Abstract
The reduced graphene oxide–strontium oxide nanorods (RGO/SrO NRs) were prepared by using simple, eco-friendly hydrothermal synthesis methods. The synthesized composite powder of RGO/SrO NRs was characterized and confirmed by using different X-ray powder diffraction (XRD), Elemental mapping, and transmission electron microscopy (TEM) characterization techniques. From TEM analysis, strontium oxide morphologies in nanorod type structure were uniformly decorated on a graphene sheet. The electrochemical performance of confirmed individual SrO NRs and RGO/SrO NRs is a potential candidate for an electrode material in supercapacitors. They were studied in three different molar concentrations of KOH electrolyte solution and tested in different techniques such as cyclic voltammetry (CV), charge–discharge (GCD), electrochemical impedance spectroscopy (EIS), and cycling stability in the aqueous three electrolyte method. It was observed that the better electrochemical performance of individual SrO NRs and novel binary nanocomposite RGO/SrO NRs could be affected 6 M KOH electrolyte solution than the 1 and 3 M KOH electrolyte solution. The RGO/SrO NRs show higher specific capacitance (Csp) of about 271 Fg−1 at 0.5 Ag−1 current density and exhibits lower solution resistance (Rs) value of about 1.12 Ω in the 6 M KOH electrolyte solution. The capacitance retention of the RGO/SrO NRs in 6 M KOH is about 89.14% for continuous 1000 cycles than in 1 and 3 M KOH electrolyte solution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nowadays, owing to the pollution problems due to burning of fossil fuels (petrol, diesel, and others), in industries and others, we are facing many health and environmental issues; to overcome these issues, developing clean and green energy storage devices such as batteries, capacitors, and supercapacitors as an alternate of fossil fuel is desired [1]. Among these energy storage devices, we preferred the supercapacitors because they have more power density and low energy density than the batteries [2]. These are dependent on the mechanism of charge storage. There are two types of supercapacitors namely electric double-layer capacitors (EDLCs) and pseudocapacitors. The EDLCs materials were related to allotrope carbon (graphene, carbon nanotubes, and activated carbon) which can be charge stored by accumulation at the interface between an electrolyte solution and electrode surface area, and the pseudocapacitors were connected to transition metal oxides/hydroxides [3, 4] and conducting polymers [5, 6], as well as these capacitances derived from the faradic reactions that occur on either the surface or a large portion of an electrode [7, 8]. The allotropes of different forms of carbon such as graphene, activated carbon and single (or) multiwall carbon nanotubes were utilized in various research applications [9,10,11]. Among these, graphene is abundantly available on earth, and is of low cost which has attracted much interest in energy storage fields because it has unique physicochemical properties such as huge surface area (2630 m2/g for monolayer graphene), excellent thermal conductivity, chemical stability and mechanical strength [12]. The individual graphene alone is not suitable for electrochemical applications because it suffers from agglomeration and restacking issues due to the van der walls interactions between layers. The reduced graphene oxide (RGO) exhibits exceptional electrical conductivity that is nearly as good as pristine graphene, in contrast to graphene oxide (GO), which exhibits insulating or semi-conducting properties. For RGO material compared to GO, the elimination of oxygen-containing functional group results in a larger specific surface area. To overcome these issues RGO combined with pseudocapacitor materials such as metal oxides or conducting polymers or alkaline earth metal oxides are used for various applications [13, 14]. These pseudocapacitive materials suffer from poor conducting nature but better storage capacitance and cyclic stability are the advantages for supercapacitor applications. These two types of materials combination are better for energy storage applications due to their synergetic effect of graphene and metal oxide. Among all of the alkaline earth metal oxides/hydroxides such as Be, Mg, Ca, Sr and Ba are utilized in different applications [15,16,17,18]. Among these, we focused on the SrO nanorods combined with graphene to form a novel binary nanocomposite material. The SrO and its different composites were synthesized by various techniques such as the combustion synthesis method [19], chemical precipitation method [20], green synthesis method [21], wet process [22], hydrothermal [23], sol–gel [24], wet impregnation [25], and sonochemical method [26]. The SrO and its composite were used in several applications [27,28,29,30].
According to previous reports, Galal et al., reported the SrRuO3-RGO compound exhibited specific capacitance in different electrolyte solutions such as 1 M NaNO3 (62.4 F/g), 1 M H3PO4 (101 F/g) and 1 M KOH (160 F/g) respectively [31]. Hyun et al., represented the SrRuO3-RuO2 compound-specific capacitance of about 192 F/g in 1 M H2SO4 [32]. Adinaveen et al., showed the SrO-ACC material specific capacitance of about 400 F/g in 1 M H2SO4 [33]. Iqbal et al., reported by a binary composite of SrO-PANI material exposed an extreme Csp of about 258 C/g in 1 M KOH [26]. Iqbal et al. prepared by novel strontium related composite showed the Csp of 175 C/g in 1 M KOH [34]. Tomar et al. presented the SrTiO3 thick and ultrathin film showing the specific capacitance of about 46 and 592 Fg−1 in 3 M KOH electrolyte [35].
In the current work, we synthesized pure SrO NRs and novel binary RGO/SrO NRs by simple hydrothermal method for supercapacitor applications. The prepared compounds were characterized by XRD, EDAX, and TEM analysis. The SrO NRs and novel binary RGO/SrO NRs composite were tested for the electrochemical properties in different concentrations 1, 3, and 6 M of KOH electrolyte solution by different parameters such as CV, GCD, and EIS were related to supercapacitors. The synthesized individual SrO NRs and novel binary RGO/SrO NRs were first time prepared and tested for supercapacitors.
2 Experimental details
2.1 Materials
Graphite flakes, strontium nitrate (Sr(NO3)2), potassium hydroxide (KOH), sodium borohydride (NaBH4), super p carbon, poly vinyl difluoride (PVDF) and NMP solution were purchased from Merck Laboratories Pvt. Ltd., Mumbai, India.
2.2 Synthesis of RGO/SrO nanorods
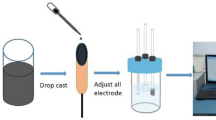
Graphite oxide (GO) was prepared through a chemical approach by modified Hummers’ method [4]. The reduced graphene oxide-strontium oxide nanorods (RGO/SrO NRs) nanocomposite synthesized through the facile hydrothermal method is as shown in Fig. 1. First, 10 mg of GO was distributed in 10 ml of distilled water through sonication for 1 h to foam a brown suspension, and then 0.2 M Sr(NO3)2 was dissolved in 35 ml of distilled water and 3 M KOH was dissolved in 35 ml of distilled water to added above suspension under stirring condition for 30 min. Then 30 mg of NaBH4 was added to the above solution and stirred for 30 min then transferred into 100 ml autoclave closed and kept in a hot air oven at 150 °C for 3 h. After the reaction was complete, the finished product was rinsed in ethanol and water. After that, the precipitate was followed by dried at 80 °C for overnight to obtain RGO/SrO composite. The same procedure was followed without GO to obtain SrO NRs.
2.3 Materials characterization
The crystal structure of SrO NRs and RGO/SrO NRs was confirmed by the Rigaku X-ray diffractometer (Miniflex 600). The surface morphology and elemental mapping of the prepared RGO/SrO NRs composite were collected by TEM (FEI Technai G2 F20 X-Twin) along with EDAX (AMETEK).
2.4 Synthesis of working electrode
The preparation of the working electrode by using the active material (70%), carbon (15%), and binder PVDF (15%). These were well grained with the use of NMP solution and coated on a nickel foam sheet followed by drying at 80 °C overnight. The electrochemical performance was tested by the three-electrode method. Platinum wire as a counter electrode, Ag/AgCl as a reference electrode, and prepared electrode as a working electrode.
3 Results and discussion
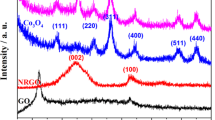
X-ray diffraction (XRD) analysis was performed to examine the crystal structure of the as-prepared SrO and RGO/SrO NRs composite as shown in the Fig. 2a, b. The observed diffraction peaks with suggestions for 2θ values with diffraction peaks at 20.19 (112), 25.28 (202), 36.39 (310), 41.40 (111), 44.20 (200), 47.81 (210), 50.01 (211), 57.27 (220), 64.01 (221), 73.67 (310) were related to SrO. The extra appear diffraction peaks 22.57 (210), 29.65 (201), 32.96 (211), 46.70 (321) related to Sr(OH)2 and 35.21 (110), 39.70 (201), 45.64 (112), (59.87) related to SrO2, respectively. All the observed peaks in the spectrum were assigned by using the JCPDS card (No: 001-1113, 6-520, 75-0263, and 01-073-0661). The strong diffraction peaks represent the crystalline nature and purity of the compounds [24, 36, 37].
The crystalline size of the synthesized materials is calculated by using the Debye Scherrer formula.
Here, the parameters such as K, λ, β and θ denote the Scherrer constant, wavelength of radiation used in recording of X-ray diffraction, Full-Width at Half Maxima (FWHM) and angular position of particular XRD peak, respectively. The crystalline sizes of the SrO and RGO/SrO at the maximum diffraction peak is about 26. 81 nm and 10.2 nm, respectively It was clearly observed that the SrO and RGO/SrO diffraction peak intensity was different.
A well-known plot called the "Williamson-Hall Plot" can be used to determine the properties of materials, which include the contributions of crystallite size and micro strain.
The broadening of peaks in this plot are expressed as;
The values of βD = \(\frac{K\lambda }{{D Cos\theta }}\) and \(\beta \varepsilon = \varepsilon \tan \theta\). Using these values in above equation, a final expression is attained as [38,39,40]
For the synthesized samples, the W–H plots have been generated and shown in Fig. 3a, b. The experimental data of synthesized samples show a straight line (\(y = mx + c)\) having slope (\(m = \varepsilon\)) and intercept \(\left( {C = \frac{K\lambda }{D}} \right)\). By using the value of intercept and slope, the micro-strain was calculated at the maximum diffraction peak of the SrO and RGO/SrO which are about 5.77⨯10–3 and 7.31⨯10–3, respectively. Here, we observed that the crystalline size was directly proportional to an intensity of diffraction peaks and the crystalline size was inversely proportional to the micro strain of the material. Figure 2b represents the RGO/SrO NRs composite which was well matched with the SrO diffraction. The GO diffraction peak appears at 11.7° but when the GO converts into RGO, the diffraction peak angle was shifted to 25.8°. The RGO peak merged with the SrO diffraction peak at 25.28°. When we added a very low quantity of GO to SrO for the preparation RGO/SrO NRs composite, an intensity of the RGO/SrO diffraction peaks were reduced and the evidence of the RGO was confirmed in the TEM and EDAX results.
The morphological analysis was confirmed by electron microscopy techniques such as the SEM and TEM. Figure 4a, b shows secondary electron micrographs of SrO material showing the rod-type structure and which was further confirmed using the TEM technique. Figure 5a–e shows TEM images of the RGO/SrO NRs composite images. The synthesized RGO/SrO nanorods through facile hydrothermal method had a well-defined rod like morphology with an average diameter of about 70 nm. The SrO nanorods were formed by non-uniform diameters. The SrO nanorods were uniform and well deposited on the RGO sheets as clearly shown in Fig. 5a–e which is in good agreement with the SEM data. Figure 5e represents the d-spacing value of about 0.468 nm of the SrO nanorod and Fig. 5f shows the SAED pattern of RGO/SrO. It was clearly shown based on the diffused ring pattern which was arrived from RGO sheets and the spotty pattern observed from SRO nanorods.
The RGO/SrO NRs composite contains elemental composition analyzed by EDAX as shown in Fig. 6. The strontium (Sr), oxygen (O) and carbon (C) elements are present in the RGO/SrO NRs composite. The weight percentage and elemental mapping of RGO/SrO nanocomposite are shown in the insight of Fig. 6. The weight percentage also exactly matched with an added weight percentage of GO and Sr precursor materials. Finally, in this nanocomposite, there were no other indicated impurity elements that indicate the purity of the synthesized RGO/SrO NRs composite.
3.1 Electrochemical performance
The electrochemical activities of SrO NRs and RGO/SrO NRs composite were tested by CV, GCD, and EIS techniques in various aqueous 1 M, 3 M, and 6 M KOH electrolyte solutions by using ZIVE Sp1 instrument. Figure 7 shows the pseudocapacitor nature CV curves of synthesized SrO NRs and RGO/SrO NRs composite material at different scan rates with a three-electrode system and potential window in the range of 0.0–0.5 V in various concentrations of aqueous electrolyte KOH solution. Figure 7a denotes the CV loops of the SrO NRs in three concentrations of KOH electrolyte solution at 100 mVs−1 scan rate. The SrO NRs were tested in various concentrations of KOH solution to achieve better supercapacitor performance and exposure to 6 M KOH electrolyte solution showed better response to more current compared to 1 M and 3 M KOH electrolyte solutions, thus 6 M KOH solution is more suitable for supercapacitive nature with SrO NRs as a to achieve a promising working electrode. Figure 7b represents the CV curves of SrO NRs in 6 M KOH electrolyte solution with various scan rates from 5 to 100 mVs−1. Figure 7c reveals the RGO/SrO NRs composite CV curves at 100 mVs−1 in 1 M, 3 M, and 6 M KOH electrolyte solutions. Among these solutions, the 6 M KOH exhibited a better current response than in 1 M and 3 M KOH solutions. Figure 7d shows the RGO/SrO NRs composite at different scan rate CV curves in a 6 M KOH solution. The SrO NRs and RGO/SrO NRs both have better current response in 6 M KOH than in the 1 M and 3 M KOH because the conductivity of electrolyte ions play a key role in a certain value of concentration and the electrolyte ion mobility also decreases at higher concentration and due to these reasons, the 6 M KOH electrolyte exposed excellent capacitive performance than in the 1 M and 3 M KOH solution [3, 41,42,43]. The specific capacitance (Csp) of the SrO and RGO/SrO NRs at different scan rates are calculated from given below formula.
where I (V) denotes the average catholic current, s denotes scan rates (V s−1), m signifies the mass of coated material (g) and ΔV indicates the potential difference (V) respectively. The Csp of the SrO NRs and RGO/SrO NRs at 5, 10, 20, 40, 60, 80 and 100 mVs−1 scan rates are about 367.51 Fg−1, 317.45 Fg−1, 273.55 Fg−1, 243.68 Fg−1, 216.14 Fg−1, 202.5 Fg−1, 164.09 Fg−1, 514.33 Fg−1, 406.47 Fg−1, 353.19 Fg−1, 309.70 Fg−1, 277.24 Fg−1, 233.75 Fg−1, 214.92 Fg−1 respectively. The Csp of the SrO NRs and RGO/SrO NRs in 1, 3 and 6 M KOH electrolyte solution at 100 mVs−1 scan rate is about 28.56 Fg−1, 85.80 Fg−1, 164.09 Fg−1 and 152.22 Fg−1, 208.77 Fg−1, 214.92 Fg−1, respectively. Finally, the 6 M KOH is better suitable for SrO and RGO/SrO NRs supercapacitor electrode materials.
In the synthesized SrO NRs and RGO/SrO NRs composite, an important parameter is GCD curves as shown in Fig. 8. These GCD curves showed the potential window range from 0.0 to 0.4 V in 1 M, 3 M and 6 M KOH electrolyte solution. Figure 8a, c denotes the SrO NRs and RGO/SrO NRs composite GCD curves in three molar concentrations of KOH electrolyte solution. Among these different concentrations of electrolyte solutions, the synthesized electrode materials such as SrO NRs and RGO/SrO NRs were exposed to high discharge time in 6 M KOH electrolyte solution at 0.5 A/g current density. The SrO NRs and RGO/SrO NRs GCD curves at different current densities is as shown in Fig. 8b, d. The specific capacitance (Csp), energy density (ED) and power density (PD) of prepared working electrode materials are calculated by the below given formula.
Here, the above formula terms are discharge time (Δt) in (s), current (I) in (Amp), potential window (ΔV) in (Volts), and mass of the active material (m) in (grams) coated on the working electrode substrate. The Csp values of the SrO NRs and RGO/SrO NRs at 0.5 Ag−1 current density in 1, 3 and 6 M KOH electrolyte solutions are 45, 136, 212 and 150, 191, 271 Fg−1 respectively. Figure 9a, b shows the SrO NRs and RGO/SrO NRs composite material Csp values at different current densities in 1, 3 and 6 M KOH electrolyte, and the Csp values are shown in Table 1. The energy and power density of SrO NRs and RGO/SrO NRs composite materials calculated at 0.5 Ag−1 current density in 6 M KOH electrolyte solution are about 11.77, 15.05, and 240.75, 248 Wkg−1 respectively. The SrO NRs and RGO/SrO NRs energy and power density at various current densities as shown in Fig. 10.
The electrochemical impedance spectra of SrO NRs and RGO/SrO NRs were examined in different concentrations of KOH electrolyte as revealed in Fig. 11. The range of frequency region taken from 0.1 Hz to 100 kHz with an AC signal at 5 mV amplitude. In Fig. 11a, b represents a very low semicircle at a high frequency region recognized to the charge transfer resistance and a straightforward line at a lower frequency region ascribed to the ion diffusion resistance was confirmed in the Nyquist plots. The semicircle loop not appearing in the Nyquist plots suggested that all electrode materials contain lower charge transfer resistance (Rct) values. In Fig. 11a, the SrO NRs in 6 M KOH electrolyte solution exposed lower solution resistance (Rs) value (1.33 Ω) than in the 1 M KOH (4.44 Ω) and 3 M KOH (1.91 Ω) electrolyte solution. In Fig. 11b, the RGO/SrO NRs also show a lower Rs value (1.12 Ω) in the 6 M KOH electrolyte solution than in the 1 M KOH (1.99 Ω) and 3 M KOH (1.62 Ω) electrolyte solution. Figure 11c represents, the RGO/SrO electrode Nyquist plot after continuous 1000 cycles in 1 M, 3 M and 6 M KOH solution. Here, the Rs values were increased compared with before continuous 1000 cycles. Among these, in 6 M KOH solution the RGO/SrO Rs value (1.54 Ω) was slightly increased compared with 1 M (2.12 Ω) and 3 M KOH (2.41 Ω) electrolyte solution. An increased Rs values effect shown on the cyclic stability performance. Finally, both electrodes exhibited lower Rs values in 6 M KOH solution so it is good conductivity nature and holds the lower Rs values.
Cyclic stability performance is another important parameter for electrochemical performance studies as shown in Fig. 12. The RGO/SrO NRs cyclic stability was examined in 1, 3, and 6 M KOH electrolyte solution at 5 Ag−1 current density as shown in Fig. 12a–c respectively. The RGO/SrO first five cycles and last five cycles in 1, 3, and 6 M KOH solution are as shown in the insight of Fig. 12a–c, respectively. The RGO/SrO NRs first and final cycle (1000) specific capacitance in 6 M KOH solution is about 175 Fg−1 and 156 Fg−1 at 5 Ag−1 current density. The decay of specific capacitance from the initial to final cycle is about 89.14%. For comparison purpose, the RGO/SrO in 6 M KOH electrolyte solution capacity retention is about 89.14% than in 1 M (58.02%) and 3 M (65.33%) KOH electrolyte solution. Finally, the novel RGO/SrO electrode in 6 M KOH shown better capacity retention percentage so it is a better evidence of the cyclic stability performance for supercapacitor application studies.
4 Conclusion
In summary, the novel binary nanocomposite of RGO/SrO NRs was prepared by a facile hydrothermal method. The structural, morphology, and elemental composition information of SrO NRs and RGO/SrO NRs were confirmed by XRD, TEM, and EDAX analysis. All electrochemical properties such as CV, GCD, and EIS were well satisfied and matched the SRO NRs and RGO/SrO NRs electrodes were exposed to better performance in 6 M KOH than the 1 and 3 M KOH electrolyte solution. These electrodes exhibit higher specific capacitance of about 212 Fg−1 and 271 Fg−1 at 0.5 Ag−1 current density and lower Rs values of about 1.33 Ω and 1.12 Ω in 6 M KOH electrolyte solution compared with in 1 and 3 M KOH. The capacitance retention of the RGO/SrO NRs in 6 M KOH is about 89.14% for continuous 1000 cycles than in 1 M and 3 M KOH electrolyte solution. Finally, we conclude that the 6 M KOH electrolyte solution is better suitable for inexpensive prepared the RGO/SrO NRs for supercapacitor application electrode material.
Data availability
Sreenivasa Kumar Godlaveeti: Conceptualization, Methodology, validation, investigation, data curation, Writing-Original draft preparation and writing–review and editing. Adinarayana Reddy Somala: Writing-Reviewing and Editing. Ramamanohar Reddy Nagireddy: Supervision, Writing-Reviewing and Editing.
References
H. Liu, X. Liu, S. Wang, H.K. Liu, L. Li, Energy Storage Mater. 28, 122 (2020)
S. Kumar, G. Saeed, L. Zhu, K.N. Hui, N.H. Kim, J.H. Lee, Chem. Eng. J. 403, 126352 (2021)
S.K. Godlaveeti, S. Jangiti, A.R. Somala, H. Maseed, R.R. Nagireddy, J. Clust. Sci. 32, 703–710 (2021)
G.S. Kumar, S.A. Reddy, H. Maseed, N.R. Reddy, Funct. Mater. Lett. 13, 1 (2020)
M. Tahir, L. He, W. Yang, X. Hong, W.A. Haider, H. Tang, Z. Zhu, K.A. Owusu, L. Mai, J. Energy Chem. 49, 224 (2020)
J. Li, J. Qiao, K. Lian, Energy Storage Mater. 24, 6 (2020)
B.E. Conway, V. Birss, J. Wojtowicz, J. Power Sources 66, 1 (1997)
P. Simon, Y. Gogotsi, P. Simon, Nat. Mater. 7, 845 (2008)
V. Dhinakaran, M. Lavanya, K. Vigneswari, M. Ravichandran, M.D. Vijayakumar, Mater. Today Proc. 27, 824 (2020)
R. Bahru, N. Shaari, M.A. Mohamed, Int. J. Energy Res. 44, 2471 (2020)
S.H.K. Yap, K.K. Chan, S.C. Tjin, K.T. Yong, Sensors. 20, 2046 (2020)
E. Oz, S. Demirel, S. Altin, J. Alloys Compd. 671, 24 (2016)
Ö. Güler, Ç. Yavuz, Ö. Başgöz, S. Altın, I.S. Yahia, J. Mater. Sci. Mater. Electron. 31, 3184 (2020)
N. Bugday, M.N. Ates, O. Duygulu, W. Deng, X. Ji, S. Altin, S. Yasar, J. Alloys Compd. 928, 167037 (2022)
V. Altunal, V. Guckan, A. Ozdemir, K. Kurt, A. Ekicibil, Z. Yegingil, Opt. Mater. (Amst). 108, 110436 (2020)
J. Yan, Z.H. Pan, C.Y. Zhao, Appl. Energy 275, 115356 (2020)
A. Cosquillo Mejia, S. Afflerbach, M. Linder, M. Schmidt, Appl. Therm. Eng. 169, 114961 (2020)
M. Appiah, H. Hao, Z. Liu, X. Jiang, M. Emmanuel, J. Abdullah, Z. Yao, M. Cao, H. Liu, J. Phys. Chem. C 124, 14089 (2020)
F. Granados-Correa, J. Bonifacio-Martínez, Mater. Sci. Pol. 32, 682 (2014)
K.R. Nemade, S.A. Waghuley, Results Phys. 3, 52 (2013)
A.A. Gungor, H. Nadaroglu, D.D. Gultekin, Chem. Sci. Int. J. 26, 1 (2019)
T. Athar, Mater. Focus 2, 450 (2013)
S. Harish, M. Sabarinathan, J. Archana, M. Navaneethan, K.D. Nisha, S. Ponnusamy, V. Gupta, C. Muthamizhchelvan, D.K. Aswal, H. Ikeda, Y. Hayakawa, Appl. Surf. Sci. 418, 147 (2017)
S. Sultana, Rafiuddin, M.Z. Khan, K. Umar, A.S. Ahmed, M. Shahadat, J. Mol. Struct. 1098, 393 (2015)
J. Tantirungrotechai, S. Thepwatee, B. Yoosuk, Fuel 106, 279 (2013)
M.Z. Iqbal, S. Alam, A.M. Afzal, M.J. Iqbal, K. Yaqoob, M.A. Kamran, M.R.A. Karim, T. Alherbi, Solid State Ionics 347, 115276 (2020)
X. Yue, S. Yi, R. Wang, Z. Zhang, S. Qiu, Nano Energy 47, 463 (2018)
V. Senthil, S. Panigrahi, Int. J. Hydrogen Energy 44, 18058 (2019)
H. Li, L. Shen, B. Ding, G. Pang, H. Dou, X. Zhang, Nano Energy 13, 18 (2015)
H. Wang, J. Liu, J. Zhai, Z. Pan, B. Shen, J. Eur. Ceram. Soc. 37, 3917 (2017)
A. Galal, H.K. Hassan, T. Jacob, N.F. Atta, Electrochim. Acta 260, 738 (2018)
T.S. Hyun, H.G. Kim, I.D. Kim, J. Power Sources 195, 1522 (2010)
T. Adinaveen, J. Judith Vijaya, M. Amal Raj, P. IyyappaRajan, L. John Kennedy, N. ClamentSagaya Selvam, J. Electroanal. Chem. 799, 222 (2017)
M.Z. Iqbal, A. Khan, A. Numan, S.S. Haider, J. Iqbal, Ultrason. Sonochem. 59, 104736 (2019)
A.K. Tomar, G. Singh, R.K. Sharma, J. Power Sources 426, 223 (2019)
M.M. Hussain, M.M. Rahman, A.M. Asiri, Microchim. Acta 183, 3265 (2016)
B. Marí, K.C. Singh, M. Moya, I. Singh, H. Om, S. Chand, Opt. Mater. (Amst). 34, 1267 (2012)
K.K. Khichar, S.B. Dangi, V. Dhayal, U. Kumar, S.Z. Hashmi, V. Sadhu, B.L. Choudhary, S. Kumar, S. Kaya, A.E. Kuznetsov, S. Dalela, S.K. Gupta, P.A. Alvi, Polym. Compos. 41, 2792 (2020)
V. Dhayal, S.Z. Hashmi, U. Kumar, B.L. Choudhary, A.E. Kuznetsov, S. Dalela, S. Kumar, S. Kaya, S.N. Dolia, P.A. Alvi, J. Mater. Sci. 55, 14829 (2020)
A. Kumari, K. Kumari, F. Ahmed, A. Alshoaibi, P.A. Alvi, S. Dalela, M.M. Ahmad, R.N. Aljawfi, P. Dua, A. Vij, S. Kumar, Vacuum 184, 109872 (2021)
Y. Zhao, M. Hao, Y. Wang, Y. Sha, L. Su, J. Solid State Electrochem. 20, 81 (2016)
F. Md Jamil, M.A. Sulaiman, S. Mohd Ibrahim, A.K. Masrom, M.Z.A. Yahya, Adv. Mater. Res. 1133, 3 (2016)
S. Kumar, G. Srivastava, G. Almutairi, F. Ahmed, N.M. Shaalan, S. Dalela, R. Kumar, A.P. Kumar, P.A. Alvi, K.H. Chae, H.H. Hammud, K. Kumari, J. Electron Spectrosc. Relat. Phenom. 253, 147138 (2021)
Acknowledgements
The authors are thankful to the RUSA facility lab for providing the electrochemical performance at Yogi Vemana University, Kadapa-516005.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors state that there is no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Godlaveeti, S.K., Somala, A.R. & Nagireddy, R.R. Efficient electrochemical performance of the RGO/SrO nanorods prepared by the hydrothermal method. Appl. Phys. A 129, 303 (2023). https://doi.org/10.1007/s00339-023-06610-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-06610-4