Abstract
This work investigates the performance of an InAs-based dielectric-modulated heterojunction charge plasma-based tunnel field-effect transistor (DMH-CP-TFET) as a label-free biosensor. The nanogap cavity is created under the source electrode by etching out gate oxide material. The target biomolecules are modeled inside the nanogap cavity. The Dielectric Modulation technique and Charge-Plasma concept are utilized to investigate the biomolecules immobilized in the nanogap cavity. The presence of biomolecule inside the nanogap cavity changes the electrostatic potential, electric field, and capacitance of the device, and hence, the threshold voltage and drain current modulated. By measuring the change in the threshold voltage and drain current, the sensitivity of the device gets evaluated. The proposed device offers high electron mobility, narrow bandgap, and the ability to engineer heterojunctions to control charge carrier injection and tunneling properties. The simulated results show the ION sensitivity of 4.08 × 106 and ION/IOFF sensitivity of 8.39 × 106 for neutral biomolecules (K = 12). The ION/IOFF sensitivity is increased by 97.43% for positively charged biomolecules (1 × 10, K = 6) and 94.98% for negatively charged biomolecules (− 1 × 10, K = 6). The linearity analysis shows a strong correlation having Pearson coefficient, r > 0.97 is achieved for threshold voltage and subthreshold slope. The proposed device reports better sensitivity characteristics than the recent published heterojunction TFET biosensors. Silvaco TCAD ATLAS 2D device simulator is used to investigate the sensitivity, selectivity, and linearity of the proposed device for both neutral and charged biomolecules.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In today's fast-paced world of technology, biosensors are becoming increasingly important for human well-being. Researchers around the world are working hard to develop new techniques to detect biomolecules [1], as the widespread use of biosensors has the potential to revolutionize many fields. Biosensors have already found useful applications in medicine for early detection and diagnosis, drug delivery, environmental monitoring, and security [2]. These sensors work by generating electrical signals from the physiochemical reactions of biomolecules [3]. The process of detecting biomolecules involves two steps: biomolecule detection and transduction. During detection, the targeted biomolecule is analyzed, and during transduction [4, 5], the physiochemical reaction is transformed into a quantifiable electrical signal [6]. Among the various types of biosensors, field-effect transistor (FET)-based biosensors have gained considerable attention from researchers due to their exceptional characteristics, such as label-free detection, compact size, faster response, and reliability [7]. FET-based biosensor offers several other advantages like low cost and capable of mass production. Ion-sensitive FETs (ISFETs), which are the part of the FET family, have evolved to be able to identify biomolecules based on the charge between the dielectric gate and the ionic solution [8]. The ISFET are only capable to detect charged biomolecules and not able to report neutral biomolecules. Additionally, the traditional FET-based biosensor has several drawbacks, including a very long detection time caused by the kT/q limit (SS > 60 mV/decade) and high power consumption because of leakage, a short-channel effect, drain-induced barrier lowering, and a poor ION/IOFF ratio [9, 10]. Tunnel FET (TFET)-based biosensors can solve the mentioned problems as the TFETs are more sensitive, quick to respond, and energy efficient. Although TFET-based biosensor acts as a suitable replacement of FET-based biosensors, it offers various shortcomings, viz., poor ON state current and negative conduction (Ambipolarity) [11]. TFET device required a highly doped source and drain region and hence results in random dopant fluctuation, device variability, and a high-temperature fabrication process. To overcome the TFET drawbacks, the charge plasma concept is used [12]. Using charge plasma technique, the doping requirement in the source and drain region gets eliminated. Hence, CP TFET-based devices are free from random dopant fluctuations, device variability, and low thermal budget, and are simpler in fabrication.
Several researchers have developed the charged plasma (CP) technique for TFET-based biosensors to simplify the fabrication process, control random dopant fluctuations, and reduce the cost of producing nanoscale devices [13]. This technique involves using a suitable electrode work function for the source and drain regions to prevent doping in those areas. To successfully implement the CP concept, the work function (ϕ) of the drain and source must follow the conditions outlined in Eqs. (1) and (2)
where Eg signifies the energy bandgap of silicon (1.1 eV) and \({\chi }_{\mathrm{Si}}\) represents the electron affinity (4.17 eV). Moreover, the thickness of the silicon body (TSi) must also be lesser than Debye's length [14]. In [15], a double-gate TFET (DG-TFET) was proposed and the simulations were carried out to validate the proposed model. Significant improvement in the performance was observed as compared to single-gate device. Recently, researchers across the globe focused on dielectric-modulated TFET (DM TFET) for biosensing applications. Wet etching and thin deposition techniques are used in DM TFETs to create nano-cavities close to the source side. On the source side, nano-cavity is typically used to facilitate the tunneling process [16]. When the biomolecules modeled inside the nanogap cavity, the overall capacitance of the device changes and this will help in recognition of biomolecules. The impact of dielectric constant (K) and charge density (Nf) on the electrical properties of dielectric-modulated junction-less TFET (DM-JLTFET) has been examined for a biosensors as a label-free detection. Additionally, the DM-JLTFET offers several other advantages such as a low thermal budget, low cost, and simpler in fabrication [17]. In another study [18], the author investigated the performance of label-free, CP TFET (CS-CPTFET)-based biosensors for both neutral and charged biomolecules. Band energy variation, subthreshold swing (SS), surface potential, sensitivity, and electric field (E) are all used to analyze the performance. In [19], the performance of a double-gate junction-less TFET was studied by changing the dielectric constant for detecting biomolecules using Silvaco TCAD. The outcomes of the study confirm that the proposed double-gate device is superior as compared to other FET-based biosensors mentioned in the literature.
This research explores the potential of a dielectric-modulated heterojunction CP TFET (DMH-CP-TFET) device, based on Indium Arsenide (InAs) material with low band-gap energy, for biosensing applications. This innovative device is capable of detecting both neutral and charged biomolecules, making it highly versatile. The study investigates the effect of different biomolecules on the electrical properties of the DMH-CP-TFET by varying the K and Nf, with a focus on sensitivity, selectivity, and linearity. Through this investigation, the authors aim to shed light on the potential applications of this device for biosensing purposes.
The rest of the manuscript is systematized as follows: In Sect. 2, the structure of the proposed device, simulation parameters, and model description is discussed. In Sect. 3, simulation results of the proposed DMH-CP-TFET device by changing the dielectric constant and charge density have been carried out. Finally, in Sect. 4, the conclusion and important findings about the proposed work are discussed.
2 Device, structure, modeling, and simulation
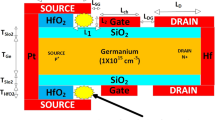
Figure 1 provides a clear cross-sectional diagram of the DMH-CP-TFET device as a biosensor. This figure helps to visualize the device's structure and understand how it functions as a biosensor. Additionally, Table 1 lists all the relevant device parameters and numerical values that were considered during the simulation of the proposed biosensor. The heavily doped p + source region and n + drain region is built using platinum electrode and hafnium electrode with ϕ of 5.93 eV and 3.9 eV, respectively, to meet the fundamental need of the CP concept [3, 20]. Additionally, it is assumed that the silicon body thickness (TSi) is 10 nm, which is smaller than Debye's length. The length of the channel (Lch) is taken as 50 nm, while the drain and source length are considered as 100 nm. For the entire device, the intrinsic doping level is assumed to be 1015 cm−3. The CP concept has decreased the thermal budget of the device by eliminating the doping steps and making the fabrication process simpler. To create source, drain, and gate electrodes, the low-pressure chemical vapor deposition technique (LP CVD) is utilized. As represented in Fig. 1, the SiO2 layer’s thickness (Tox) is taken as 0.5 nm to avoid silicide formation [4]. The length and thickness of cavities to hold the biomolecules are 25 nm and 5.5 nm, respectively. The gate metal work function (ϕG) is considered as 4.4 eV.
Indium arsenide (InAs) material having a doping concentration of 1018 cm−3 is used in the channel region as it offers a lower energy bandgap. Due to the lesser energy bandgap, tunneling of a large amount of charge carrier is possible from the source–channel junction easily [21, 22].
The proposed biosensor is simulated using the Silvaco TCAD ATLAS 2D device simulator. The selection of the BTBT model is the most important aspect of TFET simulation; in the proposed work, the tunneling rate is calculated using a non-local BTBT model. Several other models, such as the Shockley–Read–Hall and Auger model for minority carrier recombination and the Wentzel–Kramers–Brillouin theory for numerical tunneling probability, are also used to effectively carry out the simulation results.
The K and Nf play an important role in modulating tunneling width to control the tunneling at the source–channel interface in DMH-CP-TFET. The dielectric constant (K = 1) signifies an air-filled nanogap cavity, whereas the dielectric constant (K > 1) represents a biomolecule-filled nanogap cavity. The presence of biomolecules in the nanogap cavity results in increasing the gate capacitance, thereby improving the drain current and, hence, can be used for sensing the biomolecule in the label-free biosensor. The validation of the proposed model is done by comparing the transfer characteristics with the data reported in the literature [15]. Figure 2 portrays the variation of ID with VGS for the proposed model and previous work, and calibrated data closely follow the results reported in the literature [15].
Calibrated transfer characteristics of DMH-CP-TFET biosensor with the work reported [15]
3 Results and discussion
In this work, the performance of the proposed DMH-CP-TFET biosensor has been investigated for both neutral and charged biomolecules. Different biomolecules have different dielectric constant values. Figure 3 portrays the energy-band plot of a DMH-CP-TFET in the OFF state, along with the horizontal X-position. Under OFF conditions (VDS = 1 V, VGS = 0 V), the creation of heterojunction results in a large tunneling width at the interface between the source and channel. Due to this, greater energy is needed to tunnel electrons from the valance band to the conduction band. Figure 4 represents the energy-band diagram of a DMH-CP-TFET in the ON state. The tunneling width at the source and channel junction is reduced during the ON state (VDS = 1.5 V, VGS = 1 V) and K = 1 (empty cavity) in comparison to the OFF-state condition of the device. The charge carriers now tunnel through the junction, causing the drain current to flow.
The impact of increasing the K (1–12) on the proposed device's energy-band diagram is depicted in Fig. 5. The K = 1 represents an empty cavity, whereas K > 1 represents a cavity occupied with biomolecules. As shown in Fig. 5, the density of holes at the source side enhances with the dielectric constant (K) of biomolecules modeled in nano-cavities increases. This induced layer of holes improved the alignment of the source region of the valance band (VB) and the conduction band (CB) of the channel. This causes the energy band to raise upward, reducing the tunneling width and allowing a large amount of carrier flow through it. As a result, as the K increases, so does the ID.
The behavior of the electric field (E) of the DMH-CP-TFET biosensor with respect to the position along the Lch at different K values is depicted in Fig. 6. As it can be seen, the E at the tunneling junction increases with an increase in the K value of neutral biomolecules.
This effect occurs, because tunneling width reduces and more charge carriers move through the junction at higher dielectric constant values. For the K value of 12, an electric field of 3.5 × 106 V/m was obtained at the junction.
Figure 7 portrays the behavior of the proposed biosensor in terms of electric potential along with the variation in the length of the cavity (L1) at VDS = 1 V and VGS = 1.5 V.
The electric potential has been observed to shift from left to right as the value of the K increases along the L1. The existence of various biomolecules inside the cavity can be seen as a potential shift in the plot 7.
We have investigated the proposed device in terms of sensitivity, selectivity, and linearity parameters for biosensing applications.
3.1 Sensitivity analysis
The sensitivity is considered as one of the major parameters to investigate the performance of a biosensor. Generally, the size of biomolecules is quite small; therefore, each biosensor must be capable to detect the presence of such a small biomolecule. Due to this, the sensitivity of a biosensor must be as high as possible. The electrical characteristics of the device change after the biomolecules were modeled inside the cavity region. These changes will help in the finding of biomolecules. The sensitivity of a biosensor can be expressed as
where Z is the sensitivity parameter, Zair represents the sensitivity parameter value when the biomolecule is absent inside nanogap cavity, and Zbio represents the value of sensitivity parameter when biomolecule is present inside nanogap cavity. The subthreshold swing (SS) is another crucial parameter for evaluating the effectiveness of TFET-based biosensor [15]. The SS defines the gate voltage (VGS) over the channel region and can be represented as
A biosensor’s efficiency is determined by its SS value; the lower SS value, faster is the response of biosensor. In case of conventional FET, the diffusion current mechanism affects the device SS, while for TFET-based biosensor, the SS mainly depends upon tunneling mechanism. The variation of SS sensitivity (VGS = 1.5 V and VDS = 1.0) with different values of K for neutral biomolecule (Nf = 0) is represented in Fig. 8a.
As observed from Fig. 8a, the SS sensitivity improves with an increase in K value. At K = 2, the SS sensitivity value is 0.083, while at K = 12, the SS sensitivity value is 0.573. This implies superior detection ability and electrical properties of the DMH-CP-TFET biosensor. Further, the SS sensitivity curve follows the linear behavior, which is a property of a good sensing device.
The variation in threshold voltage (VTH) sensitivity with dielectric constant for neutral biomolecules (Nf = 0) is depicted in Fig. 8b. The threshold voltage (VTH) is the gate voltage that represents the transition from strong to weak control of the tunneling energy barrier width at the source tunnel junction [18, 19]. It can be perceived from Fig. 8b that VTH sensitivity increases with an increase in K value. The VTH sensitivity at K = 2 is 0.015, while at K = 12, the VTH sensitivity increases to 0.7764.
Figure 8c demonstrates the ION/IOFF sensitivity as a function of neutral biomolecules. As depicted in Fig. 8c, the ION/IOFF sensitivity improves with an increase in the value of K. This has happened due to an increase in the value of K at the source–channel junction, and a sharp doping profile is created. As the concentration of holes in the source region rises, so does the alignment between the CB of the channel and the VB of the source, this leads to more tunneling of carriers. As a result, as the K increases, so does the ION/IOFF ratio. A significant enhancement is observed in the ION/IOFF sensitivity of DMH-CP-TFET (K = 2, ION/IOFF sensitivity = 250.09) as compared with that of K = 12 (ION/IOFF sensitivity = 8.39 × 106). The performance of the DMH-CP-TFET biosensor is compared with MOSFET-based biosensor as discussed in [7], there is a slight increment in the ION/IOFF ratio of MOSFET—based biosensor with K. This outcome in ION/IOFF ratio with K advocates the superior performance of DMH-CP-TFET biosensor for label-free detection as compared to MOSFET-based biosensors.
The variation in ION sensitivity of DMH-CP-TFET biosensor as a function of the neutral biomolecule is shown in Fig. 8d. As observed from Fig. 8d, the ION sensitivity increases almost linearly with an increase in K. The ION sensitivity value is 433.33 at K = 2 and increases to 4.08 × 106 at K = 12.
It has been observed from Fig. 8c and d that both the ION and ION/IOFF ratio for DMH-CP-TFET improve with an increase in K. The high value of ION/IOFF ratio indicates lesser power loss through leakage, improved performance, and faster switching. Further, a large variation in characteristics of DMH-CP-TFET depicts the better sensitivity of the device. Table 2 portrays the variation in different sensitivity parameters of DMH-CP-TFET biosensors for neutral biomolecules. The ION sensitivity, ION/IOFF sensitivity, and SS sensitivity increase from 433.33 to 4.08 × 106, 250.09 to 8.39 × 106, and 0.083 to 0.573, respectively, as the K value increases from 2 to 12. This indicates the better detection capability and improved electrical characteristics of the DMH-CP-TFET biosensors.
Figure 9 demonstrates the variation in different sensing parameters, such as ION sensitivity, ION/IOFF sensitivity, and SS sensitivity, of DMH-CP-TFET biosensors with negatively charged biomolecules. The negative charge density (Nf) varies from − 1 × 1010 Ccm−2 to − 1 × 1012 Ccm−2. As observed from Fig. 9, the SS sensitivity improves from 0.48% to 57.29% when the Nf varies from − 1 × 1010 Ccm−2 to − 1 × 1012 Ccm−2 at dielectric constant (K = 6).
The ION/IOFF ratio decreases slightly with an increase in Nf, and this decrement in ION/IOFF ratio is because the tunneling barrier width increases with an increase in Nf. Due to this, the tunneling reduces, and ION increases. The ION sensitivity increases from 0.48 to 57.29% as the Nf increases from − 1 × 1010 Ccm−2 to − 1 × 1012 Ccm−2.
The effect of positive charge density on the sensitivity parameters of DMH-CP-TFET is displayed in Fig. 10. As perceived from Fig. 10, the SS sensitivity increases with positively charged biomolecules due to the narrowing of the barrier at the junction. The ION/IOFF ratio reduces with increase in positive charged density (from Nf = 1 × 1010 Ccm−2 to 1 × 1012 Ccm−2). This is due to the enhancement in tunneling width in the cavities.
The reflection of positive and negatively charged biomolecules on the sensitivity parameters of DMH-CP-TFET is depicted in Table 3.
The sensitivity of the proposed DMH-CP-TFET biosensor has been compared with other TFET-based biosensor reported in the literature as mentioned in Table 4
3.2 Selectivity analysis
Another critical criterion for a biosensor is selectivity. It is described as a biosensor's capacity to detect a target biomolecule in the presence of other biomolecules. The target biomolecule with dielectric constant K = 12 is studied here, and the selectivity is investigated in the presence of other biomolecules with dielectric constants K = 2, 4, 6, 8, and 10. It is stated mathematically in the form of equation as
In Fig. 11, the selectivity analysis is performed based on VTH, SS, ION/IOFF, and ION. Selectivity is crucial for accurate and reliable measurements of the target biomolecule in complex biological matrices, such as blood, urine, or saliva. In this investigation, we have chosen the desired biomolecule having dielectric constant, K = 12 for selectivity analysis. It is seen in Fig. 11 that the selectivity of the target biomolecule rises with the relative change in biomolecule dielectric constants (K). With respect to K = 2, the target biomolecule has the highest selectivity. The greater is the difference in the dielectric constant of the biomolecules, better will be the selectivity. Similarly, the selectivity for the other biomolecules may also be computed in the same way.
3.3 Linearity analysis
Linearity is a characteristic that reflects the precision of the measured response to a straight line, and it is mathematically expressed as
To analyze the linearity of the proposed device, we determined the Pearson coefficient correlation (r), which reflects the degree of the linear relationship between the two variables. Figure 12 shows the linear relationship with different biomolecule’s dielectric constants (K = 1, 2, 4, 6, 8, 10 and 12). In Fig. 12a and b, the VTH and SS slope decreases with the increase in the value of the dielectric constant. It shows a strong negative correlation with biomolecules with the Pearson coefficient, r > − 0.978. Similarly, in Fig. 12c and d, the ION and gm slope increase with the increment in the dielectric constant value. The Pearson coefficient, r > 0.913, represents a strong positive correlation with biomolecules to be detected. The strong correlation is reflecting the good linear characteristics of a biosensor. This means that the biosensor can reliably distinguish different biomolecules and produce a response that is directly proportional to those biomolecules’ dielectric constant.
4 Conclusion
In this manuscript, we have investigated the performance of the proposed device in terms of sensitivity, selectivity, and linearity parameters. The sensitivity analysis of the proposed device shows that the maximum ION and ION/IOFF sensitivity of 4.08 × 106 and 8.39 × 106 are obtained when the nano-cavity is filled with a neutral biomolecule (K = 12). The ION/IOFF sensitivity is increased by 97.43% for positively charged biomolecules (1 × 10, K = 6) and 94.98% for negatively charged biomolecules (− 1 × 10, K = 6). The linearity analysis shows a strong correlation with Pearson coefficient, r > 0.97 is achieved for threshold voltage and subthreshold slope. Moreover, the sensitivity, selectivity, and linearity analysis has proven the DMH-CP-TFET-based biosensor to be a potential choice for label-free detection of charged as well as neutral biomolecules. For future work, nanowire-based biosensors are the promising candidate of modern biosensor application. An InAs-based nanowire structure can enhance the sensitivity of the biosensor due to high surface-to-volume ratio.
Data availability
All the data and resulted are derived during the research, included in this article, and cited results have been indicated.
References
H. Im, X.-J. Huang, B. Gu, Y.-K. Choi, A dielectric-modulated field-effect transistor for biosensing. Nat. Nanotechnol. 2(7), 430–434 (2007)
A. Bandiziol, P. Palestri, F. Pittino, D. Esseni, L. Selmi, A TCAD based methodology to model the site-binding charge at ISFET/electrolyte interfaces. IEEE Trans. Electron Devices. 62(10), 3379–3386 (2015). https://doi.org/10.1109/TED.2015.2464251
M. Patil, A. Gedam, G.P. Mishra, Performance assessment of a cavity on source charge plasma TFET based biosensor. IEEE Sens. J. 21(3), 2526–2532 (2021). https://doi.org/10.1109/JSEN.2020.3027031
R.B. Peesa, D.K. Panda, Rapid detection of biomolecules in a junction less tunnel field-effect transistor (JL-TFET) biosensor. SILICON 14, 1705–1711 (2022). https://doi.org/10.1007/s12633-021-00981-0
M. Verma, S. Tirkey, S. Yadav, D. Sharma, D.S. Yadav, Performance assessment of a novel vertical dielectrically modulated TFET-based biosensor. IEEE Trans. Electron Devices 64(9), 3841–3848 (2017). https://doi.org/10.1109/TED.2017.2732820
D. Sarkar, K. Banerjee, Proposal for tunnel-field-effect-transistor as ultra-sensitive and label-free biosensors. Appl. Phys. Lett. 100(14), 143108 (2012)
A. Kumar, S. Kale, A comparative analysis of cavity positions in charge plasma based tunnel FET for biosensor applications. IETE J. Res. (2023). https://doi.org/10.1080/03772063.2023.2189631
D. Singh, S. Pandey, K. Nigam, D. Sharma, D.S. Yadav, P. Kondekar, A charge-plasma-based dielectric-modulated junctionless TFET for biosensor label-free detection. IEEE Trans. Electron Device 64(1), 271–278 (2016). https://doi.org/10.1109/TED.2016.2622403
M. Jagadesh Kumar, K. Nadda, Bipolar charge-plasma transistor: a novel three terminal device. IEEE Trans. Electron Devices 59(4), 962 (2012)
D. Sharma, D. Singh, S. Pandey, S. Yadav, P.N. Kondekar, Comparative analysis of full-gate and short-gate dielectric modulated electrically doped Tunnel-FET based biosensors. Superlattices Microstruct. 111, 767–775 (2017). https://doi.org/10.1016/j.spmi.2017.07.035
Hraziia, A. Vladimirescu, A. Amara, C. Anghel, An analysis of the ambipolar current in Si double-gate tunnel FETs. Solid State Electron. 70, 67–72 (2012). https://doi.org/10.1016/j.sse.2011.11.009
A. Anam, S. Anand, S.I. Amin, Design and performance analysis of tunnel field effect transistor with buried strained Si1−xGex source structure based biosensor for sensitivity enhancement. IEEE Sens. J. 20(22), 13178–13185 (2020). https://doi.org/10.1109/JSEN.2020.3004050
D. Soni, D. Sharma, M. Aslam, S. Yadav, Approach for the improvement of sensitivity and sensing speed of TFET-based biosensor by using plasma formation concept. Micro Nano Lett. 13(12), 1728–1733 (2018)
R. Narang, M. Saxena, M. Gupta, Comparative analysis of dielectric-modulated FET and TFET-based biosensor. IEEE Trans. Nanotechnol. 14(3), 427–435 (2015)
K. Boucart, A.M. Ionescu, Double-gate tunnel FET with High-$\kappa$ gate dielectric. IEEE Trans. Electron Devices 54(7), 1725–1733 (2007). https://doi.org/10.1109/TED.2007.899389
K. Saurabh, M.J. Kumar, Novel attributes of a dual material gate nanoscale tunnel field-effect transistor. IEEE Trans Electron Device 58(2), 404–410 (2011). https://doi.org/10.1109/TED.2010.2093142
J. Gargi, S. Dipanjan, D. Papiya, C. Manash, Power and Delay analysis of dielctric modulated dual cavity junctionless double gate field effect transistor based label free biosensor. Comput. Electr. Eng. 99, 107828 (2022)
R. Das, M. Chanda, C.K. Sarkar, Analytical modeling of charge plasma-based optimized Nanogap embedded surrounding gate MOSFET for label-free Biosensing. IEEE Trans. Electron Device 65(12), 5487–5493 (2018). https://doi.org/10.1109/TED.2018.2872466
S. Kanungo, S. Chattopadhyay, P.S. Gupta, K. Sinha, H. Rahaman, Study and analysis of the effects of SiGe source and pocket-doped channel on sensing performance of dielectrically modulated tunnel FET-based biosensors. IEEE Trans. Electron Devices 63(6), 2589–2596 (2016). https://doi.org/10.1109/TED.2016.2556081
R. Gowsami, B. Bhowmick, Comparative analysis of circular gate TFET and heterojunction TFET for dielectric modulated label free biosensing. IEEE Sens. J. 19(21), 9600–9609 (2019). https://doi.org/10.1109/JSEN.2019.2928182
R. Das, S. Baishya, Analytical modelling of electrical parameters and the analogue performance of cylindrical gate-all-around FinFET. Pramana J. Phys. (2019). https://doi.org/10.1007/s12043-018-1663-5
A. Biswas, S.S. Dan, C. Le Royer, W. Grabinski, A.M. Ionescu, TCAD simulation of SOI TFETs and calibration of non-local band-to- band tunneling model. Microelectron. Eng. 98, 334–337 (2012). https://doi.org/10.5555/2749481.2749636
A. Bhattacharya, M. Chanda, D. De, Performance assessment of new dual-pocket vertical heterostructure tunnel FET- based biosensor considering steric hindrance issue. IEEE Trans. Electron Devices 66(9), 3988–3993 (2019)
A. Chong, H. Liu, S. Wang, S. Chen, Simulation and performance analysis of dielectric modulated dual source trench gate TFET biosensor. Nanosc. Res. Lett. 16(1), 34 (2021)
Funding
Authors do not have to compete for financial interests or personal relationships that can influence the work reported in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Swati, Kaur, J. & Singh, A.K. Performance investigation of an InAs-based dielectric-modulated heterojunction TFET as a label-free biosensor. Appl. Phys. A 129, 365 (2023). https://doi.org/10.1007/s00339-023-06643-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-06643-9