Abstract
Fabrication and designing advanced porous structures are effective methods to enhance the electrochemical performance of metal organic frameworks (MOFs) for supercapacitor applications. Here, we have developed ternary metal oxide MOFs prepared by a simple chemical bath deposition method. Firstly, a chemical bath deposition process was used to fabricate ZnxCo3−xO4 petal-like nano particles (NPs). So, their structural electrochemical properties were investigated to identify the influence of metal cation contents. Then ternary metal oxides MOFs are synthesized. These binder-free Cu–Zn1.5Co1.5O4 petal-like composite electrodes exhibit high specific capacitance. Its maximum reachable specific capacitance is 330 F/g at 1 A/g. The excellent electrochemical performance of the petal-like electrodes may be attributed to the intrinsic nature of ternary metal oxide MOFs and the porous petal-like morphologies that provide enough space for the storage and diffusion of the electrolyte.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The world’s economy including industries, transport, daily life, etc. is built on producing and using energy, as the population of the world increases, countries need to produce more and more energy which leads to the global energy crisis and environmental issues [1, 2]. Although clean energy sources are expanding, the potential to produce energy is limited [3, 4]. To response to energy crisis and environmental pollution, a great number of investigations have been done on energy storage devices and their storage mechanisms [5, 6]. Supercapacitors, as the electrochemical energy storage device, have high power density, excellent reversibility, and long cycle life [7]. Capacitors can be categorized into two main groups based on their charge storage mechanisms, the first one is electrical double-layer capacitors (EDLCs) in which charge stores in the electric double layer at the electrode/electrolyte interface [8], and the second one is pseudocapacitors which store energy by reversible fast faradaic reactions mechanism [9]. The most commonly used materials for the former group are carbon-based materials [10,11,12]. On the other hand, in the latter group, conductive polymers and transition metal oxides (TMOs) support such redox reactions [13]. Although various methods have been developed to enhance the capacitive performance of TMOs, the obtained specific capacitances over time are still low. Recently, metal–organic frameworks (MOFs) materials with fantastic features such as abundant porosity, high specific surface area, and strong chemical bonds revealed great potential applications such as catalysis, sensors, electrochemical energy-storage devices, and etc. [14,15,16]. MOFs are composed of metal ions/clusters and electron-donating natural [17]. Recently, supercapacitors with MOFs structure have become some of the most investigated candidates due to their cost effectiveness, stable structure, and good porosity [18]. In the field of supercapacitors, MOFs are utilized not only as precursors for the synthesis of TMOs, carbon and composites but also as electrode materials; due to their controllable structure, high surface area, and porosity [19].
Using MOFs directly as electrode materials for supercapacitors shows lower specific capacitance because of poor electrical conductivity, and the pore size unsuitable for electrolyte diffusion. It is worth noting that poor electrical conductivity can increase the charge transfer resistance [20]. It is possible to overcome this problem, by constructing nanoscale particle size which led to a decrease in diffusion distance, or by designing and synthesizing new MOFs, in which redox metal cations provide pseudocapacitance and sufficiently large pores/space for electrolyte solution penetration and act as the diffusion channels of electrolyte ions [21]. Besides, using conductive substrate to fabricate MOFs-based electrodes can address important issues such as poor electrical conductivity, and structural instability during charge/discharge cycles [22]. For instance, Nazari et al. prepared Zn-MOF nanoparticles via the hydrothermal method [23]. It is worth noting that mixed metal oxides, especially binary or ternary metal oxides, have enough ability to improve electrochemical performance impressively. Besides, zeolitic imidazolate frameworks ZIF-67 derived from the co-precipitation of cobalt salt and 2-methylimidazole have been also utilized as direct materials or, as precursor templates for supercapacitor electrodes. For instance, Kato et al. utilized several Co-based MOFs as precursor templates to obtain leaf-like Co3O4@NiCo2O4 nanoarrays, which showed a specific capacity of 544F/g at 1 A/g [24]. The fabrication of MOFs-derived Co–Ni hollow cubic nanostructures was reported by Eskandari et al., which obtained a specific capacity of 402F/g at 1 A/g [19]. The investigation reported by Liu et al. in which dual-metal (Co/Zn) MOFs were prepared and utilized to fabricate three-dimensional porous Co@Co-NPC [25]. Moreover, Wen et al. reported the synthesis of Ni-MOF/CNTs composites for an asymmetric supercapacitor [26].
In this work, we synthesized and optimized ZnxCo3−xO4 (x = 0, 1, 1.5, 2, 3) on Ni foam as conductive substrate. Then, Cu was dopped in the Zeolitic imidazolate Frameworks (ZIF)-derived ZnxCo3−xO4, petal-like nanocomposites through a simple chemical bath deposition method [27]. So, their structural properties and morphologies were investigated by X-ray diffraction (XRD) and field emission scanning electron microscopy (FESEM) techniques, respectively. Besides, electrochemical performance studies of derived ZIF electrodes were pursued by cyclic voltammetry (CV), galvanostatic charge/discharge, and electrochemical impedance spectroscopy (EIS). Consequently, the specific capacitances of electrodes were calculated and compared.
2 Experimental section
2.1 Materials and methods
2.1.1 Synthesis of ZnxCo3−xO4 nanostructures
All chemical materials were used after purchase without further purification as follows Cobalt nitrate hexahydrate, Copper nitrate hexahydrate, Zinc nitrate hexahydrate 2-methylimidazole, and ethanol. Co–Zn ZIFs were synthesized based on the reported method by Fathi et al. [27], by adding Zn nitrate. Typically, the various amount of Co (NO3)2·6H2O (99%) and Zn (NO3)2⋅6H2O (99%), as reported in Table 1, are dissolved into 10 mL deionized (DI) water and stirred well by adding 10 mL aqueous 2-methylimidazole (C4H6N2, 98%) for 1 min. Besides, the Ni foam as conductive substrate was cleaned by HCl 1 mol, NaOH 1 mol, and ethanol to remove the impurities. Then, in order to deposit Co–Zn ZIFs on the surface of Ni foam, the cleaned Ni foam is immersed into prepared solution for 6 h. After washing and drying under vacuum overnight, the prepared electrodes were calcinated at 350 ºC for 2 h in the air to obtain ZnxCo3−xO4 arrays on Ni foam (x = 0, 1, 1.5, 2, 3).
2.1.2 Preparation of Cu-Zn1.5Co1.5O4 structured electrode
The procedure of synthesis ternary metal oxides ZIFs structure is completely the same as the previous part, the equal amounts of three metal nitrates, including Co (NO3)2·6H2O (99%), Zn (NO3)2·6H2O (99%), and Cu (NO3)2·6H2O (99%), are dissolved into 10 mL deionized (DI) water and stirred well by adding 10 mL aqueous 2-methylimidazole (C4H6N2, 98%) for 1 min with the molar ratio 1 to 15. Then, the cleaned Ni foam is immersed into prepared solution for 6 h. The prepared electrode was washed and dried under vacuum overnight. The calcination of the obtained electrode is performed at 350 ºC for 2 h in the air to obtain ternary metal oxides ZIFs on Ni foam.
2.2 Physical characterization
The structural characteristics of the obtained materials were investigated by X-ray diffractometry (XRD) (Philips PW1710, Netherlands) with Cu-Kα radiation source (λKα1 = 1.5406 Å) within the 2θ value along with the range of 10‐80º with a step size of 0.02° and counting time of 2 s per step. The morphologies of the obtained nanocomposites were investigated by field-emission scanning electron (FESEM) (ZEISS, Germany). Moreover, elemental composition identifications were measured by energy dispersive spectroscopy (EDS).
2.3 Electrochemical measurements
The electrochemical performance of the obtained ZIF-derived petal-like composite electrodes was investigated in a three-electrode system using an SP-300 Bio-Logic potentiostats/galvanostats instrument at room temperature in 2 M KOH as the electrolyte in which ZIF samples, platinum, and Ag/AgCl were selected as the working, counter, and reference electrodes, respectively. Cyclic voltammetry (CV) was measured for all prepared electrodes at diffident scan rates ranging from 5 to 100 mVs−1 in the potential window of 0.0 to 0.5 V. Galvanostatic charge/discharge (GCD) curves were obtained at various current densities, and the electrochemical impedance spectroscopy (EIS) was performed within the frequency range of 100 kHz–0.05 Hz.
3 Results and discussion
3.1 Structural characterization
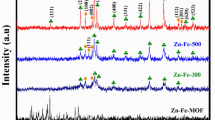
The XRD patterns of ZIF-derived ZnxCo3−xO4, and Cu–Zn1.5Co1.5O4 nanocomposites were presented in Fig. 1. As illustrated in Fig. 1, except peaks of nickel foam (peaks at 45°,52°, 77°) other peaks observed at 2θ values of 31.9°, 36.9° can be indexed to the ZnCo Oxide planes (220), (311), (JCPDS card No. 23-1390) [27]. Besides, the XRD patterns of the obtained ZnxCo3−xO4 nanostructures are illustrated in Fig. S1 in which peaks at 32°, 34.6° and 36.6° can be attributed to (100), (002), and (101) (JCPDS card No 1451–36) corresponding to Zinc oxide.
The FESEM images and EDS results of the prepared ZIF-derived petal-like ZnxCo3−xO4, and Cu–Zn1.5Co1.5O4 nanostructures are presented in Fig. 2. As illustrated in Fig. 2a–l low magnification images of ZIF-derived ZnxCo3-xO4 structures show homogeneous growth. Besides, high magnification images prove that these petal-like structures are very uniform and smooth. The FESEM image and EDS result of Cu–Zn1.5Co1.5O4 exhibit thin petal-like structures (Fig. 2m–o), which provide a suitable substrate for effective ion/electron exchange. This thin petal-like structure with high surface areas can improve the specific capacitance of the electrode. The elemental mapping on the petal-like structure in Fig. 3 clearly shows the uniform distribution of Co, Zn, and Cu (Scheme 1).
3.2 Electrochemical performance
To certify the electrochemical performance of binder free ZIF-derived ZnxCo3−xO4, and Cu–Zn1.5Co1.5O4 petal-like electrodes, comparative CV measurements with various scan rates from 5 to 100 mV/s were carried out in a three-electrode cell system. CV curves of ZnxCo3-xO4 with different scan rates from 5 to 100 mV/s were presented in Fig. S2. According to CV curves of ZnxCo3−xO4, a pair of redox current peaks were explicitly observed. Generally, the redox couples mainly correspond to the reversible reactions of M–O/M–O–OH (M demonstrates Zn or Co) in alkaline medium [28] To identify the best combination amount of Zn and Co, CV curves of ZnxCo3−xO4 at 20 mV/s were compared and exhibited in Fig. 4a. As illustrated in Fig. 4a, a significant enhancement redox current peak was occurred in sample in which Zn and Co had the same molar ratio. Besides, Cu–Zn1.5Co1.5O4 petal-like composite displayed an improvement in electrochemical performance which means that Cu not only improved the electrical response, but also decreased electrical resistance. CV curves of Cu–Zn1.5Co1.5O4 petal-like composite at various scan rates from 5 to 100 mV/s were presented in Fig. 5a. By increasing the scan rate, all electrodes show a decrease in the diffusion-controlled storage process; in other words, the outer material surfaces contribute to the charge–discharge process, which can lead to the lower effectiveness of the electro-active materials, that is, the capacitive contribution is enhanced. Besides, the comparative CV of binder free ZIF-derived ZnxCo3−xO4, and Cu–Zn1.5Co1.5O4 petal-like electrodes at a scan rate of 20 mV/s were illustrated in Fig. 6a. As presented in Fig. 6a the capacitive contribution of the Cu–Zn1.5Co1.5O4 petal-like electrode is higher than the other prepared electrodes. This feature can be ascribed to the structural properties of ternary metal oxides which give multiple electro-active sites for redox reactions [29].
Evaluation of the electrochemical performance of the nanocomposites in a three-electrode configuration: a CV at scan rates of 20 mVs−1 in an aqueous 2 M KOH electrolyte solution, b galvanostatic charge/discharge plots at a current density 1A/g; of Zn-ZIF, Zn Co (molar ratio: 2,1), Zn Co (molar ratio: 1,1), Zn Co (molar ratio: 1,2), and Co-ZIF
Comparison of the electrochemical performance of Co–ZIF, Cu–Co3O4, Zn1.5Co1.5O4, and Cu–Zn1.5Co1.5O4 Petal-like composites, a CV at scan rates of 20 mVs−1, b galvanostatic charge/discharge plots at a current density 1A/g, c specific capacitance, and d Nyquist plots [27]
Galvanostatic charge−discharge (GCD) tests were utilized to investigate the specific capacitances of the binder free ZIF-derived ZnxCo3−xO4, and Cu–Zn1.5Co1.5O4 petal-like electrodes. Fig S3 displays the GCD curves of the ZnxCo3-xO4 electrodes at various current densities in 2 M KOH electrolyte within the potential of 0−0.5 V. Besides, as illustrated in Fig. 4b, the comparison of GCD behaviors of ZnxCo3−xO4 electrodes at 1A/g presents the nonlinear curves, that is, the capacitive performance is attributed to the electric double-layer specific capacitance and the pseudocapacitance. Moreover, the specific capacitances (Csp) can be calculated by means of GCD measurements. \({C}_{\mathrm{sp}}\) can be calculated by the following equation:
where I is the current applied (A), \(\mathrm{\Delta t}\) the discharge time (s), m the mass of loaded material (g), and \(\Delta \mathrm{V}\) the window potential (V) [30, 31].
Further, as presented in Fig. 6b, the comparative GCD plots at 1A/g, the Cu–Zn1.5Co1.5O4 petal like electrode improved the energy dissemination inside the electrode; that is, ternary metal oxide composite structure leads to a quicker particle diffusion to active sites for the redox reactions, thus, improving the natural electrical conductivity. In addition, the GCD curves of the Cu–Zn1.5Co1.5O4 petal like electrode at various current densities were displayed in Fig. 5b. As illustrated in Fig. 5b, by increasing current densities, the time of discharge and consequently specific capacitance decreases. In other words, the redox reactions between metallic cations and OH anions are the diffusion-controlled process through the electrode. The specific capacitance values can be calculated from comparative GCD plots of Co–ZIF, Cu–Co3O4 [27], Zn1.5Co1.5O4, and Cu–Zn1.5Co1.5O4 Petal-like composites (Fig. 6b). They show specific capacitance values of 93, 182, 226 and 330 F/g, respectively (Fig. 6c). Besides, their coulombic efficiencies were calculated and displayed in Fig. 6c.
Another result is the coulombic efficiency (η) defined as the ratio of discharging time \({(t}_{D})\) and charging time \(({t}_{C})\) in seconds, when the charge–discharge current densities are equal [32]. It is obtained from the following relationship:
As presented in Fig. 6c the coulombic efficiency of Cu–Zn1.5Co1.5O4 is 79% higher than other samples. A higher coulombic efficiency indicates better access of ions to the electrochemical surface. This shows that in addition to increasing the specific capacitance, access to the electrochemically active surface has also been improved.
To evaluate the performance of structure, conductivity and charge transport in the electrode/electrolyte interface, the EIS analysis of samples was carried out over the frequency range of 100 kHz to 0.01 Hz with the equivalent circuit illustrated. The Nyquist plots consist of a semicircle, indicating the charge-transfer resistance (Rct) caused by Faradic reactions, the starting point of the semicircle (Rs) denotes the electrolyte resistance, Cdl is the double-layer specific capacitance [33].
The slope of the line for all samples is greater than 45 degrees, which indicates a good ionic penetration of the electrolyte in the structure. Having a higher penetration layer resistance, in the Warburg region, shows a better quasi-perpendicular slope compared to other electrodes, which indicates that it has a better quasi-capacitive behavior.
As observed in Fig. 6d, the Nyquist plot of Cu–Zn1.5Co1.5O4 Petal-like composite indicates excellent capacitive behavior in comparison with other samples because of minimal slope differences observed from the vertical diffusion lines.
4 Conclusions
In summary, ZnxCo3−xO4 (x = 0, 1, 1.5, 2, 3) composites via chemical bath deposition method were synthesized on the surface of Nickel foam as a conductive substrate. It was investigated how structural features and electrochemical performance are affected by the amount of Zn and Co. Besides, it was found that Cu–Zn1.5Co1.5O4 composite compared to ZnxCo3−xO4 is a promising electrode material, due to its outstanding electrical advantages of Cu content. The experimental results revealed that this petal- like electrode exhibited a remarkable electrochemical performance when it was used as an electrode material. The specific capacitance of Co-ZIF, Zn1.5Co1.5O4, and Cu–Co3O4 electrodes are 93, 182 and 226 F/g at a current density 1A/g in 2 M KOH, respectively. The specific capacitance achieved by the Cu–Zn1.5Co1.5O4 electrode was calculated at 330 F/g at 1A/g in 2 M KOH. The reason is as follows: ternary metal oxide structures which give multiple electro-active sites for redox reactions, and Cu content which improves electrical conductivities. Therefore, the obtained superior properties of the present active electrodes make them an interesting alternative to be used in energy storage devices.
Data availability
The data supporting the findings of this study are available within the paper, its supplementary information files.
References
A. González, E. Goikolea, J.A. Barrena, R. Mysyk, Review on supercapacitors: technologies and materials. Renew. Sustain. Energy Rev. 58, 1189–1206 (2016). https://doi.org/10.1016/j.rser.2015.12.249
A. Yadav, B. Gerislioglu, A. Ahmadivand, A. Kaushik, G.J. Cheng, Z. Ouyang, Q. Wang, V.S. Yadav, Y.K. Mishra, Y. Wu, Y. Liu, S. RamaKrishna, Controlled self-assembly of plasmon-based photonic nanocrystals for high performance photonic technologies. Nano Today 37, 101072 (2021). https://doi.org/10.1016/j.nantod.2020.101072
C. Li, X. Sun, Y. Yao, G. Hong, Recent advances of electrically conductive metal-organic frameworks in electrochemical applications. Mater. Today Nano 13, 100105 (2021). https://doi.org/10.1016/j.mtnano.2020.100105
H. Ronduda, M. Zybert, A. Szczęsna-Chrzan, T. Trzeciak, A. Ostrowski, D. Szymański, W. Wieczorek, W. Raróg-Pilecka, M. Marcinek, On the sensitivity of the ni-rich layered cathode materials for li-ion batteries to the different calcination conditions. Nanomaterials 10, 1–21 (2020). https://doi.org/10.3390/nano10102018
J. Zhang, G. Zhang, T. Zhou, S. Sun, Recent developments of planar micro-supercapacitors: fabrication, properties, and applications. Adv. Funct. Mater. 30, 1–21 (2020). https://doi.org/10.1002/adfm.201910000
S. Clark, F.L. Bleken, S. Stier, E. Flores, C.W. Andersen, M. Marcinek, A. Szczesna-Chrzan, M. Gaberscek, M.R. Palacin, M. Uhrin, J. Friis, Toward a unified description of battery data. Adv. Energy Mater. (2022). https://doi.org/10.1002/aenm.202102702
G.Z. Chen, Supercapacitor and supercapattery as emerging electrochemical energy stores. Int. Mater. Rev. 62, 173–202 (2017). https://doi.org/10.1080/09506608.2016.1240914
G.Z. Chen, Understanding supercapacitors based on nano-hybrid materials with interfacial conjugation. Prog. Nat. Sci. Mater. Int. 23, 245–255 (2013). https://doi.org/10.1016/j.pnsc.2013.04.001
J. Libich, J. Máca, J. Vondrák, O. Čech, M. Sedlaříková, Supercapacitors: properties and applications. J. Energy Storage 17, 224–227 (2018). https://doi.org/10.1016/j.est.2018.03.012
M. Qorbani, A. Esfandiar, H. Mehdipour, M. Chaigneau, A. Irajizad, A.Z. Moshfegh, Shedding light on pseudocapacitive active edges of single-layer graphene nanoribbons as high-capacitance supercapacitors. ACS Appl. Energy Mater. 2, 3665–3675 (2019). https://doi.org/10.1021/acsaem.9b00375
J. Xia, F. Chen, J. Li, N. Tao, Measurement of the quantum capacitance of graphene. Nat. Nanotechnol. 4, 505–509 (2009). https://doi.org/10.1038/nnano.2009.177
J. Chmiola, P.L.T. Celine Largeot, P. Simon, Y. Gogotsi, Monolithic carbide-derived carbon films for micro-supercapacitors. Science (80-) 328, 480–483 (2010). https://doi.org/10.1126/science.1184126
J. Xu, D. Su, W. Bao, Y. Zhao, X. Xie, G. Wang, Rose flower-like NiCo2O4 with hierarchically porous structures for highly reversible lithium storage. J. Alloys Compd. 684, 691–698 (2016). https://doi.org/10.1016/j.jallcom.2016.05.252
A. Dhakshinamoorthy, A.M. Asiri, H. Garcia, Mixed-metal or mixed-linker metal organic frameworks as heterogeneous catalysts. Catal. Sci. Technol. 6, 5238–5261 (2016). https://doi.org/10.1039/c6cy00695g
X. Liu, S. Akerboom, M. De Jong, I. Mutikainen, S. Tanase, A. Meijerink, E. Bouwman, Mixed-lanthanoid metal-organic framework for ratiometric cryogenic temperature Sensing. Inorg. Chem. 54, 11323–11329 (2015). https://doi.org/10.1021/acs.inorgchem.5b01924
Y. Mao, G. Li, Y. Guo, Z. Li, C. Liang, X. Peng, Z. Lin, Foldable interpenetrated metal-organic frameworks/carbon nanotubes thin film for lithium-sulfur batteries. Nat. Commun. (2017). https://doi.org/10.1038/ncomms14628
I. Hussain, S. Iqbal, T. Hussain, W.L. Cheung, S.A. Khan, J. Zhou, M. Ahmad, S.A. Khan, C. Lamiel, M. Imran, A. AlFantazi, K. Zhang, Zn–Co-MOF on solution-free CuO nanowires for flexible hybrid energy storage devices. Mater. Today Phys. 23, 1655 (2022). https://doi.org/10.1016/j.mtphys.2022.100655
W. Guo, W. Sun, Y. Wang, Multi-layer CuO @ NiO hollow spheres : microwave- assisted metal-organic-framework derivation and highly reversible structure-matched stepwise lithium storage graphical abstract. ACS Nano (2015). https://doi.org/10.1021/acsnano.5b05610
M. Eskandari, N. Shahbazi, A.V. Marcos, R. Malekfar, P. Taboada, Facile MOF-derived NiCo2O4/r-GO nanocomposites for electrochemical energy storage applications. J. Mol. Liq. 348, 118428 (2022). https://doi.org/10.1016/j.molliq.2021.118428
T. Liu, L. Finn, M. Yu, H. Wang, T. Zhai, X. Lu, Y. Tong, Y. Li, Polyaniline and polypyrrole pseudocapacitor electrodes with excellent cycling stability. Nano Lett. 14, 2522–2527 (2014). https://doi.org/10.1021/nl500255v
X. Liu, C. Shi, C. Zhai, M. Cheng, Q. Liu, G. Wang, Cobalt-based layered metal-organic framework as an ultrahigh capacity supercapacitor electrode material. ACS Appl. Mater. Interfaces. 8, 4585–4591 (2016). https://doi.org/10.1021/acsami.5b10781
H. Zhang, W. Zhao, M. Zou, Y. Wang, Y. Chen, L. Xu, 3D, mutually embedded MOF @ carbon nanotube hybrid networks for high-performance lithium-sulfur batteries. Adv. Energy Mater. (2018). https://doi.org/10.1002/aenm.201800013
Z. Nazari, M.A. Taher, H. Fazelirad, A Zn based metal organic framework nanocomposite: Synthesis, characterization and application for preconcentration of cadmium prior to its determination by FAAS. RSC Adv. 7, 44890–44895 (2017). https://doi.org/10.1039/c7ra08354h
K. Tao, Y. Yang, C. Yang, Q. Ma, L. Han, Construction of NiCo2O4 nanosheet-decorated leaf-like Co3O4 nanoarrays from metal-organic framework for high-performance hybrid supercapacitors. Dalt. Trans. 48, 14156–14163 (2019). https://doi.org/10.1039/c9dt02907a
B. Liu, J.Z. Li, X.F. Gong, Y.L. Zhang, Q.Y. Zhou, J.J. Cai, Z.G. Liu, X.L. Sui, Z.B. Wang, Facile synthesis of flower-like dual-metal (Co/Zn) MOF-derived 3D porous Co@Co-NPC as reversible oxygen electrocatalyst for rechargeable zinc-air batteries. Ionics (Kiel) 26, 1913–1922 (2020). https://doi.org/10.1007/s11581-019-03364-z
P. Wen, P. Gong, J. Sun, J. Wang, S. Yang, Design and synthesis of Ni-MOF/CNTs composites and rGO/carbon nitride composites for an asymmetric supercapacitor with high energy and power density. J. Mater. Chem. A. (2015). https://doi.org/10.1039/C5TA02461G
A. Fathi, M. Eskandari, P.T. Antelo, E. Saievar-Iranizad, ZIF-derived Cu doped Co3O4/RGO composites for asymmetric supercapacitors. Solid State Sci. 132, 106967 (2022). https://doi.org/10.1016/j.solidstatesciences.2022.106967
D. Li, Y. Gong, Y. Zhang, C. Luo, W. Li, Q. Fu, C. Pan, Facile synthesis of carbon nanosphere/NiCo2O4 core-shell sub-microspheres for high performance supercapacitor. Sci. Rep. (2015). https://doi.org/10.1038/srep12903
Q. Guan, J. Cheng, B. Wang, W. Ni, G. Gu, X. Li, L. Huang, G. Yang, F. Nie, Needle-like Co3O4 anchored on the graphene with enhanced electrochemical performance for aqueous supercapacitors. ACS Appl. Mater. Interfaces. 6, 7626–7632 (2014). https://doi.org/10.1021/am5009369
M. Eskandari, C.A. García, D. Buceta, R. Malekfar, P. Taboada, NiCo2O4/MWCNT/PANI coral-like nanostructured composite for electrochemical energy-storage applications. J. Electroanal. Chem. (2019). https://doi.org/10.1016/j.jelechem.2019.113481
M. Eskandari, R. Malekfar, D. Buceta, P. Taboada, NiCo2O4-based nanostructured composites for high-performance pseudocapacitor electrodes. Colloids Surf. A Physicochem. Eng. Asp. 584, 124039 (2020). https://doi.org/10.1016/j.colsurfa.2019.124039
M. Rajkumar, C.T. Hsu, T.H. Wu, M.G. Chen, C.C. Hu, Advanced materials for aqueous supercapacitors in the asymmetric design. Prog. Nat. Sci. Mater. Int. 25, 527–544 (2015). https://doi.org/10.1016/j.pnsc.2015.11.012
S. Sharifi, A. Yazdani, K. Rahimi, Effect of Co2+ content on supercapacitance properties of hydrothermally synthesized Ni1-xCoxFe2O4 nanoparticles. Mater. Sci. Semicond. Process. 108, 104902 (2020). https://doi.org/10.1016/j.mssp.2019.104902
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fathi, A., Eskandari, M., Taboada, P. et al. Mixed ternary metal (Co/Zn/Cu) MOF for electrochemical energy-storage electrodes. Appl. Phys. A 129, 387 (2023). https://doi.org/10.1007/s00339-023-06660-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-06660-8