 1037 cm−1 (C–O–C stretching), 1414 cm−1 (CO group with C=O combination/amide II, N_H bending), 1621 cm−1 (amide II group, C=O stretching), and at 2886 cm−1 that reveals the existence of C–H stretching.
The FTIR curve of GQD demonstrated a sharp peak at 3421 cm−1, which is due to the existence of hydroxyl groups [40], and 1457 and 2896 peaks, imply the presence of C–H bond. Moreover, the peaks at 1429 and 2947 cm−1 are also related to the C–H groups [46]. In addition, the presence of 1027 and 1629 cm−1 peaks maintains the presence of C–O and C=C [23, 45, 47].
In the case of the af-GQD curve, the 1362 cm−1 (vibrational stretching C–N), 1558 cm−1 (N–H bending), and 3432 cm−1 peaks indicate the existence of amino groups in af-GQD. As expected, when the spectra of HA and af-GQD were added together, they both had a clear peak in the same wavelength region, but the intensity of the peaks was not the same. Specifically, the intensity of the peaks in the 1362 cm−1 (C–N stretching vibration) and 1045 cm−1 (C–O) is reduced. Moreover, the peaks at 1623 and 1413 cm−1 represented amide I and amide II, indicating the formation of amide bonds [45, 47, 48].
Above all, the results of the FTIR spectra and zeta potential confirmed the adherence of amino groups on the surface of af-GQD and HA on the surface of GQD-HA.
UV–visible spectroscopy was also carried out to ensure that the HA binding on af-GQD was done successfully. As shown in Fig. 6, the GQD absorption peak was monitored at 200 nm owing to the \(\pi -\pi\)* transition of the aromatic C=C bond. The shoulder of the GQD-HA absorption curve displays the surface states that are contributed to a sequence of functional group absorption bands with lower energy. The position of the absorption peak between GQD and af-GQD was quite similar.
The excitation spectrum, emission spectrum, and fluorescence intensity may all be measured using a fluorescence spectrophotometer. The fluorescence properties of GQD are demonstrated in Fig. 6. With the changes in excitation wavelength from 340 to 500 nm, the maximum fluorescent emission peak of GQD was shifted to a long wavelength, and its intensity was decreased.
According to Fig. 7, the GQD (230,312 nm), af-GQD (242,324 nm), and GQD-HA (227,316 nm) samples possess excitation spectra of 200–400 nm and emission spectra of 320–550 nm with the emission peak at 421, 413, and 433 nm, respectively. The excitation curve of each nanoparticle possesses two peaks. These peaks resemble two types of electron transitions from \(\pi\) and \(\sigma \mathrm{orbital}\) to the lowest unoccupied electron orbital. The excitation peak of af-GQD was 324 nm which was lower than the other two nanoparticles because of the surface amine group electron donor properties. This fact once again confirms GQDs’ accurate amination response. Notably, af-GQDs shine more brightly than GQD and GQD-HA because of the lone pair electrons in nitrogen and donor group of the amines group.
Before using nanoparticles in biomedical applications, cytotoxicity testing must be performed to ensure that they are safe. The cytotoxicity of GQD, af-GQD, and GQD-HA is investigated by applying certain quantities of a medium comprising nanoparticles (e.g., 2, 1, 0.5, and 0.25 mg/ml) to L929 cells and incubating them. Figure 8 displays the viability of L929 cells through the MTT assay after 24 h of incubation with the nanoparticles. The outcome of the MTT test reveals high viability of the L929 cell line (> 70%) and efficient biocompatibility. GQD-HA not only exhibited maximum viability among other nanoparticles but also helped cell proliferation because of the ECM component, HA.
To evaluate the biosensor’s efficiency, several concentrations of each cell line were used. For this purpose, 500, 1000, 3000, 5000, 10,000, of each cell line were suspended in a microtubes. As shown in Fig. 9, the cancer cell concentration of 500–5000 results in the most intense quenching of the fluorescent signal in the collected upper solution. In contrast, increased cell concentration does not affect fluorescent signal quenching anymore. The reason behind this result is that when the cell’s concentration is too high, they form aggregates, and thus, many of the cell’s CD44 receptors are not available anymore to link to the GQD-HA nanoparticles.
Since the GQD-HA biosensor adheres specifically to CD44+ cells, there is a correlation between the number of surface CD44 receptors and the fluorescent intensity; thus, as the number of CD44+ cells applied with GQD-HA increases, the fluorescent intensity decreases in the collected upper solution.
As seen in Fig. 8, MDA-MB-231 cell line curve shows that different concentrations of cells resulted in different fluorescent emissions. The fluorescent emissions are obtained from evaluating the cells’ medium which is gathered after applying GQD-HA, so maximum emission means no CD44 receptors on the surface of the cells, which results in presence of all of the applied GQDs in the gathered medium. Consequently, as the number of cells with CD44 on their membrane increases, more GQDs are attached to the cells and a smaller proportion of them are present in the medium, so the GQDs emission decreases. This biosensor demonstrated a wide linear range of detection from 500 to 50,000 cells with the limit of 95, 98, 112, 123 cell/ml for MDA-MB-231, A549, MCF-7, and HeLa, respectively.
Our results clearly displayed that the control sample with no cells possessed the highest fluorescent emission. Also, the tubes containing the concentration of 5000 cells, showed the lowest fluorescent emission. The L929 curve displays no difference in fluorescent emission regarding various cell concentrations, indicating no CD44 expression on the surface of these normal cells. These results together demonstrate that CD44 expression in cancerous cell lines are different, and that MDA-MB-231 [8] and A549 express more CD44 receptors on their membrane compared to other cancer cell lines.
Bioimaging of cancer (MDA-MB-231, A549, MCF-7, HeLa) and normal (L929) cell lines with confocal laser scanning microscopy were employed to show the potential of GQD-HA in cancer cell detection. As it is mentioned, GQD-HA can bind to the CD44 receptors over expressed in specific cancer cells, and consequently entering the cytoplasm through endocytosis. After 30 min of incubation with GQD-HA, the cells were exposed to a 380 nm excitation laser light of confocal microscopy, and a blue emission was monitored (Fig. 10). The longer incubation time (2, 3 and 4 h) displayed no difference in fluorescent signal (data are not shown). As shown in the dark field images of Fig. 9, the emission fluorescence intensity in the cancer cell lines is varied due to the diversity in the expression of CD44 receptors in each cell line. Meanwhile, under the same condition, no fluorescence emission was detected in the dark field diagram of the L929 (control) normal cell line. It can be concluded that the L929 cell line either does not express CD44 receptors or that these receptors are inactivated. The bright field image in Fig. 9 exhibited no change in cell morphology, confirming that GQD-HA has minimum cytotoxicity to the cells. According to our findings, this biocompatible nano-biosensor possesses high specificity in detecting CD44 + cancer cells, which can also be employed for targeted gene/drug delivery for cancer therapy [8].
Confocal laser scanning microscope image of cancer cells with high expression of CD44 (A549, MDA-MB-231, HeLa, MCF-7) and normal cells with low expression of CD44 (L929) treated with GQD-HA at 37 °C for 50 min. (Left column: bright field image, middle column: dark field image exited with a 380 nm laser; right column: merged images)
Laser confocal microscopy imaging exhibited high sensitivity and specificity for GQD-HA in the detection of cancer cells with overexpression of CD44+ compared to CD44− L929 normal cells with a much higher population (Fig. 11). In this regard, 100 MDA-MB-231 or A549 cells were combined with 200,000 L929 normal cells and cultured. Then, our nanocomposite was applied, and GQD-HA was linked to the CD44 receptors and uptaken by the cancer cells through endocytosis. Afterward, a blue fluorescent was monitored upon 380 nm excitation through confocal microscopy. We observed that GQD-HA nanocomposite detected the cancerous cells in particular ways. Therefore, it is evident from the above phenomenon that the GQD-HA nanocomposite can detect cancerous cells with no external interference.
4 Conclusion
Herein, the successful synthesis of a GQD-HA based biosensor for efficient cancer cell detection was reported. First, af-GQD was synthesized via two successive hydrothermal processes from GO. Afterward, the resulting af-GQD was grafted with HA through ED C–N HS coupling chemistry to form the GQD-HA nanocomposite. The physiochemical analysis confirmed the successful generation of GQD-HA nanocomposite. We demonstrated this nanocomposite could effectively recognize cancer cells and build a foundation for theranostic applications. Our novel approach to identifying cancer cells acted more efficiently than conventional methods based on the EPCAM receptor because we detected Cd44 receptors that are overexpressed in many cancer cells, but the conventional methods detect EPCAM receptors that are reduced in the metastasis process. Above all, the fluorescent properties of GQD-HA can be employed as a bioimaging agent for specific detection of cancerous cells. Because of the biocompatibility and nontoxic features of GQD-HA nanocomposite, it can be used as an efficient carrier for targeted gene/drug delivery to cancer cells with minimum side effects.
Data Availability
All data generated or analysed during this study are included in this published article
Abbreviations
- CTC:
-
Circulating tumor cell
- DI:
-
Deionized
- DLS:
-
Dynamic light scattering
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- DMF:
-
Dimethyl formamide
- DMSO:
-
Dimethyl sulfoxide
- EDC:
-
1-Ethyl-3-(3-dimethyl-aminopropyl) carbodiimide
- EDX:
-
Energy-dispersive X-ray spectroscopy
- EMT:
-
Epithelial–mesenchymal transition
- FBS:
-
Fetal bovine serum
- FTIR:
-
Fourier-transform infrared spectroscopy
- GO:
-
Graphene oxide
- GQD:
-
Graphene quantum dot
- af-GQD:
-
Amino-functionalized graphene quantum dots
- GQD-HA:
-
Hyaluronic acid-amino-functionalized graphene quantum dots
- HA:
-
Hyaluronic acid
- rGO:
-
Reduced graphene oxide
- NHS:
-
N-Hydroxysuccinimide
- MES:
-
2-(N-Morpholino) ethanesulfonic acid hemi sodium salt
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PBS:
-
Phosphate-buffered saline
- PEG:
-
Polyethylene glycol
- SEM:
-
Scanning electron microscopy
- TEM:
-
Transmission electron microscopy
- UV:
-
Ultraviolet
- Vis:
-
Visible
References
H. Sung et al., Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71(3), 209–249 (2021)
S. Asghari, M. Mahmoudifard, Core-shell nanofibrous membrane of polycaprolactone-hyaluronic acid as a promising platform for the efficient capture and release of circulating tumor cells. Polym. Adv. Technol. 32(3), 1101–1113 (2020)
P. Rodrigues, S. Vanharanta, Circulating tumor cells: come together, right now, over metastasis. Cancer Discov. 9(1), 22–24 (2019)
F. Chemi et al., Early dissemination of circulating tumor cells: biological and clinical insights. Front. Oncol. 11, 1423 (2021)
Y. Yu et al., Design of a biocompatible and ratiometric fluorescent probe for the capture, detection, release, and reculture of rare number CTCs. Anal. Chem. 90(22), 13290–13298 (2018)
D. Naor et al., CD44 in cancer. Crit. Rev. Clin. Lab. Sci. 39(6), 527–579 (2002)
H. Xu et al., CD44 as a tumor biomarker and therapeutic target. Exp. Hematol. Oncol. 9(1), 1–14 (2020)
C. Chen et al., The biology and role of CD44 in cancer progression: therapeutic implications. J. Hematol. Oncol. 11(1), 1–23 (2018)
N. Al-Othman et al., Role of CD44 in breast cancer. Breast Dis. 39(1), 1–13 (2020)
O. Kearns, A. Camisasca, S. Giordani, Hyaluronic acid-conjugated carbon nanomaterials for enhanced tumour targeting ability. Molecules 27(1), 48 (2021)
N.J. Singhai, R. Maheshwari, S. Ramteke, CD44 receptor targeted ‘smart’multi-walled carbon nanotubes for synergistic therapy of triple-negative breast cancer. Colloid Interface Sci. Commun. 35, 100235 (2020)
M. Ma et al., Hyaluronic acid-conjugated mesoporous silica nanoparticles: excellent colloidal dispersity in physiological fluids and targeting efficacy. J. Mater. Chem. 22(12), 5615–5621 (2012)
X. Pan et al., Light-nutrition coupling effect of degradable fluorescent carbon dots on lettuce. Environ. Sci. Nano 10(2), 539–551 (2023)
A. Wang et al., Rare earth-free composites of carbon dots/metal–organic frameworks as white light emitting phosphors. J. Mater. Chem. C 7(8), 2207–2211 (2019)
F. Kang et al., Spectral tuning, stabilities under external exposures, and spontaneous enhancement of emission intensity in grown-into-glass all-inorganic metal halide perovskite nanocrystals. Laser Photonics Rev. 17(1), 2200166 (2022)
S. Asghari, et al., The role of the nanofibers in lateral flow assays enhancement: a critical review. Int. J. Polym. Mater. Polym. Biomater. 1–14 (2022)
M. Mahmoudifard, Thermosensetive Nanofibrous Structure for Exosome Isolation. Google Patents (2021)
E. Ekrami, M. Khodabandeh Shahraky, M. Mahmoudifard, M.S. Mirtaleb, P. Shariati, Biomedical applications of electrospun nanofibers in industrial world: a review. Int. J. Polym. Mater. Polym. Biomater. 72(7), 561–575 (2022)
M. Mahmoudifard, A.M. Shoushtari, A. Mohsenifar, Fabrication and characterization study of electrospun quantum dot—poly vinyl alcohol composite nanofiber for novel engineering applications. Fibers Polym. 13(8), 1031–1036 (2012)
P. Ranjbarvan et al., Natural compounds for skin tissue engineering by electrospinning of nylon-Beta vulgaris. ASAIO J. 64(2), 261–269 (2018)
S. Zamanlui et al., Enhanced chondrogenic differentiation of human bone marrow mesenchymal stem cells on PCL/PLGA electrospun with different alignments and compositions. Int. J. Polym. Mater. Polym. Biomater. 67(1), 50–60 (2018)
N. Borzooee Moghadam et al., Graphene family in cancer therapy: recent progress in cancer gene/drug delivery applications. J. Mater. Chem. B 11(12), 2568–2613 (2023)
N. Vahedi, F. Tabandeh, M. Mahmoudifard, Hyaluronic acid–graphene quantum dot nanocomposite: potential target drug delivery and cancer cell imaging. Biotechnol. Appl. Biochem. 69(3), 1068–1079 (2021)
M. Pouresmaieli et al., A comprehensive review on efficient approaches for combating coronaviruses. Biomed. Pharmacother. 144, 112353 (2021)
E. Ekrami et al., A review on designing biosensors for the detection of trace metals. Appl. Geochem. 127, 104902 (2021)
M. Mahmoudifard, A.M. Shoushtari, M. Shanehsaz, Quantum dot/polyvinyl alcohol composite nanofibers membrane as highly sensitive fluorescence quenching-based sensors. Fibers Polym. 15(9), 1797–1803 (2014)
E. Ekrami et al., Potential diagnostic systems for coronavirus detection: a critical review. Biol. Proced. Online 22(1), 21 (2020)
S. Asghari et al., Nanostructure materials: efficient strategies for circulating tumor cells capture, release, and detection. Biotechnol. Bioprocess. Eng. 26(4), 529–545 (2021)
S. Asghari, M. Mahmoudifard, Core-shell nanofibrous membrane of polycaprolactone-hyaluronic acid as a promising platform for the efficient capture and release of circulating tumor cells. Polym. Adv. Technol. 32(3), 1101–1113 (2021)
F. Cui et al., A novel magnetic fluorescent biosensor based on graphene quantum dots for rapid, efficient, and sensitive separation and detection of circulating tumor cells. Anal. Bioanal. Chem. 411(5), 985–995 (2019)
L. Ruiyi et al., Electrochemical detection of cancer cells in human blood using folic acid and glutamic acid-functionalized graphene quantum dot-palladium@ gold as redox probe with excellent electrocatalytic activity and target recognition. Sens. Actuators B Chem. 309, 127709 (2020)
X. Zhang et al., Shining luminescent graphene quantum dots: synthesis, physicochemical properties, and biomedical applications. Trends Anal. Chem. 116, 109–121 (2019)
J. Zhao et al., Graphene quantum dots as effective probes for label-free fluorescence detection of dopamine. Sens. Actuators B Chem. 223, 246–251 (2016)
J. Shi et al., A fluorescence turn-on biosensor based on graphene quantum dots (GQDs) and molybdenum disulfide (MoS2) nanosheets for epithelial cell adhesion molecule (EpCAM) detection. Biosens. Bioelectron. 93, 182–188 (2017)
F. Du et al., Dual-ligand functionalized carbon nanodots as green fluorescent nanosensors for cellular dual receptor-mediated targeted imaging. Analyst 144(22), 6729–6735 (2019)
Y. Liu et al., Paclitaxel delivered by CD44 receptor-targeting and endosomal pH sensitive dual functionalized hyaluronic acid micelles for multidrug resistance reversion. Colloids Surf. B 170, 330–340 (2018)
J. Yoon et al., CD44 receptor-mediated/reactive oxygen species-sensitive delivery of nanophotosensitizers against cervical cancer cells. Int. J. Mol. Sci. 23(7), 3594 (2022)
M. Mahmoudifard et al., The different fate of satellite cells on conductive composite electrospun nanofibers with graphene and graphene oxide nanosheets. Biomed. Mater. 11(2), 025006 (2016)
Z. Sun, D.K. James, J.M. Tour, Graphene chemistry: synthesis and manipulation. J. Phys. Chem. Lett. 2(19), 2425–2432 (2011)
H. Sun et al., Highly photoluminescent amino-functionalized graphene quantum dots used for sensing copper ions. Chemistry 19(40), 13362–13368 (2013)
M. Soleymani et al., One-pot preparation of hyaluronic acid-coated iron oxide nanoparticles for magnetic hyperthermia therapy and targeting CD44-overexpressing cancer cells. Carbohydr. Polym. 237, 116130 (2020)
J.-D. Xie, G.-W. Lai, M.M. Huq, Hydrothermal route to graphene quantum dots: effects of precursor and temperature. Diam. Relat. Mater. 79, 112–118 (2017)
A. Basu et al., Hyaluronic acid engrafted metformin loaded graphene oxide nanoparticle as CD44 targeted anti-cancer therapy for triple negative breast cancer. Biochimica et Biophysica Acta (BBA) Gen. Subj. 1865(3), 129841 (2021)
A.-A. Nahain et al., Target delivery and cell imaging using hyaluronic acid-functionalized graphene quantum dots. Mol. Pharm. 10(10), 3736–3744 (2013)
J. Tao, S. Feng, B. Liu, J. Pan, C. Li, Y. Zheng, Hyaluronic acid conjugated nitrogen-doped graphene quantum dots for identification of human breast cancer cells. Biomed. Mater. 16(5), 055001 (2021)
S. Asghari, M. Mahmoudifard, The detection of the captured circulating tumor cells on the core-shell nanofibrous membrane using hyaluronic acid-functionalized graphene quantum dots. J. Biomed. Mater. Res. B Appl. Biomater. 111(5), 1121–1132 (2023)
X. Hu et al., Construction and comparison of BSA-stabilized functionalized GQD composite fluorescent probes for selective trypsin detection. New J. Chem. 42(21), 17718–17724 (2018)
P. Mishra, B.R. Bhat, A study on the electro-reductive cycle of amino-functionalized graphene quantum dots immobilized on graphene oxide for amperometric determination of oxalic acid. Microchim. Acta 186(9), 1–10 (2019)
Acknowledgements
This study was made possible by a grant with the number of 750 from the National Institute of Genetic Engineering and Biotechnology (NIGEB), Tehran, Iran.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Avatefi, M., Mahmoudifard, M. & Moghadam, N.B. Amine-functionalized graphene quantum dots–hyaluronic acid nanocomposite as a high-resolution cancer cell bioimaging and biosensing system. Appl. Phys. A 129, 435 (2023). https://doi.org/10.1007/s00339-023-06700-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-06700-3